Decreased Dopamine Concentrations in the Frontal Cortex After Ablative Surgeries in Patients Exhibiting Self-Injurious Behavior: A Microdialysis Study
Abstract
The authors examined brain neurochemistry in four patients with mental retardation with self-injurious behaviors after ablative surgeries. The authors found that surgeries in the human limbic system can alter dopamine levels in the frontal cortex over a 36-hour period.
For patients with mental retardation, self-injurious behavior (SIB) is a dangerous and possibly life-threatening symptom. SIB is not relieved in some patients even after intensive pharmacotherapy and extensive behavioral and instructive measures. Physical restraint is often used to protect these patients from hurting themselves. Although it remains controversial, stereotactic ablative surgery may still be a rational choice for these patients. Moreover, there are many successful, published cases of stereotactic interventions for SIB or aggressive behavior using amygdalotomies,1,2 cingulotomies,3,4 or limbic leucotomies,3,5 but the exact mechanisms by which these interventions are effective are still unclear. In the present study, we determined quantitative neurochemical changes in the cerebral cortex of patients with mental retardation with severe SIB using microdialysis after they had undergone combined stereotactic bilateral amygdalotomies and cingulotomies. The aim of this study was to directly determine changes in cortical neurochemistry following these lesions to the human limbic system.
Patients and Methods
Case Series
This study included four male patients with severe mental retardation and SIB, from 16 to 27 years of age (mean, 19 years). They all presented with a history of frequently beating their head or banging their forehead against a wall for 6–20 years (mean, 12 years). Three patients also occasionally exhibited physically violent outbursts toward their family. They were diagnosed with mental retardation because of low intelligence. They all were repeatedly hospitalized in a psychiatric hospital for SIB, and their current medications included haloperidol, risperidone, clozapine, valproate, and clonazepam. Importantly, all these efforts failed to result in adequate alleviation of their dangerous behaviors. MRI and CT scans failed to find any brain abnormalities for these patients. Brain [18F]fluorodeoxyglucose (FDG)-positron emission tomography (PET) was performed in two patients under anesthesia, and the results showed low baseline metabolism in their left frontal lobe.
Stereotactic Surgery and Microdialysis Procedures
All four patients underwent combined stereotactic bilateral amygdalotomies and cingulotomies. Before the surgery, each patient’s case was submitted to our institution’s bioethics committee. Each surgery was undertaken only after careful review and approval by this committee. The patient’s parents were fully informed about the risks and possible benefits of the surgery, and a written consent form was obtained. Details regarding the target and the surgical techniques in general can be found elsewhere.1 There were two steps for microdialysis. First, the microdialysis cannula (CMA 70; CMA Microdialysis, Solna, Sweden) was inserted into the left frontal cortex of each patient before making the lesions. Microdialysis was performed using Ringer’s solution in the operating theater (flow rate, 0.3 μL/min) using a microdialysis pump (CMA 106; CMA Microdialysis). Samples were collected for 180 minutes, and the microvials were changed approximately every 60 minutes. The samples were stored at −80°C. Second, after surgery, the microdialysis cannula was kept in the same place, and the patient was transferred to a neurointensive care unit. Microdialysis was performed continuously in all four patients using the same procedures for at least 36 hours. The collected samples were analyzed by high-performance liquid chromatography as described previously.6 After each surgery, a brain CT scan was carried out to identify the location of the cannula tip. The microdialysis cannulae were removed from the patients at different time points.
Results
Clinical Results
After surgery, one patient had a “taming effect” following surgery, and during the 4-year follow-up period, no episode of SIB was reported. Two patients had short-term relief of SIB and resumed as before the operation. The fourth patient, the oldest among the four patients, had no benefit of the surgery during the 3-year follow-up period.
Neurotransmitter Changes
The average concentrations of dopamine, norepinephrine, 5-hydroxyindoleacetic acid, GABA, and glutamine in the left cortex at different times after the operations were analyzed. Only the dopamine concentration showed significant decreases during the 36-hour observation period. Compared with the baseline concentration, dopamine concentrations at 15–36 hours postoperatively were significant lower (Figure 1).
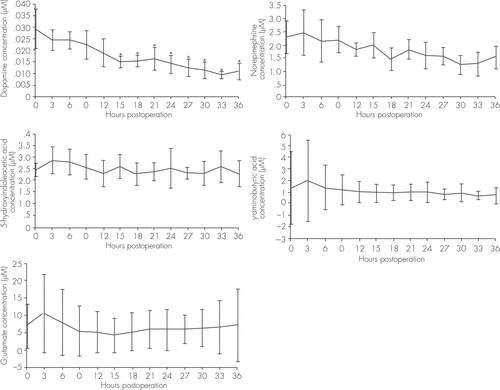
FIGURE 1. Time Course of the Concentration Changes of Neurotransmitters in the Cortices of Four Patients Over the 36 Hours After Their Surgeriesa
a Variables were compared by one-way analysis of variance and Dunnett tests. Statistical significance was determined at p<0.05, and SPSS 10.0 was used for all analysis. (A) Dopamine changes, F=3.833, *p<0.05 compared with the baseline. (B) Norepinephrine changes, F=1.307. (C) 5-Hydroxyindoleacetic acid changes, F=0.504. (D) GABA changes, F=0.404. (E) Glutamine changes, F=0.411.
Discussion
Microdialysis has provided us with a new approach for investigating the neurochemistry of the living brain. Previously, cerebral microdialysis in the human has been used to study the pathophysiology of brain injuries,7,8 cerebral vascular disease,9 and epilepsy.10 Because the microdialysis is a time-consuming procedure and only small volume of samples could be collected in one patient, amino acids, monoamines, and peptides could not be analyzed at the same time. In the present study, we used microdialysis to analyze amino acid and monoamine neurotransmitter changes in four patients with SIB after their ablative surgeries. Because the values of amino acid and monoamine neurotransmitters could be affected by sample collecting time and determination methods, the first samples collected before ablative procedures were set as baseline. Moreover, to avoid additional tissue damage in these patients by the microdialysis procedures, we used the same cortical approach for both the monopolar electrocautery probes and the microdialysis cannulae. The present results provide the first direct measures of neurochemical changes following ablative surgeries to the human limbic system.
Today, for mentally retarded patients with SIBs, the first-line medicines are classic dopamine D2 receptor antagonists, such as risperidone11 and haloperidol.12 This suggests that a high level of dopamine is involved in SIBs. In this study, we found that the average dopamine levels in the cortex were lower postoperatively in four patients. This result may partially explain the effectiveness of these ablative surgeries as loss of dopamine in the frontal cortex is functionally similar but perhaps more selective than systemic administration of a dopamine D2 receptor antagonist. We suggest that a broken Papez circuit could affect cerebral functions that influence human emotions, likely through the dopaminergic system. This finding of decreased of dopamine in the frontal cortex could also explain why most patients gain transient benefits after ablative surgeries. The transient nature of the benefits of these surgeries implies that the dopamine decrease in the frontal cortex may be temporary. This implication does not support the use of ablative surgeries such as amygdalatomies and cingulatomies in patients with SIB. Indeed, only one of the patients in this study gained long-term satisfactory outcome during the follow-up period. Therefore, we suggest a more cautious approach to the use of ablative surgeries in patients experiencing SIB.
1 : Bilateral stereotactic amygdalotomy for self-mutilation disorder: case report and review of the literature. Stereotact Funct Neurosurg 2007; 85:121–128Crossref, Medline, Google Scholar
2 : Historical evolution of stereotactic amygdalotomy for the management of severe aggression. J Neurosurg 2007; 106:710–713Crossref, Medline, Google Scholar
3 : Modern neurosurgery for psychiatric disorders. Neurosurgery 2000; 47:9–21, discussion 21–23Medline, Google Scholar
4 : Historic evolution of open cingulectomy and stereotactic cingulotomy in the management of medically intractable psychiatric disorders, pain and drug addiction. Stereotact Funct Neurosurg 2009; 87:271–291Crossref, Medline, Google Scholar
5 : Limbic leucotomy in self-mutilation. J Clin Psychiatry 2002; 63:1181, author reply 1181–1182Crossref, Medline, Google Scholar
6 : Dose- and time-dependent, context-induced elevation of dopamine and its metabolites in the nucleus accumbens of morphine-induced CPP rats. Behav Brain Res 2009; 204:192–199Crossref, Medline, Google Scholar
7 : Evidence for prolonged release of excitatory amino acids in severe human head trauma: relationship to clinical events. Ann N Y Acad Sci 1995; 765:290–297, discussion 298Crossref, Medline, Google Scholar
8 : Temporal profile of release of interleukin-1beta in neurotrauma. Neurosci Lett 2000; 284:135–138Crossref, Medline, Google Scholar
9 : Intracerebral human microdialysis: in vivo study of an acute focal ischemic model of the human brain. Stroke 1995; 26:870–873Crossref, Medline, Google Scholar
10 : Prediction of antiepileptic drug efficacy: the use of intracerebral microdialysis to monitor biophase concentrations. Expert Opin Drug Metab Toxicol 2009; 5:1267–1277Crossref, Medline, Google Scholar
11 : Risperidone use in children with Down syndrome, severe intellectual disability, and comorbid autistic spectrum disorders: a naturalistic study. J Dev Behav Pediatr 2008; 29:106–116Crossref, Medline, Google Scholar
12 : Minimally effective doses of conventional antipsychotic medications used to treataggression, self-injurious and destructive behaviors in mentally retarded adults. J Clin Psychopharmacol 2005; 25:19–25Crossref, Medline, Google Scholar



