Neural Evidence for Compromised Mental Imagery in Individuals With Chronic Schizophrenia
Abstract
Mental imagery impairment has been reported in schizophrenia. The present study aimed to investigate the neural evidence for mental imagery impairment in patients with schizophrenia. The study participants included 20 patients with chronic schizophrenia and 18 healthy control subjects. Event-related potentials were recorded during a mental hand rotation task, in which participants were instructed to judge the laterality of hands displayed in different orientations. The performances of patients were significantly less accurate and slower than control subjects on hand rotation task. Moreover, the patients showed significantly reduced rotation-related negativity amplitude for mental rotation effect. The results demonstrate mental imagery impairment in patients with schizophrenia at both the behavioral and neural levels.
Mental imagery is a higher cognitive function, which is a mental representation of physical objects and events occurring in the absence of the appropriate stimuli. A specific type of mental imagery is mental rotation, described as the ability to imagine how a misoriented object would be appeared if rotated away from the presented orientation.1 Mental rotation is typically studied by using Parson’s hand rotation paradigm in which individuals judge the laterality of pictures of left or right hands in different angles of rotation.2 In general, reaction times and error rates increase as a function of the rotation angle of the stimulus, indicating that individuals engage in a cognitive process of mental rotation.
Recent studies indicated that mental imagery ability is compromised in individuals with schizophrenia.3–6 For instance, studies have found that, although patients were slower and less accurate than control subjects on mental rotation tasks, they displayed the same decrease in speed and performance with increasing angles of rotation as control subjects.4,7 Although this information suggests impaired mental rotation in the patients, there is a scarcity of evidence about electrophysiological correlates of the mental rotation process in patients with schizophrenia, which is the main aim of the present study.
Many studies have examined mental rotation by means of event-related potentials (ERPs), because of its high temporal resolution. ERP studies have found the mental rotation process is reflected in a modulated positive wave of about 300–700 ms (P300), with decreasing amplitudes for increasing angles of rotation.8,9 This slow decrease in amplitude is supposed to be due to a superimposed negativity on the concurrently prevailing P300 complex, so-called rotation-related negativity (RRN).10–12 RRN is a parietal negative slow wave, most pronounced at the parietal electrodes, that is stronger for rotated compared with upright stimuli. RRN is assumed as an electrophysiological correlate of the mental rotation process.9,13 Decreased amplitudes and increased latencies of RRN have been reported in patients with right hemiparetic cerebral palsy and patients with multiple sclerosis.13,14 Although evidence indicates impaired mental rotation in schizophrenia, the question remains whether impaired mental imagery in patients with schizophrenia would be demonstrated by alteration of RRN in the mental hand rotation task.
In summary, the present study aimed to assess RRN during the performance of mental imagery in patients with schizophrenia. To reach this aim, the performance of a group of patients was compared with that of a group of healthy matched control subjects on a mental hand rotation task while simultaneously measuring ERP.
Methods
Participants
A group of 20 patients with schizophrenia (14 men) was recruited from outpatients of a psychiatric hospital. All patients met DSM-IV criteria for a lifetime diagnosis of schizophrenia. All patients were assessed using the Scale for Assessment of Negative Symptoms (SANS)15 and the Scale for Assessment of Positive Symptoms (SAPS).16 At the time of testing, patients were receiving antipsychotic medication (N=15, atypical; N=5, on both typical and atypical antipsychotics) and were clinically stable. The mean chlorpromazine equivalent dose was 318.5 mg.17
The control group consisted of 18 healthy participants (14 men) screened for a personal or family history of psychotic illnesses. Exclusion criteria for all participants included head injury, neurological disorder, and current substance abuse. All participants had normal or corrected-to-normal vision and were right handed. They all were competent to give written informed consent prior to the involvement in the study. The study was approved by the Ethics Committees of Kerman University of Medical Sciences.
Assessment Procedures
Hand rotation task stimuli.
The stimuli were line drawings of both back and palm views of the left and right hands, which presented in six different orientations (0°, 60°, 120°, 180°, 240°, and 300°) (see Figure 1). Each picture was shown 15 times, resulting in 360 trials (6×2×2×15). All stimuli were displayed on a 19-inch computer screen, at a distance of about 80 cm from the participants’ eyes, resulting in a visual angle of approximately 5.7°.
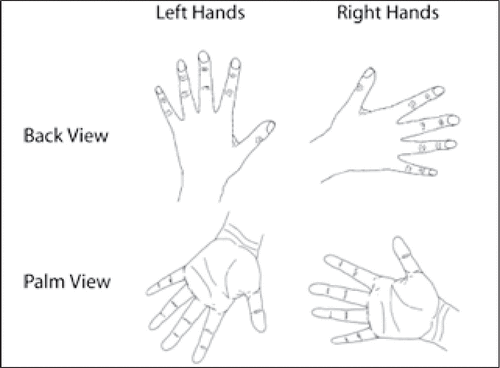
FIGURE 1. Examples of the Line Drawings of Left and Right Hands Viewed From the Palm and From the Back with Different Rotation Angles (0°, 60°, 120°, 180°, 240°, and 300°)
Experimental procedure
Parson’s hand rotation paradigm was used to implicitly evoke mental imagery.2 Participants had to judge whether a given stimulus was a left or right hand, as quickly and accurately as possible, by pressing one of two bottoms “j” or “f” for right or left hand, respectively. Laterality, view, and orientation of the hand were demonstrated in random order. The beginning of each trial was a fixation cross presented for 1500–2500 ms, followed by the presentation of a rotated hand (right or left) for 3000 ms, with interstimulus intervals of 1800–2800 ms.
Participants were seated in an electrically shielded, sound-attenuating room in front of a computer screen. They were asked not to make eye movement or blink during trials. The actual experiment was preceded by a test trial to familiarize the participants with the test procedure.
EEG recording.
EEG signals were recorded by the Mitsar 202 system (Mitsar, St. Petersburg, Russia) using 31 active electrodes according to the international 10-20 system. All electrodes were referenced to linked ear lobes, with the ground electrode placed on the medial aspect of the frontal lobe (between the FZ and FPZ electrodes). Electrode impedance was maintained below 5 kOhm. EEG was sampled at 500 Hz with filtered online 0.1- to 30-Hz band pass. The average epoch for ERP was 1000 ms (including 200-ms prestimulus baseline). Only epochs with correct responses were included in averaged ERPs. Ocular artifacts were corrected by applying the independent component analysis method. To improve the signal–noise ratio for ERP analysis, stimuli with various rotation angles were grouped in two different categories: stimuli with a small rotation angle (0°, 60° and 300°) and stimuli with a large rotation angle (120°, 180°, and 240°). Moreover, data were pooled over the stimuli of right and left hands. Inspection of ERP components showed that increasing stimulus orientation resulted in decreasing amplitude in the parietal positive component (P300 amplitude modulation) at a latency of 300–700 ms for the two groups; thus, this time window was chosen for analysis of the RRN wave (as RRN interval). Mean and peak amplitude were used to study amplitude and latency of RRN intervals. Finally, the parietal P300 amplitude modulation was calculated by subtracting the mean amplitude of large angles of rotation from small angles of rotation in the RRN interval.
Data Analysis
Data distributions were assessed for normality using the Shapiro-Wilk test. Behavioral data were analyzed using three-way analysis of variance (ANOVA), with group as the between-subject factor and anFgle of orientation displacement and hand laterality as within-subject factors. When necessary, Greenhouse-Geisser corrections were applied, and each post hoc test was Bonferroni corrected. In addition, repeated-measure ANOVA was conducted with group as the between-subject factor and electrode places and degree of rotation as within-subject factors. Pearson correlation was used to control relation between patients’ characteristics and behavioral and ERP data.
Results
The participants’ demographic and clinical characteristics are shown in Table 1. The two groups were not significantly different in age, sex, and education.
| Characteristic | Schizophrenia Patients | Control Subjects |
|---|---|---|
| Age (years) | 32.05±7.47 | 31.11±7.37 |
| Sex (men/women) | 16/4 | 14/4 |
| Education (years) | 11.7±2.79 | 12±2.48 |
| Handedness | 94.13±12.94 | 94.08±10.69 |
| Disease duration (years) | 9.98±6.41 | |
| SAPS | 22±17.75 | |
| SANS | 37.94±14.54 | |
| Chlorpromazine equivalent dose | 318.5±165.2 |
TABLE 1. Demographic and Clinical Characteristics of Participantsa
Behavioral Data
Reaction time of hand rotation.
Repeated-measures ANOVA for groups (patients with schizophrenia/control subjects) × stimulus angle (0°, 60°, 120°, 180°, 240°, and 300°) × stimulus-handedness (left/right) showed a significant main effect of group [F(1,36)=11.43, p=0.002], indicating that patients responded significantly slower than control subjects. Moreover, there were significant effects of stimulus angle [F(1,36)=49.21, p<0.001] and stimulus-handedness [F(1,36)=4.39, p=0.04], showing reaction times significantly increased with increasing the angle of rotation from upright (0°), and reaction times to the left hand stimuli were significantly slower than to the right hand stimuli. The interaction effects of group × stimulus angle and group × stimulus-handedness were not significant, indicating a similar performance of the two groups across different orientations and stimulus-handedness (Figure 2A).
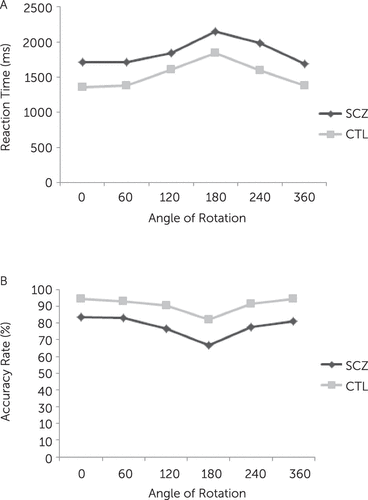
FIGURE 2. Mean Reaction Times (A) and Accuracy Rates (B) During the Mental Rotation of Hands in Patients With Schizophrenia and Control Subjects
Accuracy.
The model showed significant main effect of group [F(1,36)=5.76, p=0.02], indicating that patients were significantly less accurate than control subjects. Also, there were significant main effects of stimulus angle [F(1,36)=20.38, p<0.001] and handedness [F(1,36)=4.04, p=0.05], suggesting that accuracy rates significantly decreased with increasing angle of rotation from the upright position and were significantly less accurate in response to the left than right hand stimuli. Interaction effects between group and stimuli angle and between group and stimulus-handedness were not significant, indicating a similar performance of the two groups across different orientations and stimulus-handedness (Figure 2B).
ERP Data
Mean amplitude of RRN interval.
To increase signal–noise ratio, stimuli were grouped in two categories: small (0°, 60°, and 300°) and large angle of rotation (120°, 180°, and 240°).16 Repeated-measures ANOVA for group (patients with schizophrenia/control subjects) × electrode (P3, PZ, and P4) × angle of rotation (small/large) showed a significant main effect of group [F(1,36)=6.23, p=0.01], indicating significantly smaller mean amplitudes of RRN interval in patients with schizophrenia compared with control subjects (Figure 3). Furthermore, there were significant effects of electrode [F(1,36)=36.97, p<0.001] and angle of rotation [F(1,36)=24.72, p<0.001], indicating decreasing in amplitude with increasing angle of rotation across different electrodes . Regarding the effect of electrode, mean amplitudes in RRN interval were highest at PZ for small angles (control subjects: 7.1±3.43; patients with schizophrenia: 4.71±2.43) and lowest at P3 for large angles (control subjects: 3.27±3.4; patients with schizophrenia: 1.69±1.98). Finally, the interaction effect of group × electrode and group × angle of rotation was not significant (Table 2).
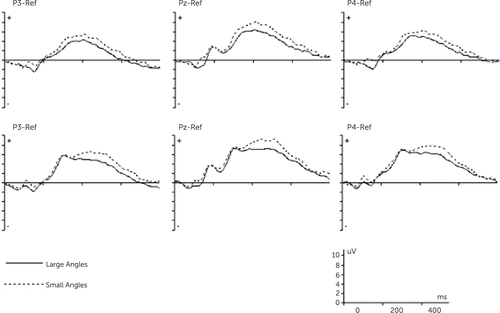
FIGURE 3. Grand-Average Event-Related Potentials During the Mental Rotation of Hands for the Control Group (A) and Schizophrenic Group (B)
| Parietal Electrode | Angle | Schizophrenia Patients | Control Subjects | ||
|---|---|---|---|---|---|
| Mean amplitude (µv) | Latency (ms) | Mean amplitude (µv) | Latency (ms) | ||
| P3 | Small angles | 2.60±2.36 | 400.0±63.4 | 4.5±3.14 | 390.6±70.8 |
| Large angles | 1.69±1.98 | 381.2±59.6 | 3.27±3.4 | 367.7±69.8 | |
| PZ | Small angles | 4.54±2.43 | 416.8±70.8 | 7.10±3.43 | 400.4±78.4 |
| Large angles | 4.01±1.82 | 414.5±79.9 | 5.81±3.43 | 373.7±69.8 | |
| P4 | Small angles | 3.42±2.7 | 405.3±60.6 | 5.93±3.40 | 384.4±67.1 |
| Large angles | 2.81±1.62 | 407.0±70.9 | 4.89±3.82 | 363.3±72.3 | |
TABLE 2. Summary of Event-Related Potential Data During Performance of Mental Rotation Taska
We then carried out a planned contrast to specifically test for amplitude difference for small and large angle of rotation between patients and controls. The results revealed that the two groups differed significantly in amplitude difference at Pz, and it was significantly lower in the patient group (0.54±0.96) than in the control group (1.22±1.01).
Latency of peak amplitude.
Repeated-measures ANOVA for group (patients with schizophrenia/control subjects) × electrode (P3, PZ, and P4) × angle of rotation (small/large) showed that the main effects of angle of rotation and group were not significant. However, there was a significant main effect of electrode [F(1,36)=3.69, p=0.03], indicating peak latency was significantly different between electrodes. Post hoc analysis showed comparison of peak latencies between P3 and PZ was near significant (p=0.06).
Correlation
To examine the effects of patient characteristics on their performances on hand rotation task, correlation coefficients (Pearson's r) were calculated between the measures (behavioral and ERP) with chlorpromazine equivalent dose, duration of disease, and SAPS and SANS scores. The results showed that the measures of performance were not associated with the chlorpromazine equivalent dose and disease characteristics (all p>0.6), except for correlation between SAPS and mean amplitude of RRN at P3 (r=−0.47, p=0.05) and PZ (r=−0.56, p=0.01).
Discussion
This study aimed to investigate the neural correlates of impaired mental imagery during the performance of the hand rotation task. The main results can be presented as follows: at the behavioral level, patients performed hand rotation less accurately and more slowly than healthy participants. At the neural level, our results showed that compared with the control subjects, the patients with schizophrenia had less modulation of P3 amplitude (decreased RRN) during mental rotation.
The behavioral data indicated response times increased linearly with increasing angle of rotation of the stimuli for the both groups. This finding is in agreement with the original finding by Coopers and Shepard,18 indicating the use of mental rotation strategy to perform the hand rotation task by our participants. Moreover, patients with schizophrenia responded relatively slower and had a higher error rate compared with control subjects, similar to findings of other studies.4,7 These results suggest patients with schizophrenia may be characterized by a compromised process of mental representation of hands.
At the neural level, consistent with previous studies,13,14 our results showed a significant general effect of increasing negativity with increasing angle of rotation (RRN) for both groups. Moreover, the observed decrease in RRN amplitude with increasing angle of rotation fits our behavioral data as they show an increase in response time as a function of angle. Accordingly, our findings further support the close relation between RRN and mental rotation process.
Our results showed that patients with schizophrenia had shallower trade-off function between angle of rotation and RRN amplitude relative to healthy participants, indicating an impaired mental imagery process at neural level. To our knowledge, this is the first study that extends previous behavioral studies in showing disruption of mental imagery at both behavioral and neural levels in patients with schizophrenia. Moreover, the pattern of P3 amplitude modulation in control subjects and the deficient P3 modulation in schizophrenia is consistent with two recent studies by Neuhaus et al.19,20 Using the Attention Network Test, they showed impairment of patients with schizophrenias to modulate P3 amplitude, which was independent of duration of illness, age, and antipsychotic medications. These findings are extended in that we showed similar pattern of impaired P3 modulation in another task (hand rotation task) in patients with schizophrenia.
A possible explanation for RRN might be the general assumption that P3 amplitude is attenuated as task difficulty increases.21,22 In our data, task difficulty increased when the orientation of hand stimuli increased from upright position, associated with smaller RRN. The less modulation of P3 in patients may indicate a failure to detect or to differentially process varying degree of task difficulty.
RRN is the standard ERP effect of mental rotation, and when superimposed on the P300 component, modulates its positivity at the parietal cortex. This modulation is associated to the degree of stimulus rotation and is suggested as a direct correlate of mental rotation.9,23 Moreover, studies have shown that the posterior parietal cortex is activated during mental rotation, and RRN reflects the stronger activation of this area for stimuli with an increased rotation of angle.9,24 Therefore, decreased RRN during mental rotation in the patients might indicate slightly less activation of posterior parietal cortex in the patients compared with control subjects, although it is activated in both groups during mental rotation.
These findings support the possible role of the posterior parietal cortex in the pathophysiology of patients with schizophrenia. Supporting evidence comes from studies suggesting that the dysfunction of the posterior parietal cortex is a putative candidate for many cognitive impairments in schizophrenia,25,26 such as impaired spatial attention and oculomotor control, as well as deficits in motor control and motor imagery.3,6,27
In agreement with previous studies, we found less accuracy in response to the left hand stimuli than to the right hand stimuli in both groups.13,14 This finding reflects the anatomical constrains of right-handed participants in the present study.
Finally, our results showed significant negative correlations between RRN amplitudes (P3 modulation) and SAPS total scores at P3 and Pz sites. As noted already, P3 modulation (RRN) reflects an electrophysiological correlate of the mental rotation process. A potential explanation for this finding might be that patients with positive symptoms have difficulties in monitoring the mental image of the rotation movement. In support, de Vignemont et al. found that hallucinatory patients were slower and less accurate than nonhallucinatory patients on mental rotation tasks.4 They suggested that impaired mental rotation can reflect a disruption of action monitoring in schizophrenia, because the same brain areas are involved in both processes. Therefore, our findings may support the assumption of link between positive symptoms and disruption of action monitoring in schizophrenia. We did not find correlation between hallucination scores of SAPS and RRN, which might be due to our small sample size. Future research is required to repeat this finding and investigate the relationship between different subscales of SAPS and mental imagery in schizophrenia.
One of the limitations of this study is that the numbers of patients and control subjects were relatively small. Another limitation is the effects of antipsychotic and anticholinergic medications on performances of patients. However, no correlation was found between chlorpromazine equivalent dose and behavioral and ERP measures. Future studies are required to examine the association between RRN across a group of drug-naïve patients and in different subtypes of schizophrenia.
In conclusion, our findings indicate compromised underlying neural processing during mental imagery in schizophrenia. Specifically, although prior behavioral studies have provided only partial evidence for impaired mental imagery in patients with schizophrenia, this study provides better insight in the neural process underlying mental imagery in patients.
1 : Cerebral processes during visuo-motor imagery of hands. Psychophysiology 2006; 43:401–412Crossref, Medline, Google Scholar
2 : Temporal and kinematic properties of motor behavior reflected in mentally simulated action. J Exp Psychol Hum Percept Perform 1994; 20:709–730Crossref, Medline, Google Scholar
3 : Exploring imagined movements in patients with schizophrenia. Neuroreport 2002; 13:605–609Crossref, Medline, Google Scholar
4 : Mental rotation in schizophrenia. Conscious Cogn 2006; 15:295–309Crossref, Medline, Google Scholar
5 : Disturbed sexual dimorphism of brain activation during mental rotation in schizophrenia. Schizophr Res 2010; 122:53–62Crossref, Medline, Google Scholar
6 : Abnormalities of motor imagery associated with somatic passivity phenomena in schizophrenia. Schizophr Res 2003; 60:229–238Crossref, Medline, Google Scholar
7 : Abnormalities of mental rotation of hands associated with speed of information processing and executive function in chronic schizophrenic patients. Psychiatry Clin Neurosci 2014; 68:410–417Crossref, Medline, Google Scholar
8 : Toward a chronopsychophysiology of mental rotation. Psychophysiology 2002; 39:414–422Crossref, Medline, Google Scholar
9 : The functional significance of ERP effects during mental rotation. Psychophysiology 2002; 39:535–545Crossref, Medline, Google Scholar
10 : Effects of dynamic rotation on event-related brain potentials. Brain Res Cogn Brain Res 2005; 24:307–316Crossref, Medline, Google Scholar
11 : An EEG study of mental rotation-related negativity in children with Developmental Coordination Disorder. Child Care Health Dev 2006; 32:649–663Crossref, Medline, Google Scholar
12 : Mental rotation of mirrored letters: evidence from event-related brain potentials. Brain Cogn 2009; 69:180–187Crossref, Medline, Google Scholar
13 : Neural evidence for compromised motor imagery in right hemiparetic cerebral palsy. Front Neurol 2010; 1:150Crossref, Medline, Google Scholar
14 : Compromised motor imagery ability in individuals with multiple sclerosis and mild physical disability: an ERP study. Clin Neurol Neurosurg 2013; 115:1738–1744Crossref, Medline, Google Scholar
15 : Scale for the Assessment of Negative Symptoms. Iowa City, IA, University of Iowa, 1983Google Scholar
16 : Scale for the Assessment of Positive Symptoms. Iowa City, IA, University of Iowa, 1984Google Scholar
17 : International consensus study of antipsychotic dosing. Am J Psychiatry 2010; 167:686–693Crossref, Medline, Google Scholar
18 : Mental transformations in the identification of left and right hands. J Exp Psychol Hum Percept Perform 1975; 104:48–56Crossref, Medline, Google Scholar
19 : Evidence of specificity of a visual P3 amplitude modulation deficit in schizophrenia. Schizophr Res 2010; 124:119–126Crossref, Medline, Google Scholar
20 : Visual P3 amplitude modulation deficit in schizophrenia is independent of duration of illness. Schizophr Res 2011; 130:210–215Crossref, Medline, Google Scholar
21 : P3a from visual stimuli: task difficulty effects. Int J Psychophysiol 2006; 59:8–14Crossref, Medline, Google Scholar
22 : Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol 2007; 118:2128–2148Crossref, Medline, Google Scholar
23 : Brain potentials during selective attention, memory search, and mental rotation. Psychophysiology 1989; 26:452–467Crossref, Medline, Google Scholar
24 : Linking performance with brain potentials: mental rotation-related negativity revisited. Neuropsychologia 2008; 46:3069–3073Crossref, Medline, Google Scholar
25 : Attention, motor control and motor imagery in schizophrenia: implications for the role of the parietal cortex. Schizophr Res 2004; 70:241–261Crossref, Medline, Google Scholar
26 : Parietal lobes in schizophrenia: do they matter? Schizophr Res Treatment 2011Google Scholar
27 : Defective recognition of one’s own actions in patients with schizophrenia. Am J Psychiatry 2001; 158:454–459Crossref, Medline, Google Scholar



