Predictors of Hypomania During Ventral Capsule/Ventral Striatum Deep Brain Stimulation
Abstract
Deep brain stimulation (DBS) of the ventral capsule/ventral striatum (VC/VS) is a novel therapy for neuropsychiatric disorders. Hypomania is a known complication of VC/VS DBS, but who is at risk is less understood. Factors such as family history, combined with details of DBS programming, might quantify that risk. The authors performed an iterative modeling procedure on a VC/VS DBS patient registry to identify key predictors. Hypomania was less common for men and for patients stimulated on the ventral right contact. It was more common with right monopolar stimulation. These findings may help to establish decision rules to reduce complications of VC/VS DBS.
Deep brain stimulation (DBS) is an emerging treatment for severe neuropsychiatric illness. DBS seeks to directly modulate dysfunctional circuits underpinning these disorders. This has worked well in movement disorders,1 fueling hope for similar success in psychiatry. Two anatomic targets have reached clinical trials: the subgenual cingulate gyrus (Cg25)2–4 and the ventral internal capsule/ventral striatum (VC/VS).5–8 The medial forebrain bundle has also shown promising results, with apparent rapid remission of depressive symptoms.9 VC/VS is of particular interest because it has shown efficacy in both treatment-resistant major depressive disorder6 and obsessive-compulsive disorder.5,7 Although the pivotal randomized trial of VC/VS DBS for major depressive disorder was stopped early,8 the authors of that report suggest that the difficulty was targeting and patient selection, not inefficacy of DBS overall. Recent imaging results at the Cg25 target support the idea that targeting is a key factor. Riva-Posse et al.10 found that response to Cg25 DBS required the electrode to access a confluence of white matter fibers, not the gray matter originally expected. VC/VS DBS for obsessive-compulsive disorder has worked in open-label studies,7 and the number of patients with obsessive-compulsive disorder who qualify for DBS is small.11 This result led to an approval by the U.S. Food and Drug Administration for VC/VS DBS in obsessive-compulsive disorder under a Humanitarian Device Exemption.
Humanitarian Device Exemption approval made DBS for obsessive-compulsive disorder available to a larger patient base, but it also exposed those patients to a therapy with mechanisms that are poorly understood. VC/VS DBS can cause neuropsychiatric complications, perhaps the most severe of which is stimulation-induced hypomania, which can progress to mania if untreated.12 The incidence is unknown because most implants are not reported in the literature; the largest case series reported one case among 26 patients.7 Nevertheless, because even brief manic behavior can be harmful, most centers consider bipolar mood disorders to be a VC/VS DBS contraindication and monitor patients for treatment-emergent hypomania.13
If clinicians could identify patients at risk for hypomania from VC/VS DBS, surveillance and counseling could be targeted specifically to such patients. Moreover, as VC/VS DBS spreads outside of major research centers under the Humanitarian Device Exemption, less experienced clinicians would benefit from guidelines. We therefore sought to identify factors that might predict hypomania in patients undergoing VC/VS DBS, leveraging our institution's relatively large cohort of patients who have received these implants. We hypothesized that indicators including previous hypomania, acute mood effects of DBS, electrode location within the VC/VS, and overall mood trajectory might have predictive power.
Methods
Participants
We studied 20 patients who underwent VC/VS DBS between 2006 and 2014. All were participants in at least one DBS trial or our Humanitarian Device Exemption protocol, and all protocols included consent to deidentified retrospective chart reviews. Protocols were approved by the Massachusetts General Hospital Institutional Review Board. The cutoff point for this study was August 31, 2014; we analyzed all documented events up to that point and all patients available in our records. The original inclusion/exclusion criteria are published elsewhere,6–8,13 and they required severe treatment-resistant major depressive disorder and/or obsessive-compulsive disorder; absence of psychotic, bipolar, or personality disorders; and good health sufficient to justify the risks of craniotomy. DBS implants were primarily the Medtronic Model 3391 (3387 IES) lead, although some patients received a Model 3387 lead.
Predictors were manually extracted from clinical records. DBS parameters at each visit were recorded by the programming clinician on study forms and in the medical record. Clinical scales, particularly the Montgomery-Åsberg Depression Rating Scale, were performed as part of the original studies by well-trained raters. Hypomania was coded if the programming clinician’s note stated that the patient was having a hypomanic episode or if an appropriate ICD-9 code (296.0 or 296.1) appeared in clinical documentation. Although the original evaluators did not specify which diagnostic system they were using, DSM-5 had not yet come into widespread use during our review period, and DSM-IV was the standard for admitting patients into clinical trials. The reported instances should be considered as DSM-IV hypomania. If the chart reviewer identified possible hypomania that had not been flagged by the original clinician, this was resolved by independent review by a trained DBS psychiatrist (A.S.W.).
Importantly, all patients received DBS programming following specific algorithms.5,6,8,13 The algorithms for major depressive disorder and obsessive-compulsive disorder were similar, except that the major depressive disorder trial favored early bipolar stimulation to improve blinding.8 The algorithms may have increased the probability of hypomania, because they were designed to target DBS to contacts with strong limbic effects, particularly acute mood elevation.
General Modeling
We fit a series of logistic regressions, predicting the probability of hypomania as a binary outcome. We considered two sets of predictors: factors intrinsic to the patient (e.g., personal history) and factors that changed from visit to visit (e.g., DBS amplitude). We assessed both predictor sets in hypothesis-driven models and a data-driven iterative procedure. In all cases (hypothesis-driven or iterative, patient-level or visit-level), we varied the inclusion/exclusion of predictor terms but not the overall form of the regression.
All analyses used R software with the “ggplot2,” “pROC,” and “texreg” packages.14–17 Predictors were modeled as fixed effects because hypomanic episodes were too rare to estimate mixed-effects models. For each set of predictors, we fit a logistic model and assessed its predictive power. We quantified this as the area under the receiver-operator characteristic curve. Area under the curve values were compared between models (two-tailed z-test) using the Hanley-McNeil correction for same-sample receiver-operator characteristic curve calculations.18,19 We further report p values (Wald test) for the coefficients within each regression. These are reported without correction, because they are not a primary study outcome.
Patient-Level Predictors
We first considered patient characteristics that might predict hypomania. These included a past history of manic/hypomanic episode (usually substance-induced), a family history of bipolar-spectrum disorder, sex, presence of obsessive-compulsive disorder, intraoperative mood effects during stimulator implantation,20 and mood improvement of any degree in response to DBS (as a proxy for the general efficacy in that patient). We also included medication changes in general, to control for the possibility that hypomania was driven more by a new medication than by DBS. The study records did not have sufficient detail to separate medication changes reliably into specific classes. We did not include major depressive disorder as a predictor, because 100% of the sample met the criteria for major depressive disorder.
Visit-Level Predictors
Patients’ risk for hypomania may correlate with their specific DBS settings. The VC/VS contains projecting fibers that reach multiple cortical regions, and differential activation of these bundles could drive complications.21,22 Parameter changes alter the volume of tissue activated and, in our clinical experience, the same DBS contact and frequency can either be therapeutic or harmful depending on applied voltage. Patients were followed for years, and age may relate to the chance of complications.23 Acute parameter changes are often accompanied by transient changes in mood or energy, which might signify greater limbic engagement and thus a higher risk for hypomania compared with settings without an acute effect. Similarly, settings that the patient has never experienced might be more risky than those that have been constant for 1 month or more. Finally, we hypothesized that the mood trajectory might be predictive. If a patient begins to show a rapid change in depressive symptoms (measured in our studies by the Montgomery-Åsberg Depression Rating Scale), then this could suggest overactivation and risk of leaving a therapeutic window, as is sometimes seen with antidepressants.
DBS parameters (active contacts, voltage, frequency, pulse width), age, presence/absence of acute in-session response, duration of exposure to the current parameters, and recent mood trajectory were therefore included as predictors. The majority of patients received DBS at 130 or 135 Hz. We therefore dichotomized frequency as ≥130 Hz versus <130 Hz. We dichotomized duration of parameter exposure as >4 weeks or <4 weeks based on clinical impressions that the “danger period” lasts up to 1 month. Mood trajectory was expressed as the slope of the Montgomery-Åsberg Depression Rating Scale in points per day since the previous clinical visit. Finally, because the four contacts of each DBS lead can be programmed in a variety of monopolar and bipolar configurations, we created dummy variables indicating whether each contact (0, 1, 2, or 3 on the right and left side; Figure 1) was in use at the end of each visit and whether stimulation was monopolar. Because some patients received a Model 3387 lead, which has smaller and denser contacts, we mapped electrodes on that lead to their “3391 equivalents”—the nearest electrode on a Model 3391 lead. This led to contact 0 and 1 mapping to 0 on a 3391 and contacts 2 and 3 mapping to 1 on a 3391 (Figure 1).
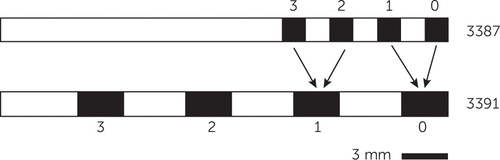
FIGURE 1. Illustration of the Distal Ends of Medtronic 3387 and 3391 DBS Leads, Demonstrating the Remapping From 3387 to Equivalent Electrodes on the 3391 Lead for Modeling Purposesa
a The scale bar illustrates the size of a 3-mm 3391 lead contact.
Hypothesis-Driven Modeling
We hypothesized that hypomania is more a function of DBS location and programming than of patients’ characteristics. We therefore split the patient-level and visit-level predictor sets further into DBS and clinical subsets. The DBS subset contained predictors that could in some way be related to the implant, whereas all others were labeled as clinical. For both the patient-level and visit-level predictors, we fit three models: one with only DBS predictors, one with clinical predictors, and a full model with both.
Iterative Discovery Modeling
From the predictors, one can construct 2,047 patient-level and >16 million visit-level models that may predict hypomania. To explore this space while avoiding overfitting, we used an iterative, information-driven, model-building approach. We added predictors stepwise to the model based on Akaike’s information criterion (AIC)24; at each step, the added predictor was the one that minimized AIC compared with other candidates. This procedure is mathematically equivalent to leave-one-out cross-validation, a common approach for guarding against overfitting.25 In situations with a rare outcome (hypomania), actual cross-validation is difficult because the outcome of interest may not appear in a random data fold. The AIC procedure achieves the same result without that difficulty.
Results
General Sample Characteristics
We reviewed 20 patients, who were aged 21–64 years at their time of entry into their original study. Full demographics are summarized in Table 1 and Table 2. Of these 20 patients, nine (45%) had hypomania during the study window, with 12 episodes occurring among 428 visits (2.8% of visits). Hypomania was managed by briefly interrupting DBS or substantially decreasing stimulation amplitude and, in most cases, an anticonvulsant or antipsychotic drug was also prescribed.
| Factor | Present | Absent |
|---|---|---|
| Hypomania | 9 (45) | 11 (55) |
| Male sex | 10 (50) | 10 (50) |
| Comorbid OCD | 6 (30) | 14 (70) |
| Personal history of hypomania | 3 (15) | 17 (85) |
| Family history of bipolar disorder | 3 (15) | 17 (85) |
| Intraoperative mood/energy change | 16 (80) | 4 (20) |
| MADRS response to DBS | 7 (35) | 13 (65) |
| Medication change post-DBS | 3 (15) | 17 (85) |
TABLE 1. Patient-Level Characteristics of the Samplea
| Factor | Present | Absent |
|---|---|---|
| Contact, left | ||
| 0 | 256 (61.2) | 162 (38.8) |
| 1 | 274 (65.6) | 144 (34.4) |
| 2 | 134 (32.1) | 284 (67.9) |
| 3 | 185 (44.3) | 233 (55.7) |
| Contact, right | ||
| 0 | 242 (58.0) | 175 (42.0) |
| 1 | 298 (71.5) | 119 (28.5) |
| 2 | 69 (16.5) | 348 (83.5) |
| 3 | 189 (45.3) | 228 (54.7) |
| Monopolar stimulation | ||
| Left | 104 (24.9) | 314 (75.1) |
| Right | 82 (19.7) | 335 (80.3) |
| Frequency <130 Hz | ||
| Left | 90 (21.5) | 328 (78.5) |
| Right | 76 (18.2) | 341 (81.8) |
| Acute mood change at visit | 19 (4.4) | 409 (95.6) |
| Acute energy change at visit | 13 (3) | 415 (97) |
| Settings constant >4 weeks | 299 (69.9) | 129 (30.1) |
| Mean age at visit (years) | 44.45 (12.83) | |
| Mean pulse width (µsec) | ||
| Left | 135.9 (53.9) | |
| Right | 134.8 (53.34) | |
| Mean MADRS slope per day | 0.03 (0.77) |
TABLE 2. Visit-Level Characteristics of the Samplea
Patient-Level Modeling
None of the patient-level models predicted hypomania well. Figure 2 presents the AIC curve for the iterative process and receiver-operator characteristic curves for all four models. The optimal iterative model is of order 3, indicated by the AIC curve’s minimum point. Area under the curve comparisons suggest that the full (all predictors) model and the optimal iterative model outperform others, with both having area under the curve values of approximately 0.95. AIC minimization favors the iterative model, which has an AIC of 13.74 compared with the full model AIC of 20.5. This trend indicates that the former fits the data equally well with fewer free parameters (3 for the iterative model and 7 for the full model). Both models, however, showed very high standard errors of their regression coefficients (all >10,000 for coefficients in the range of 0.35–60.02). Those error magnitudes suggest that the fitting algorithm could not identify a reliable model and that no coefficients provably differ from 0.
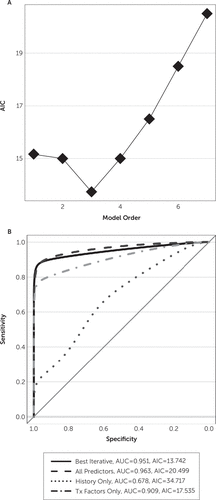
FIGURE 2. Patient-Level Predictions of Hypomaniaa
a [A] AIC curve demonstrating an optimal model order of 3 during iterative factor selection, corresponding to the following: hypomania ~ sex plus (personal history) plus (MADRS response). [B] Receiver-operator characteristic curves for the iteratively discovered optimal model compared with all possible predictors (dashed line), clinical history predictors only (dotted line), and treatment-related factors (dotted and dashed line). The iterative model outperforms all except the all-predictors model and is more parsimonious than all-predictors (AIC of 13.74 versus 20.5). AIC, Akaike’s information criterion; AUC, area under the curve; MADRS, Montgomery-Åsberg Depression Rating Scale; Tx, treatment.
Visit-Level Modeling
Figure 3 shows the results of a similar analysis on visit-level data, which include detailed DBS programming parameters. The clinical-only model, which does not take DBS settings into account, had a significantly smaller area under the receiver-operator characteristic curve (0.64, p<0.033, z<−2.23 versus other estimated models). There were no other significant area under the curve differences among models. However, the optimal iterative model had an AIC of 93.11 compared with 117.51 for the full model and 113.88 for the DBS-only model. This suggests that the latter models fit substantial noise in addition to actual trends in the data. Inspection of the regression coefficients in Table 3 further supports that conclusion. Both the full and DBS-only models again show very large (and identical) standard errors on multiple coefficients, suggesting that the data do not support these models.
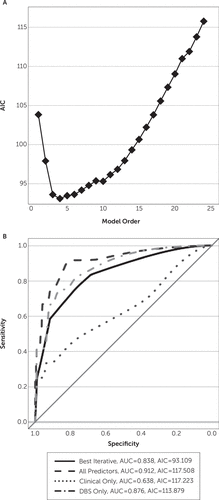
FIGURE 3. Modeling Results for Visit-Level Predictorsa
a [A] AIC curve from stepwise model building, demonstrating an optimal order of 4, the curve's minimum point. [B] Receiver-operator characteristic curves for the iterative optimal model compared with all possible predictors (dashed line), clinical history predictors only (dotted line), and DBS programming factors (dotted and dashed line). As with patient-level models, the iterative model outperforms all others in terms of accuracy-parsimony trade-off (AIC of 93.11 versus next lowest of 113.88). AIC, Akaike’s information criterion; AUC, area under the curve; DBS, deep brain stimulation.
| Coefficient | Full Model | Clinical-Only Model | DBS-Only Model | Iterative Optimal Model |
|---|---|---|---|---|
| Intercept | 65.96 (7642.33) | –3.45 (1.22)* | 63.10 (8816.64) | –1.91 (0.66)* |
| Age | –0.06 (0.06) | –0.01 (0.03) | ||
| Male sex | –3.36 (1.79) | –0.78 (0.68) | –1.61 (0.68)** | |
| Left | ||||
| Contact | ||||
| 0 | –15.62 (1827.12) | –16.44 (2245.99) | ||
| 1 | –13.60 (1827.12) | –16.02 (2245.99) | ||
| 2 | –14.60 (1827.12) | –17.04 (2245.99) | –1.23 (0.87) | |
| 3 | –13.84 (1827.12) | –16.14 (2245.99) | ||
| Monopolar stimulation | –18.42 (1827.12) | –17.12 (2245.99) | ||
| Voltage | 0.33 (0.35) | 0.25 (0.25) | ||
| Frequency <130 | –0.43 (1.58) | –1.03 (1.40) | ||
| Pulse width | –0.02 (0.02) | –0.01 (0.02) | ||
| Right | ||||
| Contact, right | ||||
| 0 | –20.99 (3356.03) | –19.22 (3793.26) | –2.89 (0.87)*** | |
| 1 | –18.41 (3356.03) | –17.87 (3793.26) | ||
| 2 | –16.11 (3356.03) | –17.08 (3793.26) | ||
| 3 | –18.88 (3356.03) | –16.86 (3793.26) | ||
| Monopolar stimulation | –1.09 (3356.03) | –15.41 (3793.26) | 1.56 (0.68)** | |
| Voltage | –0.39 (0.38) | –0.18 (0.25) | ||
| Frequency <130 | –0.69 (1.99) | 0.26 (1.61) | ||
| Pulse width | 0.03 (0.02) | 0.01 (0.02) | ||
| Constant >4 weeks | 0.89 (1.04) | 0.75 (0.77) | ||
| MADRS slope | –1.00 (0.61) | –0.71 (0.33)** | ||
| OCD | 1.06 (2.03) | –0.10 (0.88) | ||
| History of mania | –0.73 (2.75) | 0.25 (1.11) | ||
| Family history of bipolar disorder | –0.24 (1.85) | 1.21 (0.83) | ||
| Acute mood change | 2.05 (2.16) | 1.15 (1.21) | ||
| Acute energy change | 0.31 (2.10) | 1.70 (1.22) | ||
| AIC | 117.51 | 117.22 | 113.88 | 93.11 |
| Log likelihood | –32.75 | –49.61 | –38.94 | –41.55 |
TABLE 3. Regression Coefficients and AIC for Visit-Level Modeling of Hypomaniaa
Discussion
We explored the degree to which hypomania can be predicted in patients receiving VC/VS DBS for neuropsychiatric indications. The best predictions arose from considering both patient-level (sex) and DBS (contact geometry) factors. If true, that model makes interesting predictions. First, hypomania is predicted to occur more likely in female patients. Given that hypomania and rapid cycling are more common in female patients with bipolar disorder overall,26 there may be a concordant neural mechanism. Second, a trend of improving Montgomery-Åsberg Depression Rating Scale does not increase hypomania risk. This finding implies that clinicians need not become cautious as depression remits. Third, contrary to our expectation, hypomania risk did not abate when DBS settings had been constant for >4 weeks. This coefficient was not included in the best final model, but the two models that estimated it (full and DBS only under visit-level modeling) showed a positive coefficient: an increase in risk across time. This is tempered by the overall poor fit of those models but was nevertheless surprising. Fourth, obsessive-compulsive disorder was not a strong protective factor. It had a negative coefficient in all successfully fit models, but those coefficients were never significant. This trend suggests that hypomania from VC/VS DBS is a risk regardless of the clinical phenotype.
The optimal iterative model also suggests an asymmetry in DBS effects. Hypomania correlated with monopolar stimulation on the right side only. This may be consistent with the general belief that the left hemisphere supports positive mood (lesions producing depression and unopposed left hemisphere producing mania).27,28 Furthermore, there was a protective effect of stimulation at contact 0 on the right side, which presumably was deepest into the VC/VS gray matter. It is unclear whether this is a positive effect of stimulation at that contact or it reflects partial exclusivity (i.e., when contact 0 is in use, more dorsal contacts often are not).
It may be possible to clarify these results in animal models. DBS has been tested in the rat nucleus accumbens, which is believed to be an analogue of the human VC/VS target, but it has not compared the effects of unilateral left versus right stimulation or of moving stimulation more dorsally.29,30 Our results suggest that those experiments may yield clinically relevant data. Finally, although the optimal model suggested that stimulation at left contact 2 (well into dorsal striatum) may be protective, we interpret that result with caution. First, it is the only coefficient in that model without a significant p value. Second, Table 2 shows that contact 2 was used far less frequently than others. This finding may simply represent the rarity of both hypomania and the use of contact 2 rather than a true effect.
Neither a personal history of hypomania nor a family history of bipolar disorder had substantial explanatory power in this data set. If replicated, this could put clinicians at greater ease when selecting patients for VC/VS DBS implantation. In this regard, an important missing variable in our study is medications. Our clinical records did not reliably capture the presence or absence of antidepressants or mood stabilizers, either of which might have mitigated the effects of underlying genetics.
Our results highlight interesting avenues for further clinical investigation but also highlight limits of this study. First, although this is one of the largest reported populations of patients undergoing VC/VS DBS and is certainly the largest from a single center using standardized protocols, it remains a small sample. No patient-level model demonstrated predictive power. Even the visit-level modeling, with a relatively reasonable sample size of 428 participants, showed multiple cases of overfitting. Furthermore, unless the coefficients are examined, appearances are deceiving—those unreliable models still appeared to produce good prediction rules based on their receiver-operator characteristic curves. This emphasizes the limits of data-driven techniques, even with principled model selection procedures such as AIC minimization. In addition, although the AIC technically provides the benefits of cross-validation, we would strongly suggest validation of our predictions in an independent clinical cohort before they are used to guide decision making. This is also true because our sample appears to be enriched in hypomania (nine of 20 patients) relative to the reported incidence in other studies (e.g., one of 26 patients7). There are multiple possible explanations for that enrichment. First, we focused on identifying cases and we thus accepted all events that a clinician described as hypomanic. Traditional reporting of adverse events implemented in most studies would only capture severe events that carried a risk for harm. Second, participants in these studies were part of clinical trials in which programming algorithms encouraged higher voltage titration and targeted DBS to contacts that caused acute mood effects. Clinicians may have been more aggressive than in previous series, either by following the algorithm or out of a simple desire to make patients well more quickly. Finally, all VC/VS studies to date, including ours, have very small sample sizes. The variance in hypomania occurrence among these studies may be simple chance.
Finally, the predictive power of our decision rule may be limited by the imprecision of DBS contacts. Although the electrodes are at the same stereotactic coordinates in each patient, they are unlikely to affect precisely the same structures. Studies of white matter structure at the Cg25 DBS target have shown high variability in the relative location of fiber tracts.10 There is reason to believe that the same is true at the VC/VS target.22 We were not able to replace contact-related variables with distance to key fiber tracts, because we lacked the necessary imaging data. Collecting such data would be a valuable component of future DBS clinical trials, particularly at VC/VS.
In summary, we have identified a set of patient- and eDBS-related variables that may predict the development of hypomania in response to VC/VS DBS. These predictors highlight interesting hypotheses about the mechanisms of VC/VS DBS and its complications and, in particular, an asymmetry of the effects of DBS between hemispheres. If replicated or dissected further in nonhuman research, these rules may become the basis of a clinical decision support tool.
1 : Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol 2011; 68:165Crossref, Medline, Google Scholar
2 : Targeted electrode-based modulation of neural circuits for depression. J Clin Invest 2009; 119:717–725Crossref, Medline, Google Scholar
3 : Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry 2011; 168:502–510Crossref, Medline, Google Scholar
4 : Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry 2012; 69:150–158Crossref, Medline, Google Scholar
5 : Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology 2006; 31:2384–2393Crossref, Medline, Google Scholar
6 : Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry 2009; 65:267–275Crossref, Medline, Google Scholar
7 : Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry 2010; 15:64–79Crossref, Medline, Google Scholar
8 Dougherty DD, Rezai AR, Carpenter LL, et al: A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry 2015; 78:240–248Google Scholar
9 : Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry 2013; 73:1204–1212Crossref, Medline, Google Scholar
10 : Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry 2014; 76:963–969Crossref, Medline, Google Scholar
11 : Who qualifies for deep brain stimulation for OCD? Data from a naturalistic clinical sample. J Neuropsychiatry Clin Neurosci 2014; 26:81–86Link, Google Scholar
12 : A case of mania following deep brain stimulation for obsessive compulsive disorder. Stereotact Funct Neurosurg 2010; 88:322–328Crossref, Medline, Google Scholar
13 Widge AS, Dougherty DD: Managing patients with psychiatric disorders with deep brain stimulation, in Deep Brain Stimulation Management, 2nd ed. Edited by Marks WJ Jr. New York, Cambridge University Press, 2015Google Scholar
14 : ggplot2: Elegant Graphics for Data Analysis. New York, Springer, 2009Crossref, Google Scholar
15 : pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12:77Crossref, Medline, Google Scholar
16 Leifeld P: texreg: conversion of statistical model output in R to LaTeX and HTML tables. J Stat Softw 2013; 55: 1–26Google Scholar
17 : R: A Language and Environment for Statistical Computing. Vienna, R Foundation for Statistical Computing, 2014Google Scholar
18 : The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143:29–36Crossref, Medline, Google Scholar
19 : A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983; 148:839–843Crossref, Medline, Google Scholar
20 : Smile and laughter induction and intraoperative predictors of response to deep brain stimulation for obsessive-compulsive disorder. Neuroimage 2011; 54(Suppl 1):S247–S255Crossref, Medline, Google Scholar
21 : Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology 2010; 35:317–336Crossref, Medline, Google Scholar
22 : Translational research in OCD: circuitry and mechanisms. Neuropsychopharmacology 2013; 38:252–253Crossref, Medline, Google Scholar
23 : Psychosis from subthalamic nucleus deep brain stimulator lesion effect. Surg Neurol Int 2013; 4:7Crossref, Medline, Google Scholar
24 : A new look at the statistical model identification. IEEE Trans Autom Control 1974; 19:716–723Crossref, Google Scholar
25 : An asymptotic equivalence of choice of model by cross-validation and Akaike’s criterion. J R Stat Soc Ser B Methodol 1977; 39:44–47Google Scholar
26 : Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry 2010; 22:437–452Crossref, Medline, Google Scholar
27 : Mood disorders following stroke: new findings and future directions. J Geriatr Psychiatry 1989; 22:1–15Medline, Google Scholar
28 : Prefrontal cortex dysfunction in clinical depression. Depression 1994; 2:59–72Crossref, Google Scholar
29 : High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci 2007; 27:12601–12610Crossref, Medline, Google Scholar
30 : Long-term high frequency deep brain stimulation of the nucleus accumbens drives time-dependent changes in functional connectivity in the rodent limbic system. Brain Stimulat 2013; 6:274–285Crossref, Medline, Google Scholar



