Effects of Transcranial Magnetic Stimulation on the Hypothalamic-Pituitary Axis in Depression: Results of a Pilot Study
Abstract
Some studies have reported that repetitive transcranial magnetic stimulation (rTMS) applied to the dorsolateral prefrontal cortex (DLPFC) is able to induce changes in the hypothalamic-pituitary axis in subjects with major depression. The causes of these neuroendocrine effects are unknown and deserve to be studied. The authors monitored neuroendocrine hormones in 15 subjects with major depression treated by 1-Hz rTMS on the right DLPFC and explored a correlation with mood improvement. Unlike previous studies, no changes in serum cortisol, prolactin, and thyroid hormone levels were found. However, the authors did observe short-term changes in growth hormone levels in nonresponsive subjects.
Some studies have reported that repetitive transcranial magnetic stimulation (rTMS) applied to the dorsolateral prefrontal cortex (DLPFC) is able to induce changes in the hypothalamic-pituitary (HP) axis in subjects with major depression and posttraumatic stress disorder.1–6 The causes of these neuroendocrine effects, which have been reported for serum cortisol, prolactin, and thyroid hormones, are unknown. On the one hand, as studies have reported changes in the HP axis associated with the alleviation of depressive symptoms,2 we can hypothesize that alterations in the HP axis are related to a physiological response that accompanies the therapeutic effect and could thus be considered a biological marker of clinical outcome. On the other hand, rTMS may modulate brain networks implicated in the HP axis, thanks to brain connectivity. In this case, the effects of rTMS on the HP axis could be interpreted as undesirable if they make no contribution to improving the subject’s clinical condition. This is what we previously reported in a subject treated by rTMS for major depressive disorder (MDD) who presented with an increase in plasma thyroid-stimulating hormone (TSH) levels above the normal range without clinical improvement.5
In order to clarify the issue, we monitored neuroendocrine levels in subjects with MDD included in a pilot study.5,7 Our objectives were as listed below:
To screen for abnormal levels of HP axis hormones induced by rTMS in these subjects;
To explore correlations between abnormal changes in HP axis hormone levels or subclinical endocrine changes and the therapeutic efficacy of rTMS on mood.
Methods
Participants
Subjects older than 18 years and suffering from treatment-resistant depression were enrolled in the pilot study.7 To qualify for enrollment, they had to meet DSM-IV criteria for MDD,8 have a total score of ≥22 on the 21-item Hamilton Depression Rating Scale (HAM-D), and have failed to respond to a minimum of two courses of antidepressant drugs. Subjects were excluded if they had any diagnosis of bipolar I or II disorder, a history of current substance abuse (except nicotine), and a history of seizures or other neurological conditions. Additional exclusion criteria were clinically significant comorbid disease such as liver, kidney, or heart failure and a pacemaker. Female subjects were excluded if they were pregnant or lactating.
Psychotropic medications were broken off 7 days before the start of the stimulation sessions. The concomitant use of psychoactive drugs was restricted, with the exception of cyamemazine and hydroxyzine as rescue medication for severe anxiety or insomnia.
Stimulation Parameters
The subjects received once-daily rTMS sessions Monday to Friday for two weeks using MagPro×100 (MagVenture A/S, Farum, Denmark) with 75-mm figure-of-8 coils. The frequency of stimulation was 1 Hz, with an intensity at 120% of the resting motor threshold. One rTMS session consisted of six trains of 60 pulses, separated by five intertrains of 30 seconds (360 pulses per day; total duration of one rTMS session: 8 minutes, 30 seconds). Brain stimulations were applied over the right DLPFC (Brodmann’s area 9 or 46).7 These stimulation parameters have been found to be effective to treat MDD in previous studies.9
Outcome Measures
The HAM-D and Montgomery-Åsberg Depression Rating Scale (MADRS) were used to assess depressive symptoms. A therapeutic response was defined as a HAM-D reduction or MADRS reduction ≥50% relative to the pretreatment baseline.
The first and second blood samples were collected between 9:00 a.m. and 10:00 a.m. We took these blood samples within 15 minutes before and after the first and the 10th rTMS sessions, respectively. The third blood sample was taken 4 weeks later at 9:00 a.m. (Figure 1). Blood samples were centrifuged, and the serum was frozen at –20° for future analysis. Thyroid-stimulating hormone (TSH), free triiodothyronine (fT3), and free thyroxine (fT4) were quantified on a Dimension Vista analyzer with dedicated reagents (Siemens, Saint Denis, France). Cortisol, prolactin, growth hormone (GH), and estrogen were quantified on an Immulite 2000 analyzer with dedicated reagents (Siemens). Testosterone was quantified using a radioimmunoassay.
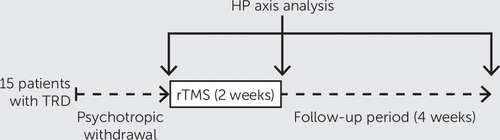
FIGURE 1. Neuroendocrine Monitoring to Assess the Effects of Repetitive Transcranial Magnetic Stimulation (rTMS) on the Hypothalamic-Pituitary (HP) Axis in Patients With Treatment-Resistant Depression (TRD)
Data Analysis
To assess the effects of rTMS on the HP axis, we first identified post-rTMS changes in HP axis values up to abnormal values. We then compared HP axis hormone levels after rTMS with baseline values using univariate nonparametric methods based on ranked hormone values (Wilcoxon signed-rank test). This comparison of HP changes was done for all 15 subjects and in a subgroup analysis according to the therapeutic results; p≤0.05 were considered significant.
Ethics
The addition of neuroendocrine monitoring to the pilot study was approved by a human research ethics committee (Committee Protection Persons number II from East of France; registration number: 2009-A00772–55). After complete description of the neuroendocrine monitoring to the subjects of the pilot study, written informed consent was obtained.
Results
Eleven women and four men were enrolled in the pilot study. Mean age was 55 years (SD=9.5; range: 41–71). Average number of hospitalizations for depression was three (SD=2.8), and mean duration of the current MDD was 20 months (SD=2.5). All but one subject was right handed.
Among the 15 subjects included in the pilot study, whatever the therapeutic result, analysis of the blood samples after rTMS revealed no significant change after rTMS in HPA hormones levels above the normal range either in short or medium term. With regard to subclinical endocrine changes induced by rTMS, no significant changes in TSH, fT3, fT4, cortisol, prolactin, estrogen, and testosterone were observed at the end of the rTMS sessions or at 4 weeks thereafter. However, we did find a trend toward a decrease in GH levels just after the 10th session (p=0.0652), but not four weeks later (p=0.3495). By analyzing levels of HP axis hormones according to the therapeutic response assessed using HAM-D scores, we found a significant decrease in GH levels at the end of the stimulation session in nonresponders (p=0.0098) (Table 1). This result tended to parallel the result for GH and MADRS scores (p=0.0547). Using effect size (Cohen's d) to measure the strength of the rTMS effect on GH levels on the basis of HRDS results, we found a large size effect (d=0.86) by comparing pre- and post-rTMS changes in GH levels between nonresponders and responders.
| Item | Group | |||||
|---|---|---|---|---|---|---|
| Responders (N=4) | Nonresponders (N=11) | |||||
| HAM-D | Mean (SD) Pretreatment | Mean (SD) Posttreatment | pa | Mean (SD) Pretreatment | Mean (SD) Posttreatment | pa |
| TSH (mUI/l) | 1.58 (1.15) | 1.39 (0.73) | 0.6250 | 1.83 (0.74) | 1.63 (0.54) | 0.4258 |
| T3 (pmol/l) | 4.87 (0.45) | 4.77 (0.46) | 1.0000 | 4.83 (0.57) | 4.74 (0.60) | 0.7266 |
| T4 (pmol/l) | 12.70 (1.31) | 12.12 (0.43) | 0.6250 | 12.70 (1.54) | 12.98 (1.76) | 0.5703 |
| Cortisol (µg/100 ml) | 15.47 (5.01) | 13.72 (2.47) | 0.6250 | 16.10 (2.76) | 14.67 (3.46) | 0.2061 |
| GH (ng/ml) | 0.26 (0.30) | 0.33 (0.32) | 0.2500 | 2.57 (3.23) | 0.46 (0.54) | 0.0098 |
| Prolactin (µg/l) | 14.72 (4.85) | 16.99 (8.43) | 0.3750 | 10.15 (4.83) | 13.65 (9.26) | 0.4131 |
| Responders (N=6) | Nonresponders (N=9) | |||||
| MADRS | Mean (SD) Pretreatment | Mean (SD) Posttreatment | pa | Mean (SD) Pretreatment | Mean (SD) Posttreatment | pa |
| TSH (mUI/l) | 1.99 (0.68) | 1.50 (0.71) | 0.0938 | 1.62 (0.66) | 1.61(0.52) | 1.000 |
| T3 (pmol/l) | 5.17 (0.37) | 5.02 (0.37) | 0.7500 | 4.62 (0.51) | 4,57(0.60) | 0.6211 |
| T4 (pmol/l) | 12.05 (0.52) | 12.13 (0.91) | 0.8750 | 13.13 (1.70) | 13.17(1,88) | 0.9375 |
| Cortisol (µg/100 ml) | 13.77 (2.14) | 12.85 (2.82) | 0.5625 | 17.39 (3.22) | 15.46(3.08) | 0.2031 |
| GH (ng/ml) | 0.64 (1.32) | 0.36 (0.58) | 1.0000 | 2.84 (3.43) | 0.47(0.45) | 0.0547 |
| Prolactin (µg/l) | 11.15 (3.74) | 12.13 (5.74) | 0.4375 | 11.51 (6.07) | 16.14(10.49) | 0.3594 |
TABLE 1. Changes in Hypothalamic-Pituitary Hormones Before and Just After 10 rTMS Sessions According to the Results of the Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Åsberg Depression Rating Scale (MADRS) (therapeutic response ≥50% improvement)
Discussion
Neuroendocrine abnormalities in depression have been reported for over 60 years and include changes in cortisol, thyroid hormones, growth hormone, and prolactin.10,11 These were identified by elevated basal levels of hormones and metabolites in blood and urine, but also by dynamic functions test of the neuroendocrine system, such as the overnight dexamethasone suppression test (DST). Indeed, some studies found abnormal DST results (nonsuppression of cortisol secretion) in psychotic depression, and such results were thus claimed to be a specific episode-related biological marker for melancholia.10,12,13 Taken as a whole, these neuroendocrine abnormalities tend to normalize with recovery from depression.12 Changes in neuroendocrine functions were also found during treatment by electroconvulsive therapy (ECT), and to date this is one of the most robust hypotheses to explain the antidepressant effects of this brain stimulation technique. According to several studies, hypothalamic stimulation occurring during ECT led to a transient release of adrenocorticotropic hormone, cortisol, and prolactin into the blood. However, the magnitude of the changes would not have led to the antidepressant effects of ECT.12 There is controversy regarding the release of the other HP axis hormones, such as GH and thyroid hormones.14–18
Compared with ECT data, there are few data on the effect of rTMS on the HP axis, probably because of the relatively recent use of this NIBS technique as a treatment for depression. These data suggest that rTMS is also associated with changes in HP axis hormones, mainly among the hypothalamic pituitary thyroid axis, compared with ECT.2,4,19,20
Our biological screening provides additional information on HP axis changes following TMS in patients with depression. First, we found no abnormal values immediately, after 10 sessions, or 4 weeks later. This led us to believe that the significant increase in plasma TSH above the normal range found using the same rTMS parameters and previously reported by our staff was probably an “accidental” event.5 Secondly, unlike previous studies that showed that rTMS did affect the HP axis, we found no changes in TSH, T4, or prolactin levels.2–4 The small size of our sample could explain why we could not replicate the previously reported data, even though it was not smaller than in some studies that found a hormone-modulating effect.2–4 The diverse stimulation parameters (frequency, left-right DLPFC) as well as the various methods used to target the DLPFC could also explain these differences in the results, even though some studies using stimulation parameters similar to ours (1 Hz, right DLPFC) have reported the effects of rTMS on serum prolactin and thyroid hormones.2,3 The concomitant use of psychotropic medications with rTMS, which varies from one study to another, should also be taken into account to explain this different result, as changes in HP axis can be attributed to brain stimulation only in a medication-free context. Thirdly, we found a significant decrease in GH levels in nonresponders. This result has never been observed, since studies assessing the effects of rTMS on the HP axis did not measure GH levels.1–4,6 As growth hormone deficiency is known to be associated with depressed mood, and as GH treatment has been associated with significant improvements in depression scales in subjects with GH deficiency, we may hypothesize that a link could exist between the effect of rTMS on GH levels and the therapeutic result. According to our results, the absence of a decrease in GH levels after rTMS among responders might be perceived as a relative increase in the levels of this hormone.21 Due to the small numbers of subjects involved in this pilot study, the results concerning GH hormone levels need to be replicated to confirm that rTMS is able to act on depression by way of the HP axis. Another limitation due to the small sample size (four men and 11 women) was that we were, unfortunately, unable to perform statistical analyses using changes in estradiol and testosterone levels according to the therapeutic results, as we did with the other HP axis hormones. Finally, caution must be exercised when linking changes in HP axis hormones with changes of mood when this device is used in subjects with depression. Indeed, changes in serum thyroid levels have been reported in healthy subjects who have undergone rTMS.19,20
In conclusion, changes in HP axis hormones in subjects with major depression treated by rTMS are unclear. Further studies using larger samples and higher levels of proof (i.e., using sham-controlled conditions) are needed to assess the effects of rTMS on HP axis hormones. Moreover, these studies must include screening for GH in addition to the other HP axis hormones to clarify the mechanisms underlying the interaction between the HP axis and depression treated by rTMS. Better understanding of the effects of TMS on HP axis hormones may also shed light on the relationship between neuronendocrine responses and the therapeutic effect of ECT.
1 : Effect of age, gender, menopausal status, and ovarian hormonal level on rTMS in treatment-resistant depression. Psychoneuroendocrinology 2008; 33:821–831Crossref, Medline, Google Scholar
2 : Changes in hypothalamic-pituitary-thyroid axis following successful treatment with low-frequency right prefrontal transcranial magnetic stimulation in treatment-resistant depression. Psychiatry Res 2010; 175:74–77Crossref, Medline, Google Scholar
3 : Repetitive TMS combined with exposure therapy for PTSD: a preliminary study. J Anxiety Disord 2009; 23:54–59Crossref, Medline, Google Scholar
4 : Acute mood and thyroid stimulating hormone effects of transcranial magnetic stimulation in major depression. Biol Psychiatry 2001; 50:22–27Crossref, Medline, Google Scholar
5 : Significant increase in plasma thyroid-stimulating hormone during low-frequency repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci 2011; 23:E12Link, Google Scholar
6 : The combined dexamethasone-CRH test before and after repetitive transcranial magnetic stimulation (rTMS) in major depression. Psychoneuroendocrinology 2003; 28:376–385Crossref, Medline, Google Scholar
7 : Interest of targeting either cortical area Brodmann 9 or 46 in rTMS treatment for depression: a preliminary randomized study. Clin Neurophysiol 2014; 125:2384–2389Crossref, Medline, Google Scholar
8 : Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. (DSM-IV). Washington, DC, American Psychiatric Association, 2000Google Scholar
9 : The efficacy and safety of low frequency repetitive transcranial magnetic stimulation for treatment-resistant depression: the results from a large multicenter French RCT. Brain Stimulat 2014; 7:855–863Crossref, Medline, Google Scholar
10 : Neuroendocrine changes in depression. Aust N Z J Psychiatry 1985; 19:120–127Crossref, Medline, Google Scholar
11 : Psychological stress and endocrine functions; blood levels of adrenocortical and thyroid hormones in acutely disturbed patients. Psychosom Med 1956; 18:324–333Crossref, Medline, Google Scholar
12 : Electroconvulsive therapy’s mechanism of action: neuroendocrine hypotheses. J ECT 2014; 30:107–110Crossref, Medline, Google Scholar
13 : Use of the dexamethasone suppression test in an inpatient setting: a replication and new findings. Psychoneuroendocrinology 1989; 14:231–239Crossref, Medline, Google Scholar
14 : Effects of ECT on pituitary hormone release: relationship to seizure, clinical variables and outcome. Br J Psychiatry 1983; 143:618–624Crossref, Medline, Google Scholar
15 : Effects of single and repeated electroconvulsive therapy sessions on plasma ACTH, prolactin, growth hormone and cortisol concentrations. Psychoneuroendocrinology 1991; 16:345–352Crossref, Medline, Google Scholar
16 : Electroconvulsive therapy (ECT) increases plasma growth hormone, prolactin, luteinising hormone and follicle-stimulating hormone but not thyrotropin or substance P. Psychoneuroendocrinology 1981; 6:261–267Crossref, Medline, Google Scholar
17 : Selective effects of ECT on hypothalamic-pituitary activity. Psychol Med 1987; 17:319–328Crossref, Medline, Google Scholar
18 : Effects of electroconvulsive therapy on hypothalamic-pituitary-thyroid axis activity in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 2002; 26:1171–1175Crossref, Medline, Google Scholar
19 : Atypical depression in growth hormone deficient adults, and the beneficial effects of growth hormone treatment on depression and quality of life. Eur J Endocrinol 2004; 151:325–332Crossref, Medline, Google Scholar
20 : Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsychiatry Clin Neurosci 1996; 8:172–180Link, Google Scholar
21 : Suprathreshold repetitive transcranial magnetic stimulation elevates thyroid-stimulating hormone in healthy male subjects. J Nerv Ment Dis 2001; 189:393–397Crossref, Medline, Google Scholar



