Preliminary Evidence on Cannabis Effectiveness and Tolerability for Adults With Tourette Syndrome
Abstract
The authors retrospectively evaluated effectiveness and tolerability of cannabis in 19 adults with Tourette syndrome. Tics scores decreased by 60%, and 18 of the 19 participants were at least “much improved.” Cannabis was generally well tolerated, although most participants reported side effects.
Tourette syndrome (TS) is an inherited neuropsychiatric disorder characterized by motor tics and at least one vocal tic with childhood onset that has persisted for more than 1 year.1 TS has a high comorbidity with other neuropsychiatric disorders, including obsessive-compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), and rage attacks. When active treatment of tics is required, comprehensive behavioral intervention for tics based on habit reversal training is the first-line treatment.2 Mainstay pharmacological agents are high-potency antipsychotics and the alpha-2 receptor agonists. These drugs are limited by significant side effects. Other pharmacological treatments have only limited evidence.
Cannabinoids have been explored as a treatment for TS since the 1980s.3–5 Delta−9-tetrahydrocannabinol (THC), a major psychoactive ingredient of cannabis, is thought to account for many of the pharmacological actions of cannabis. In recent years, there has also been interest in cannabidiol (CBD), a primary cannabinoid in cannabis, which has been found to have antiemetic, anticonvulsant, neuroprotective, and anti-inflammatory properties.6 The earliest case series reported on inhaled cannabis resulting in improvement in tics and comorbid symptoms in three patients with TS.3
Subsequently, a survey of TS patients found that 17 out of 64 consecutive respondents had used cannabis, and the majority of these (82%) reported that cannabis was effective in reducing tics, premonitory urges, and comorbidities.7 Later, several open uncontrolled studies with THC showed similar results.5 Two controlled trials have been reported to date, both of which investigated the effect of oral THC in TS patients. In a randomized double-blind placebo-controlled crossover single-dose trial with 12 adult patients, a significant global tic improvement was observed with the self-rating Tourette Syndrome Symptom List (TSSL) after treatment with oral THC compared with placebo.8 In another randomized double-blind, parallel group placebo-controlled study over 6 weeks with 24 adult patients, a significant difference was found in the TSSL between the THC and placebo groups after 10 treatment days.9 No serious side effects occurred during the study. These two studies are limited by small sample sizes and short treatment duration. It is noteworthy that the improvements seen in THC trials were not as large as those described in the case series with cannabis. Given this limited evidence, several systematic reviews have concluded that there is currently insufficient evidence to support the use of cannabinoids for tics.10–13
The goal of the present retrospective study was to evaluate the effectiveness and tolerability of cannabis treatment in adult patients with TS. We hypothesized that cannabis would be effective and well tolerated in the treatment of tics and comorbid symptoms. We add to the emerging literature on the topic by reporting the findings from semistructured assessments of 19 adult patients with TS treated with inhaled cannabis to help control their tics.
Methods
We identified 22 patients who had been using cannabis for TS in our Tourette Syndrome Neurodevelopmental Clinic at the Toronto Western Hospital (Toronto, Ontario, Canada). Our inclusion criteria were diagnosis of TS according to DSM-5 criteria and using cannabis regularly for 6 months or longer. Exclusion criteria were significant cognitive delay, current polysubstance abuse, or current alcohol abuse. Of the 22 patients we identified, one was excluded because the patient had been using cannabis for less than 6 months. Twenty-one patients met our inclusion criteria and were invited to take part in the study. Of these, 19 were willing to participate (a response rate of 90%) and were recruited into the study after signing informed consent forms. The study was approved by the institutional research ethics board.
Clinical charts for all participants were reviewed for demographic, medical and medication history, psychiatric history, and symptomatology of tics and comorbidities. All 19 participants were invited to the clinic for semistructured interviews, which were carried out by two clinic psychiatrists who have extensive experience with TS (EA-J and PS). The following standardized questionnaires (current and prior to cannabis use) were administered: the Yale Global Tic Severity Scale (YGTSS)14; PUTS [Premonitory Urges for Tics Scale]15; Y-BOCS [Yale-Brown Obsessive Compulsive Scale]16; the Adult ADHD Self-Report Scale, version 1.117 (ASRS); the Structured Clinical Interview for DSM-IV Axis I Disorders, with psychotic screen, Patient Edition18 (SCID-I/P); and the short version of the Marijuana Effect Expectancy Questionnaire (MEEQ),19 a structured list of specific effects previously reported with the use of cannabis or THC. Assessment for adverse effects associated with cannabis was based on open-ended questions, as well as the SCID-I/P psychosis interview and the MEEQ. Finally, clinicians completed the Clinical Global Impressions Improvement (CGI-I) and Clinical Global Impressions Severity (CGI-S) scales.
YGTSS questionnaires were completed by the study psychiatrists retrospectively to assess tic severity prior to treatment with cannabis and for current symptom severity during cannabis treatment. The retrospective YGTSS questionnaires were based on clinical information in the patient’s chart supplemented by patient recollection. The primary efficacy endpoints were the mean change from baseline in YGTSS-total tic score (YGTSS-TTS) and the percentage of patients rated as “much improved” or “very much improved” on the CGI-I. Tolerability endpoints included spontaneous and structured adverse effects reporting via the MEEQ and the SCID-I/P psychosis interview. In addition, the percentage of patients experiencing side effects leading to discontinuation of treatment was noted.
Data analysis was conducted with SPSS 22 (IBM SPSS Statistics for Windows, Version 22.0. Armonk, N.Y., IBM Corp.). Continuous variables were compared with paired sample two-tailed t tests, and categorical variables were compared using chi-square tests. A threshold value of p<0.05 was used for statistical significance.
Results
Clinical Characteristics and Cannabis Use
Out of the 21 patients who were contacted, 19 took part in the study, for a response rate of 90%. The demographic and clinical characteristics, as well as cannabis history for each participant, are shown in Table 1. The sample consisted of 16 males and three females. The mean age of the participants was 32 years (±12.3 years). Most participants had comorbidities; in particular, 13 patients had a diagnosis of OCD, and 11 had a diagnosis of ADHD.
| ID | Age (Years)/Gender | Comorbidities | Current and Past Medications | How Participant Learned About Cannabis for Tics | Pharmaceutical Cannabinoid | Cannabis | |||
|---|---|---|---|---|---|---|---|---|---|
| Current Source | Dosing | Route | Duration of Single Dose Effect on Tics (Hours) | ||||||
| 1 | 19/M | OCD, ADHD, restless leg syndrome, nocturnal myoclonus | Past: clonidine, risperidone, sertraline, amphetamine/dextroamphetamine, atomoxetine, clonazepam, desmopressin | Mother found information online | None | Approved supplier | 0.5 g four times daily | Smoked | 2–3 |
| 2 | 40/F | OCD, depression | Current: citalopram, clonazepam; Past: clonidine, risperidone, pimozide, aripiprazole, quetiapine, tetrabenazine, paroxetine, fluoxetine | Family friend with TS | Past: nabiximols, nabilone | Approved supplier | 0.125–0.5 g in food every night; 0.5 g smoked/day | Smoked, ingested with food | 6 |
| 3 | 21/M | OCD, anxiety | Past: olanzapine, quetiapine, methylphenidate, atomoxetine, fluoxetine, bupropion | Personal experience | None | Unapproved supplier | 1 g three times daily | Vaporized, ingested with food | 3–4 |
| 4 | 32/M | ADHD, ASD, hypertension | Past: clonidine, risperidone, haloperidol, pimozide, fluoxetine | TS clinic | Past: nabiximols | Approved supplier | 0.5 g every night for 1 week, then discontinued for 3 weeks | Vaporized | 24 |
| 5 | 34/M | OCD, social phobia | Current: venlafaxine Past: clonidine, pimozide | Friend | Past: dronabinol | Compassion clinic | 0.25 g daily | Vaporized | Uncertain |
| 6 | 51/M | OCD | Current: clonazepam Past: clonidine, mirtazapine, amytriptyline, lorazepam, valerian | Read research by Muller-Vahl and colleagues online | none | compassion clinic | 3 g in food, twice weekly | ingested with food | 5 |
| 7 | 28/M | OCD, ADHD, platelet abnormality | Past: risperidone | Personal experience | None | Unapproved supplier | <3 g spread over entire day | Smoked | Uncertain |
| 8 | 27/M | HIV positive | Current: elvitegravir/cobicistat/emtricitabine/tenofovir, guanfacine, clonazepam Past: clonidine | Personal experience | None | Compassion clinic | 0.1 g three times daily | Smoked | 2–3 |
| 9 | 18/M | OCD, ADHD, panic disorder | Current: sertraline Past: clonidine, methlyphenidate | Online | None | Unapproved supplier | 0.3 g spread over entire day | Smoked | 1.5–2 |
| 10 | 22/M | ADHD | Past: clonidine, risperidone, pimozide, quetiapine, methylphenidate, atomoxetine, bupropion, fluoxetine, clonazepam | Online | Past: nabilone | Unapproved supplier | 1.5 g spread over entire day | Smoked, ingested with food | 2 |
| 11 | 27/M | OCD, ADHD | Past: clonidine, methlyphenidate, fluoxetine, clobazam, prindolol | Personal experience | None | Approved supplier | 1.5–2 g 4–5×/daily | Smoked, ingested with food | 1–2 |
| 12 | 64/M | OCD, ADHD, COPD, type 2 diabetes | Current: citalopram, acetylsalicylic acid, clopidogrel, metformin, bisoprolol, amlodipine, atorvastatin, hydrochlorothiazide, omeprazole; Past: clonidine, amytriptyline, bupropion | Personal experience | Past: nabiximols | Approved supplier | 10 g/day | Smoked, ingested with food | 1–2 |
| 13 | 24/M | OCD, ADHD, depression | Past: clonidine, risperidone, haloperidol, methlyphenidate, atomoxetine, valproate, topiramate, fluoxetine, fluvoxamine, bupropion, zopiclone, clonazepam, lorazepam, diphenhydramine | Magazine | Past: nabiximols, nabilone | Unapproved supplier | 1 g/day smoked, 2 g/day vaporized | Smoked, vaporized | 2–3 |
| 14 | 30/F | Anxiety | Current: ziprasidone, clonazepam, sertraline, pimozide Past: risperidone, lurasidone, quetiapine, tetrabenazine, pramipexole, clomipramine, trazodone, zoplicone, lorazepam, melatonin, tetracycline, isotretinoin | Personal experience | None | Approved supplier | 0.2–0.4 g 1–2 times/week | Smoked | 2–4 |
| 15 | 24/M | ADHD, anxiety, depression | Current: fluoxetine Past: haloperidol, risperidone, olanzapine, quetiapine, clonidine, topiramate, fluoxetine, fluvoxamine, citalopram, methylphenidate, dextroamphetamine, atomoxetine, clonazepam, diazepam | Friend | Past: nabiximols, nabilone | Unapproved supplier | 0.6 g 4–5 times/day | Vaporized | 3 |
| 16 | 42/F | OCD, ADHD, PTSD, hepatitis C | Current: clonidine, aripiprazole, lisdexamfetamine, paroxetine, trazodone, clonazepam Past: ziprasodone, topiramate, bupropion | Personal experience | Past: nabilone | Approved supplier | 0.25 g 4–6 times/day | Smoked | 2 |
| 17 | 25/M | OCD | Current: haloperidol, fluvoxamine, acetaminophen/dextromethorphan/doxylamine Past: aripiprazole, lorazepam | Personal experience | None | Unapproved supplier | 1 g three times daily | Smoked | 4–6 |
| 18 | 32/M | OCD, ADHD, specific phobia (needle) | Past: clomipramine, imipramine, methylphenidate, sertraline, fluoxetine, paroxetine | TV: news report | None | Approved supplier | 1 g/day total: small amount ingested every few hours, vaporizer in PM | Vaporized, ingested with food | 3 |
| 19 | 49/M | none | Current: sildenafil Past: clonidine, pimozide, tetrabenazine, clonazepam, notriptyline, gabapentin, naproxen | TS clinic | Past: dronabinol, nabiximols, nabilone | Approved supplier | 0.5 g twice daily | Vaporized | 8–12 |
TABLE 1. Demographic and Clinical Characteristics and Cannabis Use in the Study Participants (N=19)a
Except for one patient, all had previously been treated with medications for tics, including 14 with clonidine, 13 with at least one antipsychotic, and nine with both. Nine patients had previously participated in trials with one or more pharmaceutical cannabinoid. While pharmaceutical cannabinoids had been helpful for tics, the patients reported that these were not nearly as effective as cannabis.
All study patients had been using cannabis regularly for at least 2 years. Eight of the patients serendipitously discovered the use of cannabis for tics after personally experiencing notable reductions in their tics following the use of cannabis recreationally. Given the varied sources of medical cannabis among patients, many of which used numerous and idiosyncratic names to refer to their different cannabis products, we were unable to obtain reliable information with regard to cannabis strain or THC/CBD content.
The frequency of use varied significantly, from frequent usage of small doses throughout the day, to twice weekly in the case of two patients, and one participant reporting daily use for 1 week followed by 3 weeks off. The estimated average total daily dose also varied substantially, from less than 0.1 g to 10 g, for a median of 1 g daily. The tic-reducing effects of each cannabis dose lasted a median of 3 hours; there were two notable outliers who reported effects lasting 10 and 24 hours, respectively.
Effect of Cannabis on Tics and Related Symptoms
All study participants experienced clinically significant symptom relief. Eighteen out of 19 patients experienced a decrease in YGTSS total tic severity and impairment scores (Table 2). Fifteen patients reported obsessive-compulsive symptoms at baseline, and among all of these, YBOCS total scores improved after starting cannabis (Table 2). Of the 13 patients who met criteria for ADHD according to the ASRS, only one continued to do so while using cannabis (p<0.001) (Table 3). All patients reported improvement in comorbid symptoms with cannabis, including obsessive-compulsive symptoms, attention, impulsivity, anxiety, irritability, rage outbursts, and sleep (Table 2). Group average YGTSS baseline scores prior to cannabis use all decreased substantially during cannabis use (Table 3), including total tic severity score (from 30.5±7.2 to 12.2±8.6 [60% reduction], p<0.001) (Figure 1) and impairment score (from 35.0±12.9 to 11.7±11.5 [67.1% reduction], p<0.001). Based on the CGI-I, 18 out of 19 patients (94.7%) were rated as being “very much improved” or “much improved” (Table 3).
| ID | YGTSS Total Tic Score | YGTSS Impairment | Y-BOCS Total | ASRS Criteria for ADHD | CGI-S Score | CGI-I Score | Cannabis Impact on Comorbid Conditions | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Cannabis Use | During Cannabis Use | Before Cannabis Use | During Cannabis Use | Before Cannabis Use | During Cannabis Use | Before Cannabis Use | During Cannabis Use | Before Cannabis Use | During Cannabis Use | |||
| 1 | 26 | 0 | 40 | 0 | 17 | 0 | Yes | No | 5 | 1 | 1 | Decreased OCS, ADHD and rage |
| 2 | 46 | 20 | 50 | 40 | 27 | 16 | Yes | No | 7 | 4 | 2 | Decreased OCS and rage |
| 3 | 26 | 10 | 30 | 10 | 22 | 11 | Yes | No | 3 | 1 | 2 | Improved attention; decreased restlessness, impulsivity and rage |
| 4 | 44 | 18 | 50 | 0 | 12 | 12 | Yes | No | 4 | 2 | 1 | Improved attention and communication skills |
| 5 | 36 | 11 | 20 | 10 | 28 | 16 | Yes | Yes | 4 | 3 | 1 | Decreased OCD and anxiety; improved attention |
| 6 | 26 | 17 | 30 | 20 | 21 | 19 | Yes | No | 4 | 3 | 2 | Decreased OCD, anxiety, and rage; improved sleep |
| 7 | 21 | 8 | 20 | 0 | 0 | 0 | No | No | 4 | 2 | 2 | Reduced rage and irritability |
| 8 | 26 | 13 | 30 | 10 | 20 | 14 | Yes | No | 4 | 3 | 2 | Decreased ADHD and rage; improved sleep |
| 9 | 23 | 6 | 0 | 20 | 23 | 19 | No | No | 4 | 2 | 2 | Reduced rage |
| 10 | 30 | 18 | 30 | 10 | 0 | 0 | Yes | No | 5 | 3 | 2 | Reduced restlessness and anxiety; improved attention |
| 11 | 37 | 24 | 40 | 20 | 23 | 20 | No | No | 5 | 3 | 2 | Reduced OCD and ADHD; improved concentration and organization |
| 12 | 26 | 28 | 40 | 30 | 26 | 18 | No | No | 4 | 4 | 3 | Reduced OCD and restlessness; improved concentration and organization |
| 13 | 31 | 0 | 50 | 0 | 25 | 0 | Yes | No | 5 | 1 | 1 | Decreased OCD, ADHD and rage |
| 14 | 24 | 0 | 40 | 0 | 0 | 0 | Yes | No | 5 | 3 | 1 | Reduced rage |
| 15 | 35 | 0 | 50 | 0 | 35 | 0 | Yes | No | 6 | 1 | 1 | Decreased OCD, ADHD, and rage |
| 16 | 33 | 15 | 40 | 20 | 38 | 12 | Yes | No | 5 | 2 | 2 | Decreased symptoms of OCD and rage |
| 17 | 25 | 7 | 40 | 10 | 0 | 6 | No | No | 6 | 2 | 1 | Decreased anxiety and rage |
| 18 | 39 | 15 | 40 | 10 | 25 | 13 | Yes | No | 5 | 3 | 1 | Reduced anxiety and improved productivity |
| 19 | 26 | 21 | 30 | 10 | 14 | 3 | No | No | 4 | 3 | 1 | Decreased OCS |
TABLE 2. Cannabis Effectiveness in Individual Patientsa
| Instrument and Symptoms | Baseline (Mean±SD) | During Cannabis Treatment (Mean±SD) | Percentage Reduction From Baseline (%) | p | |
|---|---|---|---|---|---|
| YGTSS | Motor tic severity | 16.7±3.3 | 7.3±5.2 | 56.3 | <0.001 |
| Vocal tic severity | 13.8±4.4 | 4.8±4.4 | 65.2 | <0.001 | |
| Impairment | 35.3±12.6 | 11.6±11.2 | 67.1 | <0.001 | |
| Total tic severity | 30.5±7.2 | 12.2±8.6 | 60 | <0.001 | |
| Total severity scale | 65.8±17.7 | 23.7±18.1 | 64 | <0.001 | |
| Y-BOCS | Obsession subtotal | 9.5±6.0 | 5.0±4.5 | 47.4 | 0.001 |
| Compulsion subtotal | 9.2±5.8 | 4.5±3.7 | 51.1 | 0.003 | |
| Total | 18.7±11.7 | 9.4±7.8 | 49.7 | 0.001 | |
| PUTS | 26.2±4.9 | 18.6±4.6 | 29 | <0.001 | |
| Baseline(Number of Participants) | During Cannabis Treatment(Number of Participants) | Percentage Reduction From Baseline(%) | |||
| ASRS: Symptoms highly consistent with ADHD | 13 | 1 | 92.3 | <0.001 | |
| CGI-I: Much improved or very much improved | N/A | 18 | N/A | N/A | |
| CGI-S: Moderately to extremely ill | 18 | 2 | 88.9 | <0.001 | |
TABLE 3. Cannabis Effectiveness Group Averagesa
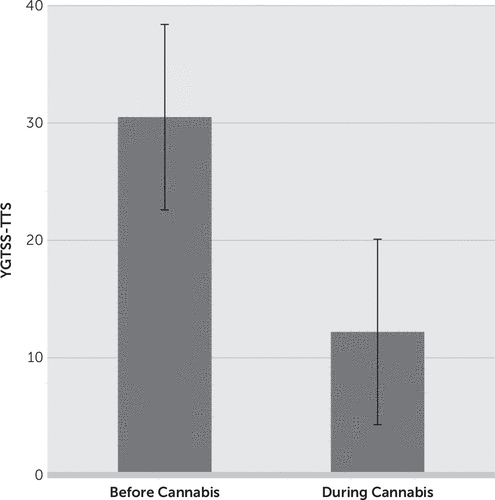
FIGURE 1. Effect of Cannabis on the Yale Global Tic Severity Scale Total Tic Score (YGTSS-TTS)
Cannabis Tolerability
The MEEQ short version is comprised of 48 items rated on a 5-point Likert scale, from disagree strongly to agree strongly, covering 6 areas. The ratings for each area on the MEEQ for each individual participant are shown in Table 4. Average ratings across individuals for each scale were as follows: cognitive and behavioral impairment, 2.4±0.9; relaxation and tension reduction, 3.9±0.5; social and sexual facilitation, 3.0±0.7; perceptual and cognitive enhancement, 3.0±0.7; global negative effects, 1.5±0.6; and craving and physical effects, 3.3±1.0. The maximum rating reported by an individual patient for the cognitive and behavioral impairment was 3.8 (i.e., between “uncertain” and “agree somewhat”), and the maximum rating for the global negative effects was 2.8 (i.e., between “disagree somewhat” and “uncertain”).
| ID | Marijuana Effect Expectancy Questionnaire | Adverse Effects Based on Open-Ended Questions | |||||
|---|---|---|---|---|---|---|---|
| Cognitive and Behavioral Impairment | Relaxation and Tension Reduction | Social and Sexual Facilitation | Perceptual and Cognitive Enhancement | Global Negative Effects | Craving and Physical Effects | ||
| 1 | 2.6 | 2.3 | 2.3 | 2.3 | 1.0 | 2.5 | None reported |
| 2 | 3.3 | 3.1 | 3.0 | 3.3 | 2.7 | 4.2 | Increased sleepiness and reduced concentration |
| 3 | 2.4 | 2.4 | 1.7 | 2.1 | 1.3 | 3.0 | None reported |
| 4 | 3.2 | 3.3 | 3.0 | 3.3 | 2.8 | 3.3 | Increased irritability and appetite |
| 5 | 2.6 | 3.8 | 2.8 | 3.3 | 2.3 | 3.3 | Dry mouth and eyes |
| 6 | 2.4 | 3.3 | 2.4 | 2.4 | 1.4 | 2.7 | None reported |
| 7 | 1.7 | 3.1 | 2.0 | 2.8 | 1.1 | 2.5 | Less motivated, reduced short-term memory |
| 8 | 3.3 | 3.0 | 2.9 | 2.9 | 2.1 | 3.7 | Higher doses cause increased social anxiety and appetite |
| 9 | 3.3 | 2.9 | 1.9 | 2.8 | 1.3 | 3.0 | Decreased concentration and worsening of symptoms at higher doses |
| 10 | 3.8 | 3.9 | 2.2 | 2.8 | 1.4 | 3.0 | May impair attention, short-term memory, information processing |
| 11 | 1.9 | 2.9 | 1.9 | 1.8 | 1.1 | 2.3 | None reported |
| 12 | 2.7 | 3.1 | 2.1 | 2.9 | 1.7 | 3.2 | None reported |
| 13 | 1.7 | 1.4 | 1.9 | 2.0 | 1.3 | 2.0 | None reported |
| 14 | 3.5 | 3.4 | 2.2 | 3.4 | 1.4 | 3.5 | Increased anxiety if around others |
| 15 | 2.7 | 2.3 | 1.7 | 2.0 | 1.1 | 2.5 | None reported |
| 16 | 2.2 | 3.0 | 1.7 | 1.5 | 1.0 | 2.3 | None reported |
| 17 | 3.0 | 3.0 | 2.0 | 2.5 | 1.2 | 2.8 | Higher doses cause wheezing and the sensation of being “high” |
| 18 | 3.2 | 3.4 | 2.1 | 2.0 | 1.1 | 3.5 | Higher doses cause sleep problems, confusion, and “high” sensation |
| 19 | 2.6 | 2.6 | 2.3 | 2.4 | 1.3 | 2.8 | Feels “high” sometimes |
TABLE 4. Cannabis Tolerability
In response to open-ended questions, the following adverse effects were reported: feeling of a “high” (N=3), decreased concentration (N=3), decreased short-term memory (N=2), increased social anxiety (N=2), increased appetite (N=2), confusion with higher doses (N=1), sedation (N=1), irritability (N=1), dry mouth (N=1), dry eyes (N=1), decreased motivation (N=1), increased tics with higher doses (N=1), and wheezing (N=1). Eight of the 19 participants did not report any adverse effects in response to open-ended questions (Table 4). One patient out of the 19 had previously discontinued cannabis due to experiencing severe irritability. However, he subsequently noted that by using cannabis for only 1 week at a time, he avoided the problem of irritability while still getting a tic suppressant effect lasting up to 3 weeks after the last dose. Finally, none of the patients met criteria for a psychotic disorder based on the SCID-I/P.
Discussion
We conducted a retrospective study of cannabis effectiveness and tolerability in adult patients with TS by interviewing eligible patients in our clinic. Of the 21 patients who were contacted, 19 participated in the study, for a response rate of 90%. These patients had moderate to severe symptoms overall, based on the baseline severity scores of their tic and comorbid symptoms, their baseline CGI-S ratings, and their complex medication histories (Table 1). Based on our two primary outcome measures, the patients appear to have had striking improvements in symptoms, with an average 60% reduction in YGTSS-TTS scores (from 30.5±7.2 to 12.2±8.6, p<0.001) (Table 3, Figure 1) and 18 of the 19 participants (94.7%) being rated as “very much improved” or “much improved” on the CGI-I (Table 3). In addition, there were substantial improvements in comorbid symptomatology (Tables 2 and 3).
It appears that medical cannabis has been generally well tolerated by this group of patients, all of whom had been using cannabis for at least 2 years. The MEEQ did not suggest ongoing adverse effects overall, and the SCID-I/P was negative for psychotic disorders. Nevertheless, on open-ended questioning, most patients did report one or more adverse effect, including feeling “high,” cognitive effects, and anxiety. One patient had to temporarily discontinue treatment due to difficulties with irritability.
Overall, these study participants experienced substantial improvements in their symptoms. This is particularly striking given that almost all participants had failed at least one anti-tic medication trial. Our findings are consistent with those of Müller-Vahl and colleagues, who found that most patients in their case series had reported at least a moderate improvement in tic symptoms.7 It is noteworthy that the improvements reported in that study, as well as those described in ours, are much larger than what was seen in the trials with oral THC.8,9 This may be due to the uncontrolled nature of the observational studies. Nevertheless, it is interesting that our patients appear to have had much greater improvement in their symptoms using inhaled cannabis compared with pure oral THC, THC/CBD oromucosal spray, or the oral cannabinoid nabilone. Thus, one might wonder whether inhaled medical cannabis is more effective for tics, possibly as a result of the impact of one or more of the various different compounds that it contains in addition to THC and CBD, and perhaps enhanced by a route that avoids first-pass hepatic metabolism. It is also worth considering the issue of dosing. There is little information on comparing oral and inhaled doses of cannabinoids, and the limited information available is specific to THC. If one accepts various assumptions, a conversion factor of 2.5 can be used to estimate an oral THC equivalent of a certain quantity of inhaled cannabis.20,21 If we further assume that the average percentage of THC in the cannabis used by our participants to be 10%, their median total daily amount of 1 g of cannabis would entail 100 mg of THC. Multiplying 100 mg by the conversion factor of 2.5 would yield 250 mg equivalent of oral THC. This would be much higher than the up to 10 mg used in the previous trials with oral THC.
Nevertheless, it is important to highlight the limitations of our retrospective observational study. There is likely a selection bias, as patients who would have tried cannabis and found it to be ineffective or intolerable would be unlikely to remain on it for long or may not be receptive to it as a treatment option. Such patients would not have been eligible for our study. On the other hand, according to the case series of consecutively interviewed patients by Müller-Vahl and colleagues, 82% of patients who had tried cannabis had experienced improvement in symptoms.7 Another limitation to our study is the potential for recall bias given the retrospective nature of the baseline assessments. These assessments included reviewing the patient charts for supporting clinical notes, including objective clinical examinations at various clinical visits. Still, this cannot completely eliminate the effect of recall bias in the assessments for the present study. An additional limitation of an observational study is the lack of a control. Therefore, it is possible that some of the improvement, perhaps in our younger adult patients, is related to the natural history of tics, which typically improves by early adulthood for most patients. However, this is unlikely to account for the improvements seen in older patients, most of whom had tried multiple other treatments with limited benefit. Still, there could be a regression to the mean effect, especially given the known waxing and waning nature of tics. In addition, there could be an enhanced placebo effect at play with inhaled cannabis, especially given the sociocultural issues surrounding the use of medical cannabis, the administrative process to obtain it, and the ritualized procedure to utilize it. It is noteworthy, however, that eight of our patients had discovered the use of cannabis for tics only serendipitously when they personally experienced notable reduction in their tics after using cannabis recreationally. Nevertheless, to adequately address this issue and other limitations raised here, one would need to conduct a randomized trial that is properly controlled and blinded for inhaled cannabis.
In conclusion, cannabis seems to be a promising treatment option for tics and associated symptoms. However, despite the substantial improvements reported here, some patients continued to take other medications in addition to cannabis. Moreover, while cannabis appears to be generally well tolerated, side effects were common. Importantly, the strength of our conclusions is limited by the retrospective nature of our study. Therefore, to better characterize the benefits and risks of medical cannabis in TS, including the roles of various cannabinoid compounds, there is a need for well-designed prospective, well-controlled studies.
1 : Tourette syndrome: A model of integration, in Handbook of Integrative Clinical Psychology, Psychiatry and Behavioral Medicine: Perspectives, Practices and Research. Edited by Carlstedt R. New York, Springer Publishing Company, 2009, pp 549–588Google Scholar
2 : Canadian guidelines for the evidence-based treatment of tic disorders: behavioural therapy, deep brain stimulation, and transcranial magnetic stimulation. Can J Psychiatry 2012; 57:144–151Crossref, Medline, Google Scholar
3 : Marijuana and Tourette’s syndrome. J Clin Psychopharmacol 1988; 8:444–445Crossref, Medline, Google Scholar
4 : Nicotine and cannabinoids as adjuncts to neuroleptics in the treatment of Tourette syndrome and other motor disorders. Life Sci 1989; 44:1521–1525Crossref, Medline, Google Scholar
5 : Treatment of Tourette syndrome with cannabinoids. Behav Neurol 2013; 27:119–124Crossref, Medline, Google Scholar
6 : The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int 2012; 109:495–501Medline, Google Scholar
7 : Cannabinoids: possible role in patho-physiology and therapy of Gilles de la Tourette syndrome. Acta Psychiatr Scand 1998; 98:502–506Crossref, Medline, Google Scholar
8 : Treatment of Tourette’s syndrome with delta 9-tetrahydrocannabinol (THC): a randomized crossover trial. Pharmacopsychiatry 2002; 35:57–61Crossref, Medline, Google Scholar
9 : Delta 9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. J Clin Psychiatry 2003; 64:459–465Crossref, Medline, Google Scholar
10 : Cannabinoids for Tourette’s syndrome. Cochrane Database Syst Rev 2009; (4):CD006565Medline, Google Scholar
11 : Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014; 82:1556–1563Crossref, Medline, Google Scholar
12 : Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015; 313:2456–2473Crossref, Medline, Google Scholar
13 : A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry 2016; 77:1050–1064Crossref, Medline, Google Scholar
14 : The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989; 28:566–573Crossref, Medline, Google Scholar
15 : Premonitory Urge for Tics Scale (PUTS): initial psychometric results and examination of the premonitory urge phenomenon in youths with tic disorders. J Dev Behav Pediatr 2005; 26:397–403Crossref, Medline, Google Scholar
16 : The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
17 : The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 2005; 35:245–256Crossref, Medline, Google Scholar
18 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN). New York, Biometrics Research, New York State Psychiatric Institute, 2002Google Scholar
19 : Marijuana and cocaine effect expectancies and drug use patterns. J Consult Clin Psychol 1991; 59:558–565Crossref, Medline, Google Scholar
20 : Biomarkers for the effects of cannabis and THC in healthy volunteers. Br J Clin Pharmacol 2009; 67:5–21Crossref, Medline, Google Scholar
21 : http://www.hc-sc.gc.ca/dhp-mps/marihuana/med/infoprof-eng.phpGoogle Scholar



