Complex Dynamics in the Basal Ganglia: Health and Disease Beyond the Motor System
Abstract
The rate and oscillatory hypotheses are the two main current frameworks of basal ganglia pathophysiology. Both hypotheses have emerged from research on movement disorders sharing similar conceptualizations. These pathological conditions are classified either as hypokinetic or hyperkinetic, and the electrophysiological hallmarks of basal ganglia dysfunction are categorized as prokinetic or antikinetic. Although nonmotor symptoms, including neurobehavioral symptoms, are a key manifestation of basal ganglia dysfunction, they are uncommonly accounted for in these models. In patients with Parkinson’s disease, the broad spectrum of motor symptoms and neurobehavioral symptoms challenges the concept that basal ganglia disorders can be classified into two categories. The profile of symptoms of basal ganglia dysfunction is best characterized by a breakdown of information processing, accompanied at an electrophysiological level by complex alterations of spiking activity from basal ganglia neurons. The authors argue that the dynamics of the basal ganglia circuit cannot be fully characterized by linear properties such as the firing rate or oscillatory activity. In fact, the neuronal spiking stream of the basal ganglia circuit is irregular but has temporal structure. In this context, entropy was introduced as a measure of probabilistic irregularity in the temporal organization of neuronal activity of the basal ganglia, giving place to the entropy hypothesis of basal ganglia pathology. Obtaining a quantitative characterization of irregularity of spike trains from basal ganglia neurons is key to elaborating a new framework of basal ganglia pathophysiology.
Models of basal ganglia physiopathology are traditionally founded on sensorimotor functions of this circuit.1 However, the role of the basal ganglia has been dramatically extended into nonmotor domains during the past decades.2 The basal ganglia play a critical role in cognitive functions, such as language, attention, memory and executive function, and other nonmotor domains.3,4 Indeed, disorders of the basal ganglia produce a combination of sensorimotor, autonomic, cognitive, and psychiatric symptoms.5–12 The high incidence of cognitive deficits and/or neuropsychiatric symptoms in basal ganglia disorders reflects the multiple functions of the basal ganglia in these nonmotor domains.8,9,13 An obvious example is Gilles-de-la-Tourette syndrome, which is both a neuropsychiatric disorder and a movement disorder. However, this is not an isolated case.
Most basal ganglia disorders have an impact on the three main categories of neuropsychiatric symptoms: cognition, emotion, and behavior. A majority of patients with either Parkinson’s disease, Huntington’s disease, Gilles-de-la-Tourette syndrome, supranuclear palsy, or corticobasal degeneration14–18 exhibit neuropsychiatric symptoms. Ninety percent of parkinsonian patients and nearly all patients with Huntington’s disease (98%) exhibit at least one neuropsychiatric manifestation.17,19 While most movement disorders, including Parkinson’s disease, involve extrabasal ganglia lesions, imaging studies have shown a correlation between basal ganglia lesions and cognitive as well as noncognitive neuropsychiatric symptoms.20 Affective, apathetic and cognitive symptoms, including obsessive-compulsive disorder (OCD), suggest that basal ganglia lesions reduce the connectivity of the prefrontal and frontal cortex (connectivity disconnection syndrome), and thus lead to executive dysfunction.21–23 Another key symptom of Parkinson’s disease, supranuclear palsy and Huntington’s disease is loss of motivation, which could be linked to pathological activity in the input nuclei (the striatum) and/or the output nuclei of the basal ganglia (the globus pallidus pars interna [GPi]).24–26 Additionally, in parkinsonian patients, the incidence of neuropsychiatric symptoms correlates with cognitive deterioration.19,27 About half of these patients suffer depression, sometimes as a prodromal manifestation, which is often associated with faster worsening of cognitive and motor functions.28–30 Less frequently, anxiety can dominate the neuropsychiatric spectrum in parkinsonian patients, while a smaller fraction of this population develops psychosis with hallucinations and delusions.19,31 Although a more detailed description is out of the scope of this review, it is worth noticing that basal ganglia dysfunction is also related to bipolar disorder, schizophrenia, mania, and delusion.32–35
Overview of Basal Ganglia Circuitry
The basal ganglia (Figure 1) are part of the cortico-subcortical circuits and the networks to which they contribute.1,36 The striatum is the most prominent input nucleus to the basal ganglia and receives dense excitatory (glutamatergic) topographic projections from virtually all cortical areas. The striatum can be subdivided into a ventral part (nucleus accumbens), the dorsomedial striatum ([DMS] or caudate), and the dorsolateral striatum ([DLS] or putamen). The putamen receives sensorimotor and thalamic projections, while the nucleus accumbens and the caudate receive input from cognitive and limbic areas including those from the frontal cortex, prefrontal cortex, the insula, and temporal areas.37 The subthalamic nucleus (STN) is a second input nucleus to the basal ganglia, which also receives topographic projections from most cortical areas.38 The topographic organization of projections between nuclei is a critical anatomical feature of the cortico-basal ganglia-thalamo-cortical (Cx-BG-Th-Cx) circuits. An effect of this organization is the functional segregation of cortical downstream, although cross-talk between parallel pathways is also present.39
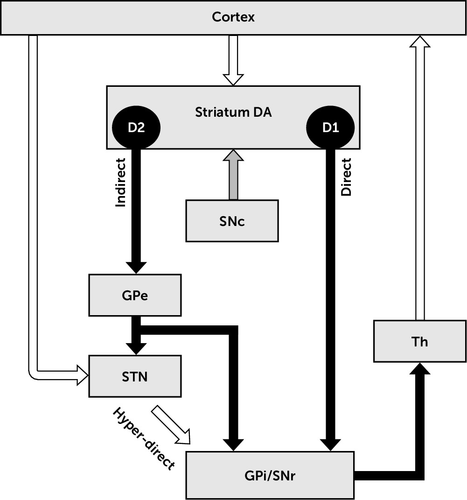
FIGURE 1. Schematization of the Basal Ganglia Circuita
a The basal ganglia network includes the striatum, the globus pallidus externa (GPe) and globus pallidus interna (GPi), the substantia nigra (pars substantia nigra compacta [SNc] and pars substantia nigra reticulata [SNr]), and the subthalamic nucleus (STN). These subcortical nuclei are interconnected and form a part of the frontal-subcortical loops (see Gatev et al.1). The striatum is a prominent input nucleus to the basal ganglia, which receives glutamatergic projections from virtually every cortical area and dopaminergic inputs from the SNc. The cortex exerts an excitatory influence over striatal efferent neurons (medium spiny neurons [MSNs]), which project to the basal ganglia output nuclei (GPi and SNr) via two pathways: The “direct” pathway is a GABAergic monosynaptic projection (striato-GPi/SNr), and the indirect “pathway” is a GABAergic multisynaptic projection (striato-GPe-GPi/SNr [see Frank280]). These two pathways arise from two populations of MSNs, expressing different dopaminergic receptors. While the MSNs of the direct pathway are under excitatory modulation from D1 [D1-like dopamine receptors] family receptors, the MSNs of the indirect pathway are under inhibitory influence from D2 [D2-like dopamine receptors] family receptors. The output nuclei of the basal ganglia are also under excitatory influence arising from the cortex via a relay in the subthalamic nucleus (STN, hyperdirect pathway) (see Nambu279). White arrows represent inhibitory GABAergic projection, black arrows represent excitatory glutamatergic projection, and the gray arrow represents dopaminergic projection. Abbreviations: DA=dopamine; Th=thalamus.
Regarding sensorimotor channels, somatotopic organization of cortical projections to the striatum and STN is maintained along Cx-BG-Th-Cx circuits.39 These circuits are characterized by consecutive segments of inhibitory (GABAergic) and excitatory (glutamatergic) projections between nuclei, driving cortical neuronal activity downstream. Briefly, the cortical glutamatergic drive exerts excitatory control over striatal efferent neurons (medium spiny neurons [MSN]), which project to the basal ganglia output nuclei via two pathways.40 The direct pathway is a GABAergic monosynaptic projection, while the indirect pathway is a GABAergic multisynaptic projection. At the level of the striatum, these two pathways are mostly segregated in two populations of MSN, expressing mostly different types of dopaminergic receptors.41 The MSN of the direct pathway express D1 dopaminergic receptors, the activation of which by dopamine has a facilitatory effect on these neurons. In contrast, the MSN of the indirect pathway express D2 dopaminergic receptors, the activation of which by dopamine has an inhibitory effect on these neurons. In human and nonhuman primates, the output nuclei of the basal ganglia consist of the GPi and the pars substantia nigra reticulata (SNr). The GPi receives its major inputs from the striatum.40 Additionally, the hyperdirect pathway bypasses the striatum, conveying excitatory impulses from the cortex to the globus pallidus through the STN.42
This anatomic-functional model stresses the importance of the balance of activity between the direct, indirect, and hyperdirect pathways on basal ganglia processing.1 In conditions with lesion of the dopaminergic nigrostriatal pathway (i.e., parkinsonism), the model predicts that loss of dopamine results in an imbalance in favor of the indirect pathway, leading to decreased inhibition and increased neuronal firing activity in the output basal ganglia.1,43 Secondarily, this imbalance leads to an inhibition of the thalamus and cortex.44 Excitation and inhibition of the thalamus are traditionally considered to promote the selection and inhibition of movement, respectively; therefore, pathologic thalamic inhibition produces akinetic symptoms, according to this model.1
While traditional models of the basal ganglia have shown extraordinary resilience to time, anatomic details have gained spectacular complexity during the past decades.37,45,46 Today, it must be acknowledged that the structure and function of the basal ganglia are more complicated than described in the aforementioned models. In a seminal review article in this journal, Mega and Cummings47 described five parallel anatomic circuits linking frontal to subcortical regions in the human brain. Each circuit was named according to its cortical site of origin, and their descriptions included not only anatomic details but also their neurochemical modulation and their clinical correlates. In Table 1, we replicate this approach, updating the information as needed. The following paragraph introduces some of the most relevant new information about these frontal-subcortical circuits.
| Frontal-Subcortical Circuit Identification | Motor Circuit | Oculomotor Circuit | Dorsolateral Prefrontal | Lateral Orbitofrontal | Anterior Cingulate |
|---|---|---|---|---|---|
| Pathway Classification | Motor | Nonmotor | |||
| Anatomical site of origin | Supplementary motor area | Frontal eye fields | Brodmann’s areas 9, 10 | Brodmann’s areas 10, 11 | Anterior cingulate |
| Major functions | Motor control, selection of motor plans109,255 | Oculomotor control | Executive, cognitive, strategy generation, learning, working memory, verbal skills, pain modulation256,257 | Personality, social behavior, empathy, prediction of outcome258,259 | Motivation, decision making, conflict monitoring, attention260,261 |
| Neurochemical modulation | Dopamine, 5-HT, norepinephrine, acetylcholine, neuroactive peptides80,82,87,173 | Dopamine, acetylcholine262 | Dopamine, glucocorticoids, estrogen, norepinephrine263 | Dopamine, 5-HT264,265 | Dopamine, 5-HT, μ-opioid, norepinephrine266,267 |
| Associated disorders | Movement disorders: Parkinson’s disease, Huntington’s chorea, dystonia, Tourette’s syndrome, essential tremor | Abnormal saccades, ophthalmoparesis, nystagmus, gaze anomalies. Can be affected in Parkinson's disease, Huntington's chorea, autism, and Lewy body dementia.268,269 | Executive dysfunction, reduced verbal fluency, poor organizational strategies, altered learning, depression, anxiety, schizophrenia270,271 | Disinhibition, irritability, lability, tactlessness, euphoria, unempathic behavior, undue familiarity, impulsivity, OCD, drug addiction, Tourette’s syndrome272–274 | Apathy, akinetic mutism, psychic emptiness, indifference to pain, poverty of spontaneous speech, inattention, emotional instability, OCD, ADHD275–277 |
TABLE 1. Main Cortico-Subcortical Circuits Serve Specific Functions and Are Affected by Specific Neurochemical Modulatorsa
The striatal microcircuitry, once considered as a simple structure converging cortical input on deep nuclei, is now viewed as a complex network processing efference from not only the cortex and the basal ganglia but also from extrabasal ganglia origins, and that includes multiple interneuron types.48–52 GABAergic interneurons play an important role in the striatum. While a subpopulation of GABAergic interneurons exerts their inhibition on a short distance, another subpopulation bridges distant striatal territories.53–55 These newly discovered anatomic details underlie complex processing of motor and cognitive information in this major input center of the basal ganglia circuit.49 Furthermore, the density of gap junctions that interconnect striatal interneurons, also connecting them electrically to MSN, underscores the scale of integration of cortical drive across different functional territories of the striatum.46 In addition, anatomical and pharmaco-biochemical studies have accumulated a large body of evidence showing that glutamatergic, GABAergic, and dopaminergic interactions are insufficient to characterize neuromodulation in the striatum.50,51
The basal ganglia are not an isolated set of nuclei. They receive indolaminergic, catecholaminergic, and acetylcholinergic projections from extrabasal ganglia regions, all of which contribute to modulation of the activity of basal ganglia neurons and interneurons. In the case of Parkinson’s disease, loss of neurons in the pedunculopontine nucleus (PPN),56 the locus coeruleus,57–60 and the substantia nigra pars compacta57–60 is characteristic and contributes to both motor and neuropsychiatric symptoms.
The dorsal raphe nucleus (DRN) sends serotoninergic projections to the basal ganglia including the striatum; the globus pallidus; the substantia nigra; and, to a lower extent, the STN. Serotoninergic receptors are expressed all along the Cx-BG-Th-Cx circuits, if with some heterogeneity. The decrease in serotonin levels in the basal ganglia is well documented both in postmortem studies on parkinsonian patients61,62 and in animal models.62,63 Clinically, the alteration of serotoninergic neurotransmission contributes to the high incidence of depression in Parkinson’s disease.64
The PPN projects acetylcholinergic fibers diffusely throughout the Cx-BG-Th-Cx circuits (striatum,65,66 STN,65–74 substantia nigra mostly pars compacta,65,66,75 globus pallidus65,66,71 including external66,73 and internal segments,66,73 thalamus66,71,73,76,77 including the central medial-parafascicular complex,73,74 and primary motor cortex74), in addition to brainstem nuclei, cerebellum, hypothalamus, and spinal cord.78 While the PPN has been considered mostly involved in the regulation of locomotion and sleep, there is an accumulation of evidence favoring its role in action-reward prediction, learning, attention and decision making.79
The locus coeruleus (A6) projects noradrenergic fibers to the Cx-BG-Th-Cx circuits, including the intralaminar complex of the thalamus and the cortex as well as the brainstem nuclei, cerebellum, hypothalamus, and spinal cord.80 Aberrancy of noradrenergic neurotransmission to these circuits may contribute to depressive symptoms related to parkinsonism, but the modulation of basal ganglia functions by the locus coeruleus remains poorly understood.81
Several neuroactive peptides modulate the Cx-BG-Th-Cx circuits, including tachykinins, enkephalins, dynorphin, somatostatin, and neuropeptide Y, all of which are used by distinct subsets of basal ganglia neurons.82 Moreover, the function and structure of higher cortical areas, such as the insula and other areas of cortex, are also affected by functional and structural abnormalities of the frontal-subcortical circuits, and their secondary dysfunction has the potential to further contribute to neurobehavioral symptoms among persons with disorders affecting the basal ganglia.60,83
The basal ganglia are complex architecturally, neurochemically, and with respect to their structural and functional connectivity. The simplified, traditional structural-functional model of the basal ganglia has provided a useful heuristic for research and education.1 However, it is naïve anatomically and functionally, particularly with respect to its reliance on linear dynamics in explorations of the pathophysiology of basal ganglia disorders. Developing new pharmacological and procedural approaches that more effectively treat basal ganglia disorders requires new heuristics that acknowledges and incorporates more fully the complexity of these structures and the circuits and networks to which they contribute.
Rate-Based Model of the Basal Ganglia
The description of summed excitatory/inhibitory drives onto the output nuclei of the basal ganglia provides a framework for considering firing rate as the coding scheme of neural information in the Cx-BG-Th-Cx circuits. In neuronal systems, a rate code assumes proportionality between the firing rate of neurons and some environmental clue or input signal. If this proportionality holds, the state of the neuronal system can be characterized by the central tendency of the firing rate, defined over a given time window.84,85 A trait of such a rate code is its robustness to noise, since information is carried by the central tendency and not by the precise timing of single spikes.
Evidence in favor of a rate code in the basal ganglia links striatal dopamine function to firing rate and motor activity as well as nonmotor activity, in normal and pathological conditions.86–89 In the nonmotor domain, a rate code plays a role in aversion learning and reward encoding in the ventral pallidum, probably accompanied by a population code.90,91 In the sensorimotor system, a rate code is supported by the negative correlation between the rate of discharge of the GPi and specific features of motor activity, like movement onset and velocity.92 In parkinsonism, two main categories of clinical and experimental studies support the rate hypothesis: 1) studies reporting higher firing rate in the GPi of parkinsonian subjects and 2) studies reporting that prodopaminergic therapies reduce the GPi firing rate.93–97 Extensive discussion about these studies can be found in the literature.1,98,99
On the other hand, a large body of experimental and clinical evidence contradicts the rate-based model at every level of the Cx-BG-Th-Cx circuit. In the primate GPi, the predicted proportionality between firing rate and severity of parkinsonian symptoms is absent, and hypokinetic symptoms can be observed without any increased firing rate.100 Direct measurements from the human STN also contradict the rate hypothesis: the firing rate in the STN of parkinsonian and epileptic patients without movement disorders is similar.101 In the striatum, MSN show local hypermetabolism and higher firing rate in Parkinson’s disease, but prodopaminergic treatments further increase their firing rate, contradicting the predictions of the rate-based model once again.102,103 At the cortical level, parkinsonian symptoms can occur without change in the mean spontaneous discharge rate of neurons.104 Finally, the rate hypothesis fails to explain the benefits of deep brain stimulation (DBS) across the spectrum of movement disorders.105–107 This growing body of controversies around the rate-based model of the basal ganglia has weakened the hypothesis of rate coding in the basal ganglia.108–110
Oscillatory Model of the Basal Ganglia
The second main hypothesis of processing of information in the basal ganglia is based on oscillatory phenomena, which can be identified both in single neurons and network activity (local field potentials [LFP]) along the Cx-BG-Th-Cx circuit.100–104 Oscillatory activity has been considered a coding scheme in some work, but more often it is regarded as an emergent property.1,115–118 In movement disorders, experimental and clinical measurements have reported positive correlations between β-band power (10–30 Hz) and hypokinetic conditions,1,113,119–131 as well as positive correlations between γ-band power (over 40 Hz) and motor activity.132–136
Regarding parkinsonian symptoms, β-band power in the basal ganglia correlates with tremor,137 rigidity,138 bradykinesia,139 and freezing of gait.140,141 As could be expected, oscillations in the basal ganglia correlate not only with motor function/dysfunction but also with neuropsychiatric symptoms.114 In the α-band (8–12 Hz), oscillations are influenced by emotional stimuli and depend on the affective state, being altered in depressed patients with Parkinson’s disease.142 Impulse control disorders have characteristic oscillations in the theta-alpha band (4–10 Hz) in the STN in patients with Parkinson’s disease.143 Theta and beta power in the STN are also related to decision making and conflict evaluation.144,145 Oscillations in the β-band in the cortex and the basal ganglia are associated with behavioral manifestations, such as behavioral stopping, semantic encoding, and memory.117,146,147 Finally, γ-band activity is related to cognitive processing.148,149
Although oscillatory activity has been identified over a broad spectrum of frequencies in the basal ganglia, the oscillatory hypothesis focuses mostly on two frequency bands, the β-band and the γ-band, resembling the notion of two opposite control subcircuits of the rate-based model: prokinetic and akinetic. However, evidence is contradictory with respect to this hypothesis. The relation between high β-band power and hypokinetic states is challenged by studies comparing LFP in different movement disorders.150–153 The proportionality between β-band power and the severity of symptoms remains debated as well.100 In the cortex, findings are also contradictory: The effect of L-DOPA on β-band activity reports either no change, increased activity, decreased activity, or mixed effects.154,155 Concerning the γ-band, although γ-band activity has been reported as stronger in the parkinsonian state, γ-band power can be further increased by antiparkinsonian treatments.155,156
Adaptive mechanisms have been proposed to explain these discrepancies and integrate these studies into the framework of the oscillatory hypothesis.157 In the Cx-BG-Th-Cx loop, the increase in γ-band activity was suggested as a prokinetic adaptive mechanism to oppose the putative antikinetic effects of the pathological increase in β-band activity.156 At a larger scale, the increased connectivity in the cerebello-thalamo-cortical loops is envisaged as a prokinetic mechanism to compensate the dysfunctional Cx-BG-Th-Cx loops.158
The anatomical origin and mechanisms of propagation of oscillatory activity across the frontal-subcortical circuit remain unclear, in health and in Parkinson’s disease.159 Indeed, a unifying hypothesis of the causal relation between oscillatory power at different frequency bands and clinical manifestations of disease remains undeveloped. The challenge to its development is the multiplicity of the phenomena for which it needs to account, including attention processing, periodic sampling of the state of the periphery, increased response inhibition, and mechanisms to maintain the neurobehavioral and motoric status quo.1,119,120,147,160–163
Pitfalls of the Rate and Oscillatory Models
The preceding review suggests that the pathophysiology of the basal ganglia is associated with alterations in the neuronal firing rate and oscillatory activity98,110,164,165 but neither model—both of which are predicated on linear measures of single unit activity and/or LFP—provides satisfactory accounts of Cx-BG-Th-Cx circuit function and dysfunction in health and disease. In particular, Parkinson’s disease, the classic basal ganglia disorder, challenges the utility of these models.
Critics of linear dynamic models of the basal ganglia have pointed out that Parkinson’s disease may be understood as a breakdown of information processing in cortico-subcortical circuits with a wide range of motor symptoms (including tremor, rigidity, bradykinesia, and hypokinesia), autonomic dysfunctions (orthostatic hypotension, altered heart rate variability), sleep disturbance, pain, and cognitive and neuropsychiatric manifestations.166 In the sensorimotor domain, neither a change in firing rate nor a change in oscillatory activity can at once explain rigidity, difficulty initiating movement, rhythmic muscle contractions (i.e., tremor), and problems with integrative motor function (i.e., coordination). Similarly, these models also cannot account for the concurrent manifestation of the broad range of nonmotor symptoms—including cognitive, emotional, behavioral, and autonomic symptoms, both hypokinetic and hyperkinetic—produced by Parkinson’s disease. As mentioned by Montgomery,167 the rate and oscillatory hypotheses attempt to account for such symptoms by dichotomizing basal ganglia control mechanisms into two opposing stable states (i.e., “on” and “off”). Therefore, both hypotheses fail for the same reason as explanatory models of basal ganglia pathophysiology: They are unable to account for the complex and inherently mixed nature of parkinsonian symptoms as well as their responses to treatment.167
During the past two decades, new mathematical frameworks have been introduced in the field to characterize the dynamics of the basal ganglia in normal and pathological conditions. These new frameworks show that central tendency measures (e.g., firing rate or frequency power)—and linear features, more generally—cannot fully characterize the dynamics of physiological signals in the Cx-BG-Th-Cx circuits.168–170 Conversely, mathematical methods developed in line with Shannon’s171 theory of information allow the quantification of features related to information transmission in complex systems. An analysis of information content in the Cx-BG-Th-Cx circuits might provide new models to describe the whole spectrum of basal ganglia disorders, as opposed to classic models, which force a classification of movement disorders in two categories depending only on the amount of motor activity produced (hypo- and hyperkinetic disorders).167 Additionally, nonlinear analysis methods provide alternative approaches to investigate the effects of treatments that cannot be reduced to either gross excitation or gross inhibition, such as DBS or new therapies targeting the catecholaminergic (i.e., adrenergic) or indolaminergic (e.g., serotoninergic) pathways.87,165,172–175
From studies using nonlinear methods of analysis, the temporal structure of spike trains reveals patterns that are characteristic of the activity of healthy and diseased basal ganglia.176 The presence of these patterns implies that: 1) a rate code cannot fully account for the transmission/processing of information in the basal ganglia; and 2) symptoms of Cx-BG-Th-Cx circuit dysfunction are related to a breakdown of temporal organization in the spiking activity of the basal ganglia.85,177
Conceptualization of Basal Ganglia Function Based on Nonlinear Dynamics
The term nonlinear system refers to organized systems generating an output that is not linearly proportional to the system’s input. Nonlinear systems exhibit complex behavior, which cannot be fully described by a linear combination of the individual behavior of their constituent parts.178 While linear dynamic systems are fully predictable (consider the distance that a car travels in 1 hr at 50 km/hour), and stochastic systems are fully unpredictable (consider the lottery), nonlinear systems may exhibit complexity and sensitivity to initial conditions and, hence, limited predictability.179 A historically relevant example in the field of nonlinear dynamics is the weather forecast: while short-term predictions are robust, long-term predictions remain uncertain.180,181 The complex temporal output of nonlinear systems results from the activity of multiple, sometimes parallel or nested, control loops, acting at different time scales.179,181,182
In the basal ganglia, such loops have been identified at the microcircuitry183,184 and macrocircuitry levels.2,86,185,186 The resultant irregularity observed in the signal is not stochastic, but chaotic (see Figure 2). Chaoticity (i.e., the extent to which something is chaotic) can be measured from time series with diverse methods and is directly proportional to the rate of information loss (i.e., entropy) and inversely proportional to predictability.182 While linear methods of analysis focus on central tendencies to describe the state of a system in time and/or frequency domains, nonlinear methods measure the persistence of certain time patterns or “shift” in the irregularity of the time series (related to entropy) and uncover hidden, complex patterns of temporal organization.
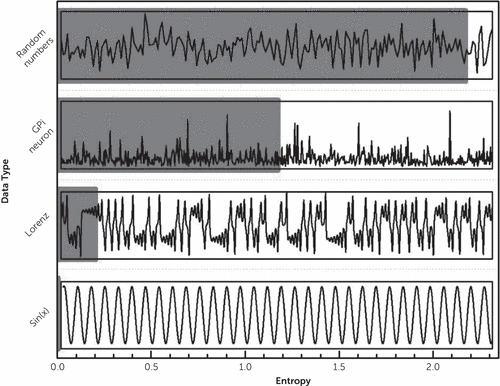
FIGURE 2. Entropy Values of Sample Time Seriesa
a Predictability increases from top to bottom. Note the high irregularity of the neuronal signal and the Lorenz attractor. Using a purely statistical approach, these signals can be easily mistaken for a random system, although both correspond to nonlinear systems, in which complex time patterns take place in an organized manner. The panels from top to bottom are as follows: uniform random numbers; neuronal GPi recording of interspike intervals, obtained from a dopamine depleted rat (see Andres et al.242); time evolution for the x variable of the Lorenz attractor (nonlinear dynamical system); and oscillatory signal, y=sin(x). The red bars represent the value of entropy, calculated with the sample entropy method (see Richman and Moorman281): entropy(random numbers)=2.17; entropy(GPi neuron)=1.17, entropy(Lorenz attractor)=0.21; and entropy(sin[x])=0.00.
Normal and pathologic human behaviors are manifestations of the complexity of the nervous system.187,188 This complexity is reflected in brain activity at every scale and can be measured by a variety of methods, including spike trains (i.e., time-series electrical signals) recorded from individual neurons in the brain, electroencephalographic and magnetoencephalographic techniques (EEG and MEG, respectively), and functional magnetic resonance imaging (fMRI).189–192 Use of these methods to describe normal complexity in brain activity is essential to understanding and treating changes in the complexity of brain activity wrought by disease, including mental illness.
In a thought-provoking paper, Yang and Tsai193 proposed that mental illness is related to the loss of brain complexity and that it can therefore be measured with complex dynamics’ tools. The foundation for this proposal is evidence of modifications of normal brain complexity in schizophrenia, anxiety disorders, dementias, autistic spectrum conditions, attention deficit/hyperactivity disorder, and sleep disorders,194–204 in which contexts, alterations in the complexity of brain activity are related to cognitive and emotional symptoms, may provide biomarkers of disease, and may be remediated by pharmacotherapies.205 In Alzheimer’s disease, cognitive deterioration correlates with a reduction of complexity of brain signals.200,206–208 Alterations of the complexity of brain activity are associated with depressive symptoms, including reduced complexity of the EEG in patients with depression,209,210 increased complexity of the MEG in younger patients with depression,195 and increased complexity in the EEG in patients with chronic stress.211 Reduction of MEG-determined complexity correlated with pharmacologically-induced remission of depressive symptoms.195 In summary, a healthy psyche is related to a certain degree of age-dependent complexity in brain activity,212 which can be measured with nonlinear methods from different invasive, as well as noninvasive, recordings of neurophysiologic signals.
In the case of the basal ganglia, a growing body of evidence shows that irregularity in physiological signals is not random in nature but exhibits complex, nonlinear temporal organization in neuronal firing activity,195,213–217 LFP/EEG,218–223 electromiography224,225 and movement kinetics.226–229 Stam et al.230 identified nonlinear features in the EEG recorded from parkinsonian patients.230 Later, entropy of EEG was reported to be higher in parkinsonian patients compared with healthy controls.231,232 This finding was confirmed by Han et al., who further indicated that Parkinson’s disease is associated with increased complexity of the EEG’s rhythm.233 Hohlefeld et al.234 reported a positive correlation between long-range temporal correlations ([LRTC] a measure of temporal organization over different time scales) in EEG recordings and deep LFP from parkinsonian patients, supporting that complex patterns can be repeated across time scales ranging from milliseconds to tens of seconds.234 Further studies have suggested that LRTC is a potential biomarker of pathological processing in basal ganglia disorders.235,236 In Parkinson’s disease, chaoticity in the activity of GPi neurons diminishes as the severity of motor symptoms increases.237 However, since normal brain complexity also diminishes with age, these effects could well depend on age or cognitive decline, a phenomenon that has not been studied yet.238 Using a neural network model, Li and Sikström239 modeled aging-dependent reduction of dopaminergic modulation by reducing neuronal nonlinearity, suggesting a mechanism that could be shared by normal aging and neurodegeneration.239
At the neuronal level, nonlinear temporal organization has been identified in interspike intervals (ISI) recorded from basal ganglia neurons in rodents, primates, and patients with movement disorders.234–237,240–242 The irregularity of ISI from basal ganglia neurons results from the replication of complex patterns, which cannot be statistically explained from the probability distribution around a central tendency.108,170,177,243,244 Neurons in the striatum code reward of actions and integrate reward into movement, leading to action selection in specific social situations and participation in reward-based learning.245,246 As is the case with motor symptoms, complex electrophysiological manifestations accompany neurobehavioral manifestations of basal ganglia dysfunction.247,248 In light of the evidence, it is clear that any linear model is inadequate to characterize the irregularity produced by the basal ganglia and the information they convey.169,249 The notion of a complex “neuronal language,” which has emerged from direct observations on the temporal organization of ISI from basal ganglia neurons, can help in building a new conceptual framework to study the pathophysiology of this circuit.85,170,182,237,250
Entropy Hypothesis
The term entropy comes from the field of thermodynamics, where it is related to the number of possible states of a system. Applied to the analysis of spike trains, entropy measures temporal irregularity. Statistical quantities, such as the standard deviation (SD) or the coefficient of variation (CV), measure dispersion of a signal from its central tendency, independently from the temporal order of the data. Something similar happens with frequential analyses, where the temporal domain is lost (e.g., the Fourier spectrum).
By contrast, the order of events in the time series is the crucial factor for the calculation of entropy, as well as other nonlinear measures (temporal structure, LRTC, among others). The value of entropy increases when an observed pattern is not followed by similar patterns in a time series. A time series that is highly predictable has relatively low entropy; a less predictable, more irregular process has higher entropy.
For example, consider the following sequences: A=[2 2 2 1 2 1 1 1 1 2], and B=[1 2 1 2 1 2 1 2 1 2]. Simple statistical measures are equal for both data streams: mean(A)=mean(B)=1.50, SD(A)=SD(B)=0.53, and CV(A)=CV(B)=0.35. However, entropy is higher in the first case; i.e., the more irregular time series (A). The following definitions apply, where x is the independent variable and n is the number of data samples:
Mean:  ; SD:
; SD:  ; CV:
; CV: 
Approximate entropy algorithm, m and r fixed at 2 and 0.1, respectively: ApEn(A)=0.51 and ApEn(B)=0.01.
Since linear measures are insufficient to fully characterize the complex nature of neural signals, entropy, among other nonlinear quantities, needs to be incorporated to describe the dynamics of the basal ganglia.170,240,241,243,251,252Figure 2 presents an example of a time series produced by a random number generator; a GPi neuron; a nonlinear system (Lorenz attractor); and a fully periodic system, sin(x), with their respective entropy values. The irregularity of ISI stands out as a striking feature of neuronal activity in the basal ganglia. This is similar in both normal and pathologic conditions. In the example shown, the entropy of a typical “parkinsonian” GPi neuron is above that measured from the low-dimensional nonlinear system but below the entropy of a stochastic system (a random number generator). As we discussed earlier, while linear models are unable to explain cognitive and behavioral symptomatology of basal ganglia disease, complexity measures show great potential as hallmarks of mental illness. Our proposal is that the deficiencies observed with linear models with respect to the motor, cognitive, emotional, and behavioral manifestations of frontal-subcortical circuit disorders can be addressed with nonlinear techniques. Hyperkinetic and akinetic motor disturbances are related to alterations in the complexity of activity in the frontal-subcortical circuits: neuronal entropy seems to be directly related to akinetic disorders and inversely so to hyperkinetic disorders.250 How to reconcile excesses and deficits of cognition, emotion, and behavior associated with disturbances of these circuits and nonlinear measures is a promising field of research for the next years.
Experimental and clinical investigations show that dopamine modulates neuronal entropy in the basal ganglia. In Sprague-Dawley rats, lesions of the nigrostriatal pathway modify the relation between alertness and entropy at the entopeduncular nucleus (EPN, equivalent to the GPi in primates).242,253 In this species’ EPN, the effect of dopamine depletion is a simultaneous increase of neuronal entropy and firing rate, plus an altered relationship between both.254 Specifically, under nigrostriatal lesion, neurons with high firing rate in the output basal ganglia fail to downregulate entropy, offering a functional hallmark of chronic dopamine depletion.244 Importantly, antiparkinsonian treatment (apomorphine, as well as DBS) reduces basal ganglia entropy.251,252 Therefore, temporal organization of spike trains needs to be investigated as a hidden feature behind the effects of antiparkinsonian drugs. In contrast to the rate and oscillatory hypotheses, the entropy hypothesis supports the idea that irregularity in the time series of ISI is the result of dynamic processing of information within the basal ganglia. The conceptual weight of the model is shifted from anatomical connectivity to information processing.
Conclusions
Integration of the complexity of neuronal activity into new pathophysiological models of the basal ganglia based on information theory and nonlinear dynamics is needed to account for the broad spectrum of motor and neuropsychiatric symptoms associated with frontal-subcortical circuit disorders. We propose that alterations of the temporal organization of spike trains in the basal ganglia imply that neuronal information is transmitted aberrantly and produces alterations of the function of these circuits that manifest as cognitive, emotional, behavioral, motor, and autonomic disturbances. The neuronal language analogy illustrates how the breakdown of communication between subcortical, cortical, and peripheral components of the nervous system results in the abnormal transmission of neuronal information, of which entropy is a global measure, and offers promising new avenues for the study of therapeutics and biomarkers for basal ganglia disorders.
1 : Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord 2006; 21:1566–1577Crossref, Medline, Google Scholar
2 : Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev 2000; 31:236–250Crossref, Medline, Google Scholar
3 : Sensory and cognitive functions of the basal ganglia. Curr Opin Neurobiol 1997; 7:157–163Crossref, Medline, Google Scholar
4 : The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum Brain Mapp 2013; 34:3031–3054Crossref, Medline, Google Scholar
5 : Fatigue and basal ganglia. J Neurol Sci 2000; 179(S 1-2):34–42Crossref, Medline, Google Scholar
6 : Selective dysfunction of basal ganglia subterritories: From movement to behavioral disorders. Mov Disord 2015; 30:1155–1170Crossref, Medline, Google Scholar
7 : Affective symptoms and cognitive functions in Parkinson’s disease. J Neurol Sci 2012; 317:97–102Crossref, Medline, Google Scholar
8 : Acquired obsessive-compulsive disorder associated with basal ganglia lesions. J Neuropsychiatry Clin Neurosci 2000; 12:269–272Link, Google Scholar
9 : Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am J Psychiatry 2009; 166:74–82Crossref, Medline, Google Scholar
10 : Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 2006; 5:235–245Crossref, Medline, Google Scholar
11 : Non-motor symptoms in Parkinson’s disease. J Neurol 2009; 256(Suppl 3):293–298Crossref, Medline, Google Scholar
12 : Olfactory dysfunction evaluation is not affected by comorbid depression in Parkinson’s disease. Mov Disord 2015; 30:1275–1279Crossref, Medline, Google Scholar
13 : The expanding universe of disorders of the basal ganglia. Lancet 2014; 384:523–531Crossref, Medline, Google Scholar
14 : The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
15 : Neuropsychiatric symptoms of patients with progressive supranuclear palsy and Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2001; 13:42–49Link, Google Scholar
16 : Neuropsychiatric assessment of Gilles de la Tourette patients: comparative study with other hyperkinetic and hypokinetic movement disorders. Mov Disord 2001; 16:1098–1104Crossref, Medline, Google Scholar
17 : Neuropsychiatric aspects of Huntington’s disease. J Neurol Neurosurg Psychiatry 2001; 71:310–314Crossref, Medline, Google Scholar
18 : Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry 1998; 65:717–721Crossref, Medline, Google Scholar
19 : Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry 2007; 78:36–42Crossref, Medline, Google Scholar
20 : Evolutional aspects of Alzheimer’s disease pathogenesis. J Alzheimers Dis 2012; 33(Suppl 1):S155–S161Crossref, Google Scholar
21 : Dysexecutive behaviour following deep brain lesions--a different type of disconnection syndrome? Cortex 2012; 48:97–119Crossref, Medline, Google Scholar
22 , et al: Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions. A neuropsychological, magnetic resonance imaging and positron tomography study Brain 1989; 112:699–725Crossref, Medline, Google Scholar
23 : Cognitive slowing in Parkinson’s disease is related to frontostriatal dopaminergic dysfunction. J Neurol Sci 2013; 329:23–28Crossref, Medline, Google Scholar
24 : Apathy and the basal ganglia. J Neurol 2006; 253(Suppl 7):VII54–VII61Crossref, Medline, Google Scholar
25 : Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 2006; 16:916–928Crossref, Medline, Google Scholar
26 : Neuropsychiatry of Huntington’s disease and other basal ganglia disorders. Psychosomatics 2000; 41:24–30Crossref, Medline, Google Scholar
27 : The neuropsychiatric profile of Parkinson’s disease subjects with and without mild cognitive impairment. J Neural Transm (Vienna) 2013; 120:607–611Crossref, Medline, Google Scholar
28 : The impact of depressive symptoms in early Parkinson disease. Neurology 2007; 69:342–347Crossref, Medline, Google Scholar
29 : Occurrence risk and structure of depression in Parkinson disease with and without dementia: results from the GEPAD Study. J Geriatr Psychiatry Neurol 2010; 23:27–34Crossref, Medline, Google Scholar
30 : Depression in Parkinson’s disease. Eur J Neurol 2002; 9(Suppl 3):44–54Crossref, Medline, Google Scholar
31 : Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson’s disease. Mov Disord 2009; 24:1333–1338Crossref, Medline, Google Scholar
32 : The clinical significance of bilateral basal ganglia calcification presenting with mania and delusions. J Neuropsychiatry Clin Neurosci 2013; 25:68–71Link, Google Scholar
33 : Basal ganglia shape alterations in bipolar disorder. Am J Psychiatry 2006; 163:276–285Crossref, Medline, Google Scholar
34 : Functional magnetic resonance imaging evidence for disrupted basal ganglia function in schizophrenia. Am J Psychiatry 2001; 158:646–649Crossref, Medline, Google Scholar
35 : Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biol Psychiatry 2013; 74:122–129Crossref, Medline, Google Scholar
36 : Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 1990; 85:119–146Crossref, Medline, Google Scholar
37 : The cortico-striate projection in the monkey. Brain 1970; 93:525–546Crossref, Medline, Google Scholar
38 : Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol 2000; 84:289–300Crossref, Medline, Google Scholar
39 : Somatotopic organization of the primate basal ganglia. Front Neuroanat 2011; 5:26Crossref, Medline, Google Scholar
40 : Electrophysiological interactions between striatal glutamatergic and dopaminergic systems. Ann N Y Acad Sci 2003; 1003:53–74Crossref, Medline, Google Scholar
41 : Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 2011; 34:441–466Crossref, Medline, Google Scholar
42 : Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res 2002; 43:111–117Crossref, Medline, Google Scholar
43 : The aging striatal dopamine function. Parkinsonism Relat Disord 2012; 18:426–432Crossref, Medline, Google Scholar
44 : A new approach to understand the pathophysiology of Parkinson’s disease. J Neurol 2005; 252(Suppl 4):IV1–IV4Crossref, Medline, Google Scholar
45 : Basal ganglia circuits as targets for neuromodulation in Parkinson disease. JAMA Neurol 2015; 72:1354–1360Crossref, Medline, Google Scholar
46 : Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 2010; 35:27–47Crossref, Medline, Google Scholar
47 : Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 1994; 6:358–370Link, Google Scholar
48 : Origin and molecular specification of striatal interneurons. J Neurosci 2000; 20:6063–6076Crossref, Medline, Google Scholar
49 : Local and afferent synaptic pathways in the striatal microcircuitry. Curr Opin Neurobiol 2015; 33:182–187Crossref, Medline, Google Scholar
50 : Arkypallidal cells send a stop signal to striatum. Neuron 2016; 89:308–316Crossref, Medline, Google Scholar
51 : Cognitive and behavioral dysfunction in Parkinson’s disease: neurochemical and clinicopathological contributions. J Neural Transm (Vienna) 2004; 111:1287–1301Crossref, Medline, Google Scholar
52 : Depression in Parkinson’s disease: its prevalence, diagnosis, and neurochemical background. J Neurol 2001; 248(Suppl 3):III5–III11Medline, Google Scholar
53 : Tonic inhibition of striatal dopamine transmission: effects of benzodiazepine and GABAA receptor antagonists on extracellular dopamine levels. Brain Res 1992; 599:51–56Crossref, Medline, Google Scholar
54 : Effects of striatal GABA A-receptor blockade on striatal and cortical activity in monkeys. J Neurophysiol 2008; 99:1294–1305Crossref, Medline, Google Scholar
55 : Distinct corticostriatal GABAergic neurons modulate striatal output neurons and motor activity. Cell Reports 2017; 19:1045–1055Crossref, Medline, Google Scholar
56 : Lewy pathology and neurodegeneration in premotor Parkinson’s disease. Mov Disord 2012; 27:597–607Crossref, Medline, Google Scholar
57 : Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol 1990; 27:373–385Crossref, Medline, Google Scholar
58 : Disease-specific patterns of locus coeruleus cell loss. Ann Neurol 1992; 32:667–676Crossref, Medline, Google Scholar
59 : Pathological basis for neurotransmitter changes in Parkinson’s disease. Neuropathol Appl Neurobiol 1983; 9:3–19Crossref, Medline, Google Scholar
60 : Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003; 24:197–211Crossref, Medline, Google Scholar
61 : Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res 1983; 275:321–328Crossref, Medline, Google Scholar
62 : Serotonin in Parkinson’s disease. Behav Brain Res 2015; 277:136–145Crossref, Medline, Google Scholar
63 : Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson’s disease. Neuroscience 2008; 156:830–840Crossref, Medline, Google Scholar
64 : Depression and Parkinson’s disease: a review. Am J Psychiatry 1992; 149:443–454Crossref, Medline, Google Scholar
65 : Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. J Comp Neurol 1994; 344:210–231Crossref, Medline, Google Scholar
66 : Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry. Brain Res 1982; 252:367–372Crossref, Medline, Google Scholar
67 : Direct projections from the pedunculopontine tegmental nucleus to the subthalamic nucleus in the cat. Brain Res 1980; 196:223–227Crossref, Medline, Google Scholar
68 : The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. J Comp Neurol 1983; 217:187–215Crossref, Medline, Google Scholar
69 : Anatomical and electrophysiological studies on the reciprocal projections between the subthalamic nucleus and nucleus tegmenti pedunculopontinus in the rat. Neuroscience 1983; 9:41–52Crossref, Medline, Google Scholar
70 : Cholinergic, GABAergic, and glutamate-enriched inputs from the mesopontine tegmentum to the subthalamic nucleus in the rat. J Neurosci 1995; 15:7105–7120Crossref, Medline, Google Scholar
71 : Connectivity of the human pedunculopontine nucleus region and diffusion tensor imaging in surgical targeting. J Neurosurg 2007; 107:814–820Crossref, Medline, Google Scholar
72 : Cholinergic and non-cholinergic mesopontine tegmental neurons projecting to the subthalamic nucleus in the rat. Eur J Neurosci 2011; 33:433–443Crossref, Medline, Google Scholar
73 : The organization of nucleus tegmenti pedunculopontinus neurons projecting to basal ganglia and thalamus: a retrograde fluorescent double labeling study in the rat. Neurosci Lett 1987; 79:11–16Crossref, Medline, Google Scholar
74 : Organization and efferent projections of nucleus tegmenti pedunculopontinus pars compacta with special reference to its cholinergic aspects. Neuroscience 1984; 11:931–946Crossref, Medline, Google Scholar
75 : Pedunculopontine nucleus in the squirrel monkey: cholinergic and glutamatergic projections to the substantia nigra. J Comp Neurol 1994; 344:232–241Crossref, Medline, Google Scholar
76 : The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci 2004; 27:520–527Crossref, Medline, Google Scholar
77 : Cholinergic and non-cholinergic projections from the upper brainstem core to the visual thalamus in the cat. Exp Brain Res 1988; 70:166–180Medline, Google Scholar
78 : Topographical organization of the pedunculopontine nucleus. Front Neuroanat 2011; 5:22Crossref, Medline, Google Scholar
79 : The pedunculopontine tegmental nucleus-A functional hypothesis from the comparative literature. Mov Disord 2016; 31:615–624Crossref, Medline, Google Scholar
80 : Noradrenaline in Parkinson’s disease: from disease progression to current therapeutics. Curr Med Chem 2007; 14:2330–2334Crossref, Medline, Google Scholar
81 : Cortico-basal ganglia circuit function in psychiatric disease. Annu Rev Physiol 2016; 78:327–350Crossref, Medline, Google Scholar
82 : Chemical anatomy of primate basal ganglia. Prog Neurobiol 1995; 46:131–197Crossref, Medline, Google Scholar
83 : Limbic neuropathology in idiopathic Parkinson’s disease with concomitant dementia. Folia Neuropathol 2004; 42:141–150Medline, Google Scholar
84 : Sensory neural codes using multiplexed temporal scales. Trends Neurosci 2010; 33:111–120Crossref, Medline, Google Scholar
85 : The language of neurons: theory and applications of a quantitative analysis of the neural code. Int J Med Biol Front 2015; 21:133–148Google Scholar
86 : The functional anatomy of basal ganglia disorders. Trends Neurosci 1989; 12:366–375Crossref, Medline, Google Scholar
87 : Serotonin innervation of basal ganglia in monkeys and humans. J Chem Neuroanat 2011; 41:256–265Crossref, Medline, Google Scholar
88 : Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci 2012; 15:816–818Crossref, Medline, Google Scholar
89 : Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci 2013; 33:18531–18539Crossref, Medline, Google Scholar
90 : Ventral pallidal coding of a learned taste aversion. Behav Brain Res 2016; 300:175–183Crossref, Medline, Google Scholar
91 : Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci 2004; 24:1058–1069Crossref, Medline, Google Scholar
92 : The basal ganglia: an overview of circuits and function. Neurosci Biobehav Rev 2008; 32:333–342Crossref, Medline, Google Scholar
93 : Effects of dopamine agonists on the spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res 1991; 547:152–161Medline, Google Scholar
94 : Effects of apomorphine on globus pallidus neurons in parkinsonian patients. Ann Neurol 1997; 42:767–775Crossref, Medline, Google Scholar
95 : Apomorphine induces changes in GPi spontaneous outflow in patients with Parkinson’s disease. Mov Disord 1999; 14:45–49Crossref, Medline, Google Scholar
96 : By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science 2004; 306:1940–1943Crossref, Medline, Google Scholar
97 : Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res 1991; 547:142–151Medline, Google Scholar
98 : Seven problems on the basal ganglia. Curr Opin Neurobiol 2008; 18:595–604Crossref, Medline, Google Scholar
99 : Reassessing models of basal ganglia function and dysfunction. Annu Rev Neurosci 2014; 37:117–135Crossref, Medline, Google Scholar
100 : Physiological changes in the pallidum in a progressive model of Parkinson’s disease: Are oscillations enough? Exp Neurol 2016; 279:187–196Crossref, Medline, Google Scholar
101 : Subthalamic nucleus neuronal activity in Parkinson’s disease and epilepsy subjects. Parkinsonism Relat Disord 2008; 14:120–125Crossref, Medline, Google Scholar
102 : Dopamine regulates distinctively the activity patterns of striatal output neurons in advanced parkinsonian primates. J Neurophysiol 2015; 113:1533–1544Crossref, Medline, Google Scholar
103 : Human striatal recordings reveal abnormal discharge of projection neurons in Parkinson’s disease. Proc Natl Acad Sci USA 2016; 113:9629–9634Crossref, Medline, Google Scholar
104 : Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson’s disease. J Neurosci 2002; 22:4639–4653Crossref, Medline, Google Scholar
105 : Biochemical and electrophysiological changes of substantia nigra pars reticulata driven by subthalamic stimulation in patients with Parkinson’s disease. Eur J Neurosci 2006; 23:2923–2928Crossref, Medline, Google Scholar
106 : The clinical efficacy of L-DOPA and STN-DBS share a common marker: reduced GABA content in the motor thalamus. Cell Death Dis 2011; 2:e154Crossref, Medline, Google Scholar
107 : Reduced GABA content in the motor thalamus during effective deep brain stimulation of the subthalamic nucleus. Front Syst Neurosci 2011; 5:17Crossref, Medline, Google Scholar
108 : Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci 2000; 23(Suppl):S8–S19Crossref, Medline, Google Scholar
109 : Functional anatomy of the basal ganglia. Mov Disord 2002; 17(Suppl 3):S15–S21Crossref, Medline, Google Scholar
110 : Basal ganglia physiology and pathophysiology: a reappraisal. Parkinsonism Relat Disord 2007; 13:455–465Crossref, Medline, Google Scholar
111 : Oscillatory activity in the basal ganglia. Parkinsonism Relat Disord 2007; 13(Suppl 3):S434–S436Crossref, Medline, Google Scholar
112 : A basis for the pathological oscillations in basal ganglia: the crucial role of dopamine. Neuroreport 2011; 22:151–156Crossref, Medline, Google Scholar
113 : β oscillations in the cortico-basal ganglia loop during parkinsonism. Exp Neurol 2013; 245:52–59Crossref, Medline, Google Scholar
114 : Oscillations and the basal ganglia: motor control and beyond. Neuroimage 2014; 85:637–647Crossref, Medline, Google Scholar
115 : Stimulus-specific oscillatory responses of the brain: a time/frequency-related coding process. Clin Neurophysiol 2000; 111:565–583Crossref, Medline, Google Scholar
116 : Cortico-striatal representation of time in animals and humans. Curr Opin Neurobiol 2008; 18:145–152Crossref, Medline, Google Scholar
117 : Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb Cortex 2009; 19:1631–1640Crossref, Medline, Google Scholar
118 : A θ-γ oscillation code for neuronal coordination during motor behavior. J Neurosci 2013; 33:18515–18530Crossref, Medline, Google Scholar
119 : Pathological subthalamic nucleus oscillations in PD: can they be the cause of bradykinesia and akinesia? Exp Neurol 2009; 219:58–61Crossref, Medline, Google Scholar
120 : Synchronisation in the beta frequency-band--the bad boy of parkinsonism or an innocent bystander? Exp Neurol 2009; 217:1–3Crossref, Medline, Google Scholar
121 : High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci 2000; 20:7766–7775Crossref, Medline, Google Scholar
122 : Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci 2002; 25:525–531Crossref, Medline, Google Scholar
123 : A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res 2004; 143:461–466Crossref, Medline, Google Scholar
124 : Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci 2006; 23:1956–1960Crossref, Medline, Google Scholar
125 : Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. J Neurophysiol 2006; 96:3248–3256Crossref, Medline, Google Scholar
126 : Neuronal firing rates and patterns in the globus pallidus internus of patients with cervical dystonia differ from those with Parkinson’s disease. J Neurophysiol 2007; 98:720–729Crossref, Medline, Google Scholar
127 : Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci 2007; 30:357–364Crossref, Medline, Google Scholar
128 : Pathophysiology of parkinsonism. Clin Neurophysiol 2008; 119:1459–1474Crossref, Medline, Google Scholar
129 : The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann Neurol 2008; 64(Suppl 2):S30–S46Crossref, Medline, Google Scholar
130 : High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci 2008; 28:6165–6173Crossref, Medline, Google Scholar
131 : Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry 2011; 82:569–573Crossref, Medline, Google Scholar
132 : γ oscillations in the human basal ganglia. Exp Neurol 2013; 245:72–76Crossref, Medline, Google Scholar
133 : Oscillatory activity in the basal ganglia--relationship to normal physiology and pathophysiology. Brain 2004; 127:721–722Crossref, Medline, Google Scholar
134 : Different functional loops between cerebral cortex and the subthalmic area in Parkinson’s disease. Cereb Cortex 2006; 16:64–75Crossref, Medline, Google Scholar
135 : From symphony to cacophony: pathophysiology of the human basal ganglia in Parkinson disease. Neurosci Biobehav Rev 2008; 32:378–387Crossref, Medline, Google Scholar
136 : Increased gamma oscillatory activity in the subthalamic nucleus during tremor in Parkinson’s disease patients. J Neurophysiol 2009; 101:789–802Crossref, Medline, Google Scholar
137 : Time-frequency analysis of transient neuromuscular events: dynamic changes in activity of the subthalamic nucleus and forearm muscles related to the intermittent resting tremor. J Neurosci Methods 2005; 145:151–158Crossref, Medline, Google Scholar
138 : β band stability over time correlates with Parkinsonian rigidity and bradykinesia. Exp Neurol 2012; 236:383–388Crossref, Medline, Google Scholar
139 : Local field potential beta activity in the subthalamic nucleus of patients with Parkinson’s disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol 2008; 213:108–113Crossref, Medline, Google Scholar
140 : Freezing of gait-related oscillatory activity in the human subthalamic nucleus. Basal Ganglia 2013; 3:25–32Crossref, Google Scholar
141 : High beta activity in the subthalamic nucleus and freezing of gait in Parkinson’s disease. Neurobiol Dis 2014; 64:60–65Crossref, Medline, Google Scholar
142 : Modulation of subthalamic alpha activity to emotional stimuli correlates with depressive symptoms in Parkinson’s disease. Mov Disord 2011; 26:477–483Crossref, Medline, Google Scholar
143 : Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain 2011; 134:36–49Crossref, Medline, Google Scholar
144 : Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci 2011; 14:1462–1467Crossref, Medline, Google Scholar
145 : Conflict-dependent dynamic of subthalamic nucleus oscillations during moral decisions. Soc Neurosci 2011; 6:243–256Crossref, Medline, Google Scholar
146 : Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci 2009; 29:12675–12685Crossref, Medline, Google Scholar
147 : The role of the subthalamic nucleus in response inhibition: evidence from local field potential recordings in the human subthalamic nucleus. Neuroimage 2012; 60:271–278Crossref, Medline, Google Scholar
148 : A gamma band specific role of the subthalamic nucleus in switching during verbal fluency tasks in Parkinson’s disease. Exp Neurol 2011; 232:136–142Crossref, Medline, Google Scholar
149 : Subthalamic nucleus gamma oscillations mediate a switch from automatic to controlled processing: a study of random number generation in Parkinson’s disease. Neuroimage 2013; 64:284–289Crossref, Medline, Google Scholar
150 : Neuronal activity of the human subthalamic nucleus in the parkinsonian and nonparkinsonian state. J Neurophysiol 2008; 100:2515–2524Crossref, Medline, Google Scholar
151 : Patterning of globus pallidus local field potentials differs between Parkinson’s disease and dystonia. Brain 2003; 126:2597–2608Crossref, Medline, Google Scholar
152 : Oscillatory activity in the globus pallidus internus: comparison between Parkinson’s disease and dystonia. Clin Neurophysiol 2012; 123:358–368Crossref, Medline, Google Scholar
153 : Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J Neurophysiol 2005; 93:3165–3176Crossref, Medline, Google Scholar
154 : Dopaminergic therapy in Parkinson’s disease decreases cortical beta band coherence in the resting state and increases cortical beta band power during executive control. Neuroimage Clin 2013; 3:261–270Crossref, Medline, Google Scholar
155 : Effects of L-dopa priming on cortical high beta and high gamma oscillatory activity in a rodent model of Parkinson’s disease. Neurobiol Dis 2016; 86:1–15Crossref, Medline, Google Scholar
156 : Does increased gamma activity in patients suffering from Parkinson’s disease counteract the movement inhibiting beta activity? Neuroscience 2013; 237:42–50Crossref, Medline, Google Scholar
157 : Imaging of compensatory mechanisms in Parkinson’s disease. Curr Opin Neurol 2010; 23:407–412Crossref, Medline, Google Scholar
158 : Joint amplitude and connectivity compensatory mechanisms in Parkinson’s disease. Neuroscience 2010; 166:1110–1118Crossref, Medline, Google Scholar
159 : Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain 2006; 129:1748–1757Crossref, Medline, Google Scholar
160 : Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol 2010; 20:156–165Crossref, Medline, Google Scholar
161 : Basal ganglia oscillations and pathophysiology of movement disorders. Curr Opin Neurobiol 2006; 16:629–637Crossref, Medline, Google Scholar
162 : Neural oscillations: beta band activity across motor networks. Curr Opin Neurobiol 2015; 32:60–67Crossref, Medline, Google Scholar
163 : Synchronized neuronal oscillations and their role in motor processes. Trends Cogn Sci 1997; 1:176–183Crossref, Medline, Google Scholar
164 : Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord 2003; 18:357–363Crossref, Medline, Google Scholar
165 : Cause of parkinsonian symptoms: firing rate, firing pattern or dynamic activity changes? Basal Ganglia 2015; 5:1–6Crossref, Google Scholar
166 : Non-motor Parkinson’s: integral to motor Parkinson’s, yet often neglected. Pract Neurol 2014; 14:310–322Crossref, Medline, Google Scholar
167 : Modeling and theories of pathophysiology and physiology of the basal ganglia-thalamic-cortical system: critical analysis. Front Hum Neurosci 2016; 10:469Crossref, Medline, Google Scholar
168 : Non-linear dynamics in parkinsonism. Front Neurol 2013; 4:211Crossref, Medline, Google Scholar
169 : Multiplexed coding in the human basal ganglia. J Phys Conf Ser 2016; 705:012049Crossref, Google Scholar
170 : Nonlinear analysis of discharge patterns in monkey basal ganglia. Brain Res 2006; 1118:84–93Crossref, Medline, Google Scholar
171 : A mathematical theory of communication. Mob Comput Commun Rev 2001; 5:3–55Crossref, Google Scholar
172 : Dopaminergic control of striatal 5-HT level at normobaric condition and at pressure. Undersea Hyperb. Med. 2010; 37:159–166Medline, Google Scholar
173 : Serotonergic action on dorsal striatal function. Parkinsonism Relat Disord 2012; 18(Suppl 1):S129–S131Crossref, Medline, Google Scholar
174 : One view of the current state of understanding in basal ganglia pathophysiology and what is needed for the future. J Mov Disord 2011; 4:13–20Crossref, Medline, Google Scholar
175 : Disrupting neuronal transmission: mechanism of DBS? Front Syst Neurosci 2014; 8:33Crossref, Medline, Google Scholar
176 : Multiple-time-scale framework for understanding the progression of Parkinson’s disease. Phys Rev E Stat Nonlin Soft Matter Phys 2014; 90:062709Crossref, Medline, Google Scholar
177 : Neural code alterations and abnormal time patterns in Parkinson’s disease. J Neural Eng 2015; 12:026004Crossref, Medline, Google Scholar
178 : Chaos: An Introduction to Dynamical Systems. New York, Springer, 1996Crossref, Google Scholar
179 : Interdisciplinary application of nonlinear time series methods. Phys Rep 1999; 308:64Crossref, Google Scholar
180 : Deterministic nonperiodic flow. J Atmos Sci 1963; 20:130–141Crossref, Google Scholar
181 : Nonlinear Dynamics and Chaos. Cambridge, Perseus, 1994Google Scholar
182 : Practical implementation of nonlinear time series methods: The TISEAN package. Chaos 1999; 9:413–435Crossref, Medline, Google Scholar
183 : Striatal microcircuitry and movement disorders. Trends Neurosci 2012; 35:557–564Crossref, Medline, Google Scholar
184 : Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 2014; 17:1022–1030Crossref, Medline, Google Scholar
185 : Organization of two cortico–basal ganglia loop circuits that arise from distinct sectors of the monkey dorsal premotor cortex, in Basal Ganglia: An Integrative View. Edited by Barrios FA, Bauer C. InTech,
186 : Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9:357–381Crossref, Medline, Google Scholar
187 : Complexity of mental illness: a new research dimension. Prog Neuropsychopharmacol Biol Psychiatry 2013; 45:251–252Crossref, Medline, Google Scholar
188 : Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009; 10:186–198Crossref, Medline, Google Scholar
189 : Multiscale Lempel-Ziv complexity for EEG measures. Clin Neurophysiol 2015; 126:541–548Crossref, Medline, Google Scholar
190 : Complexity measures in magnetoencephalography: measuring “disorder” in schizophrenia. PLoS One 2015; 10:e0120991Crossref, Medline, Google Scholar
191 : Optimizing complexity measures for FMRI data: algorithm, artifact, and sensitivity. PLoS One 2013; 8:e63448Crossref, Medline, Google Scholar
192 : Distinguishing cognitive state with multifractal complexity of hippocampal interspike interval sequences. Front Syst Neurosci 2015; 9:130Crossref, Medline, Google Scholar
193 : Is mental illness complex? From behavior to brain. Prog Neuropsychopharmacol Biol Psychiatry 2013; 45:253–257Crossref, Medline, Google Scholar
194 : Lempel-Ziv complexity in schizophrenia: a MEG study. Clin Neurophysiol 2011; 122:2227–2235Crossref, Medline, Google Scholar
195 : Complexity analysis of spontaneous brain activity: effects of depression and antidepressant treatment. J Psychopharmacol 2012; 26:636–643Crossref, Medline, Google Scholar
196 : Complexity of spontaneous brain activity in mental disorders. Prog Neuropsychopharmacol Biol Psychiatry 2013; 45:258–266Crossref, Medline, Google Scholar
197 : A shift to randomness of brain oscillations in people with autism. Biol Psychiatry 2010; 68:1092–1099Crossref, Medline, Google Scholar
198 : Complexity and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2013; 45:267–276Crossref, Medline, Google Scholar
199 : Dimensional complexity of the EEG in patients with posttraumatic stress disorder. Psychiatry Res 2004; 131:79–89Crossref, Medline, Google Scholar
200 : Assessment of EEG dynamical complexity in Alzheimer’s disease using multiscale entropy. Clin Neurophysiol 2010; 121:1438–1446Crossref, Medline, Google Scholar
201 : Complexity analysis of spontaneous brain activity in attention-deficit/hyperactivity disorder: diagnostic implications. Biol Psychiatry 2009; 65:571–577Crossref, Medline, Google Scholar
202 : Reduced physiologic complexity is associated with poor sleep in patients with major depression and primary insomnia. J Affect Disord 2011; 131:179–185Crossref, Medline, Google Scholar
203 : Atypical EEG complexity in autism spectrum conditions: a multiscale entropy analysis. Clin Neurophysiol 2011; 122:2375–2383Crossref, Medline, Google Scholar
204 : Linear and non-linear EEG analysis of adolescents with attention-deficit/hyperactivity disorder during a cognitive task. Clin Neurophysiol 2010; 121:1863–1870Crossref, Medline, Google Scholar
205 : Antipsychotics reverse abnormal EEG complexity in drug-naive schizophrenia: a multiscale entropy analysis. Neuroimage 2010; 51:173–182Crossref, Medline, Google Scholar
206 : Cognitive and neuropsychiatric correlates of EEG dynamic complexity in patients with Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 2013; 47:52–61Crossref, Medline, Google Scholar
207 : EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol 2004; 115:1490–1505Crossref, Medline, Google Scholar
208 : Global dynamical analysis of the EEG in Alzheimer’s disease: frequency-specific changes of functional interactions. Clin Neurophysiol 2008; 119:837–841Crossref, Medline, Google Scholar
209 : Decrease of complexity in EEG as a symptom of depression. Neuroreport 1994; 5:528–530Crossref, Medline, Google Scholar
210 : Neural complexity in patients with poststroke depression: A resting EEG study. J Affect Disord 2015; 188:310–318Crossref, Medline, Google Scholar
211 : A method of identifying chronic stress by EEG. Pers Ubiquitous Comput 2013; 17:1341–1347Crossref, Google Scholar
212 : Fractals in Affective and Anxiety Disorders, in The Fractal Geometry of the Brain. Edited by Di Ieva A. New York, Springer, 2016, pp 471–483Crossref, Google Scholar
213 : Evidence for chaos in spike trains of neurons that generate rhythmic motor patterns. Brain Res Bull 1988; 21:529–538Crossref, Medline, Google Scholar
214 : Is there chaos in the brain? II. Experimental evidence and related models. C R Biol 2003; 326:787–840Crossref, Medline, Google Scholar
215 : Dendritic computation. Annu Rev Neurosci 2005; 28:503–532Crossref, Medline, Google Scholar
216 : Decoding of dopaminergic mesolimbic activity and depressive behavior. J Mol Neurosci 2007; 32:72–79Crossref, Medline, Google Scholar
217 : Neuronal arithmetic. Nat Rev Neurosci 2010; 11:474–489Crossref, Medline, Google Scholar
218 : The brain as a dynamic physical system. Neuroscience 1994; 60:587–605Crossref, Medline, Google Scholar
219 : Nonlinear EEG analysis and its potential role in epileptology. Epilepsia 2000; 41(Suppl 3):S34–S38Crossref, Medline, Google Scholar
220 : Synchronization in monkey visual cortex analyzed with an information-theoretic measure. Chaos 2008; 18:037130Crossref, Medline, Google Scholar
221 : Advances in neural networks research: an introduction. Neural Netw 2009; 22:489–490Crossref, Medline, Google Scholar
222 : Modeling of movement-related potentials using a fractal approach. J Comput Neurosci 2010; 28:595–603Crossref, Medline, Google Scholar
223 : Synchronous chaos and broad band gamma rhythm in a minimal multi-layer model of primary visual cortex. PLOS Comput Biol 2011; 7:e1002176Crossref, Medline, Google Scholar
224 : Surface EMG and acceleration signals in Parkinson’s disease: feature extraction and cluster analysis. Med Biol Eng Comput 2008; 46:849–858Crossref, Medline, Google Scholar
225 : Sample entropy analysis of surface EMG for improved muscle activity onset detection against spurious background spikes. J Electromyogr Kinesiol 2012; 22:901–907Crossref, Medline, Google Scholar
226 : Nonlinear dynamics indicates aging affects variability during gait. Clin Biomech (Bristol, Avon) 2003; 18:435–443Crossref, Medline, Google Scholar
227 : Nonlinear dynamical model of human gait. Phys Rev E Stat Nonlin Soft Matter Phys 2003; 67:051917Crossref, Medline, Google Scholar
228 : An improved surrogate method for detecting the presence of chaos in gait. J Biomech 2006; 39:2873–2876Crossref, Medline, Google Scholar
229 : Linear and nonlinear tremor acceleration characteristics in patients with Parkinson’s disease. Physiol Meas 2012; 33:395–412Crossref, Medline, Google Scholar
230 : Investigation of EEG non-linearity in dementia and Parkinson’s disease. Electroencephalogr Clin Neurophysiol 1995; 95:309–317Crossref, Medline, Google Scholar
231 : Specific patterns of cortical dysfunction in dementia and Parkinson’s disease demonstrated by the acceleration spectrum entropy of the EEG. Clin Electroencephalogr 1995; 26:188–192Crossref, Medline, Google Scholar
232 : Investigation of non-linear properties of multichannel EEG in the early stages of Parkinson’s disease. Clin Neurophysiol 2001; 112:38–45Crossref, Medline, Google Scholar
233 : Investigation of EEG abnormalities in the early stage of Parkinson’s disease. Cogn Neurodyn 2013; 7:351–359Crossref, Medline, Google Scholar
234 : Correlation between cortical and subcortical neural dynamics on multiple time scales in Parkinson’s disease. Neuroscience 2015; 298:145–160Crossref, Medline, Google Scholar
235 : Long-range temporal correlations in the subthalamic nucleus of patients with Parkinson’s disease. Eur J Neurosci 2012; 36:2812–2821Crossref, Medline, Google Scholar
236 : Modulation of cortical neural dynamics during thalamic deep brain stimulation in patients with essential tremor. Neuroreport 2013; 24:751–756Crossref, Medline, Google Scholar
237 : Finite dimensional structure of the GPI discharge in patients with Parkinson’s disease. Int J Neural Syst 2011; 21:175–186Crossref, Medline, Google Scholar
238 : Age-related variation in EEG complexity to photic stimulation: A multiscale entropy analysis. Clin Neurophysiol 2009; 120:476–483Crossref, Medline, Google Scholar
239 : Integrative neurocomputational perspectives on cognitive aging, neuromodulation, and representation. Neurosci Biobehav Rev 2002; 26:795–808Crossref, Medline, Google Scholar
240 : Deterministic dynamics in neuronal discharge from pallidotomy targets. J Int Med Res 2008; 36:979–985Crossref, Medline, Google Scholar
241 : Nonlinear temporal organization of neuronal discharge in the basal ganglia of Parkinson’s disease patients. Exp Neurol 2010; 224:542–544Crossref, Medline, Google Scholar
242 : Neuronal entropy depends on the level of alertness in the parkinsonian globus pallidus in vivo. Front Neurol 2014; 5:96Crossref, Medline, Google Scholar
243 : Neuronal activity in the substantia nigra in the anaesthetized rat has fractal characteristics. Evidence for firing-code patterns in the basal ganglia. Exp Brain Res 2003; 151:167–172Crossref, Medline, Google Scholar
244 Darbin O, Dees D, Martino A, et al: An entropy-based model for basal ganglia dysfunctions in movement disorders. BioMed research international 2013; 2013:742671Google Scholar
245 : Reward functions of the basal ganglia. J Neural Transm (Vienna) 2016; 123:679–693Crossref, Medline, Google Scholar
246 : Dopamine reward prediction-error signalling: a two-component response. Nat Rev Neurosci 2016; 17:183–195Crossref, Medline, Google Scholar
247 : EEG markers are associated to gray matter changes in thalamus and basal ganglia in subjects with mild cognitive impairment. Neuroimage 2012; 60:489–496Crossref, Medline, Google Scholar
248 : Modulation of beta oscillations in the subthalamic area during action observation in Parkinson’s disease. Neuroscience 2009; 161:1027–1036Crossref, Medline, Google Scholar
249 : Cracking the neuronal code. Science 1995; 270:756–757Crossref, Medline, Google Scholar
250 : Globus pallidus internus neuronal activity: a comparative study of linear and non-linear features in patients with dystonia or Parkinson’s disease. J Neural Transm (Vienna) 2016; 123:231–240Crossref, Medline, Google Scholar
251 : Deep brain stimulation reduces neuronal entropy in the MPTP-primate model of Parkinson’s disease. J Neurophysiol 2008; 100:2807–2818Crossref, Medline, Google Scholar
252 : Apomorphine reduces subthalamic neuronal entropy in parkinsonian patients. Exp Neurol 2010; 225:455–458Crossref, Medline, Google Scholar
253 : Structure function revisited: a simple tool for complex analysis of neuronal activity. Front Hum Neurosci 2017; 11:409Crossref, Medline, Google Scholar
254 : Neuronal entropy-rate feature of entopeduncular nucleus in rat model of Parkinson’s disease. Int J Neural Syst 2016; 26:1550038Crossref, Medline, Google Scholar
255 : Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol 1996; 6:751–758Crossref, Medline, Google Scholar
256 : Dorsolateral prefrontal cortex GABA concentration in humans predicts working memory load processing capacity. J Neurosci 2016; 36:11788–11794Crossref, Medline, Google Scholar
257 : Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003; 126:1079–1091Crossref, Medline, Google Scholar
258 : A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci 2009; 10:885–892Crossref, Medline, Google Scholar
259 : The functions of the orbitofrontal cortex. Brain Cogn 2004; 55:11–29Crossref, Medline, Google Scholar
260 : Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci 2007; 7:356–366Crossref, Medline, Google Scholar
261 : Electrophysiological measurements of anterior cingulate function. J Neural Transm (Vienna) 2002; 109:977–988Crossref, Medline, Google Scholar
262 : Medications influencing central cholinergic neurotransmission affect saccadic and smooth pursuit eye movements in healthy young adults. Psychopharmacology (Berl) 2017; 234:63–71Crossref, Medline, Google Scholar
263 : Stress-induced cognitive dysfunction: hormone-neurotransmitter interactions in the prefrontal cortex. Front Hum Neurosci 2013; 7:123Crossref, Medline, Google Scholar
264 : The roles of the orbitofrontal cortex via the habenula in non-reward and depression, and in the responses of serotonin and dopamine neurons. Neurosci Biobehav Rev 2017; 75:331–334Crossref, Medline, Google Scholar
265 : Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex 2006; 16:106–114Crossref, Medline, Google Scholar
266 : Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry 2003; 60:1145–1153Crossref, Medline, Google Scholar
267 : Selective antagonism at dopamine D3 receptors enhances monoaminergic and cholinergic neurotransmission in the rat anterior cingulate cortex. Neuropsychopharmacology 2003; 28:839-849Crossref, Medline, Google Scholar
268 : Oculomotor dysfunction in amyotrophic lateral sclerosis: a comprehensive review. Arch Neurol 2011; 68:857–861Crossref, Medline, Google Scholar
269 : Diffuse Lewy body disease with dementia and oculomotor dysfunction. Mov Disord 1990; 5:143–147Crossref, Medline, Google Scholar
270 : Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Brain Res Rev 2000; 31:138–146Crossref, Medline, Google Scholar
271 : Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000; 10:1078–1092Crossref, Medline, Google Scholar
272 : Meta-analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cereb Cortex 2014; 24:232–248Crossref, Medline, Google Scholar
273 : Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr Opin Neurobiol 2015; 30:59–65Crossref, Medline, Google Scholar
274 : Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 2000; 10:318–325Crossref, Medline, Google Scholar
275 : Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000; 4:215–222Crossref, Medline, Google Scholar
276 : An examination of rostral anterior cingulate cortex function and neurochemistry in obsessive-compulsive disorder. Neuropsychopharmacology 2015; 40:1866-1876Crossref, Medline, Google Scholar
277 : Impaired reward processing by anterior cingulate cortex in children with attention deficit hyperactivity disorder. Cogn Affect Behav Neurosci 2014; 14:698–714Crossref, Medline, Google Scholar
278 : An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson’s disease. J Clin Exp Neuropsychol 2006; 28:1127–1144Crossref, Medline, Google Scholar
279 : Functional circuitry of the basal ganglia, in Deep Brain Stimulation for Neurological Disorders Edited by Itakura T. New York, Springer, 2015, pp 1–11Crossref, Google Scholar
280 : Computational models of motivated action selection in corticostriatal circuits. Curr Opin Neurobiol 2011; 21:381–386Crossref, Medline, Google Scholar
281 : Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 2000; 278:H2039–H2049Crossref, Medline, Google Scholar



