Pseudo-Charles Bonnet Syndrome With a Frontal Tumor: Visual Hallucinations, the Brain, and the Two-Hit Hypothesis
We describe the case of a 52-year-old woman with blindness and visual hallucinations, suggestive of Charles Bonnet syndrome. The patient was very distressed regarding the visual hallucinations, which were often in the form of humans and animals the patient thought were real. Imaging revealed a large meningioma located in the orbital frontal area and causing significant mass effect on the adjacent brain parenchyma and optic nerves. Upon questioning, the patient’s initial history was notable for apathy and personality change, according to family members. After resection of the mass, her hallucinations resolved, although her blindness remained. The patient’s personality returned to baseline, according to her family, and her score on the Montreal Cognitive Assessment (MoCA) for the blind improved. The resolution of hallucinations after intracranial mass resection provides support for a two-hit hypothesis for psychotic hallucinations.
Case Report
A 52-year-old right-handed woman presented to a large academic medical center with visual hallucinations in the context of recent-onset blindness. Beginning 2½ years earlier, she noticed blurry vision, which progressed to complete visual loss in her left eye. Two years earlier, she had similar symptoms in her right eye that also progressed to visual loss. One year earlier, she was diagnosed with bilateral optic atrophy. She now presented after 6 months of visual hallucinations. A recent brain MRI was performed and demonstrated a 6.3-cm extra-axial enhancing mass, thought to be a meningioma, causing significant mass effect on the surrounding brain parenchyma (Figure 1).
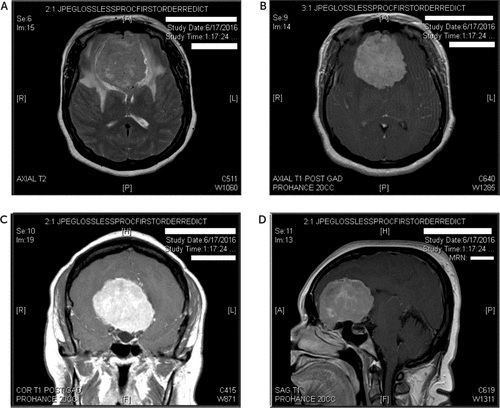
FIGURE 1. MRI of Lesion in the Patient’s Orbital Frontal Areaa
a The panels show A) axial T2, B) axial T1, C) coronal T1, and D) sagittal view of the mass.
Upon examination, the patient could be heard crying out to others in the room, even though she was alone. She was found speaking to visual hallucinations in the room, referencing men and “black snakes.” Upon interview, she stated that for the last 6 months, her visual hallucinations included images of humans and animals. She described that full-sized men were holding snakes, that the snakes were coming out of their bodies, or that the men were attempting to place the snakes on her, all of which led her to experience increasing distress and paranoia. She endorsed that these hallucinations were very distressing to her, as she had no privacy from “these men” during the day. There was no specific spatial distribution of the hallucinations. She denied any auditory hallucinations, and she stated that the men did not speak to her and did not answer her questions.
Upon questioning, the patient’s family said that her hallucinations had worsened and her personality had changed, with the patient becoming more apathetic. Her husband noted that while the family encouraged her to seek help and tried to intervene in her health, the patient did not act concerned regarding these recent changes and neglected to care for herself. He also noted that while she was previously kind and quiet, she had become much less kind toward others and spoke increasingly often about her hallucinations. He denied that she had showed any episodes of impulsive behavior.
An ophthalmology examination revealed that the patient was unable to indicate the position of objects in front of her but that her extraocular motility was full in both eyes. She had no light perception bilaterally and no relative afferent pupillary defect bilaterally, suggesting equal to nearly equal visual function loss. She was found to have bilateral optic nerve atrophy. Of note during the ophthalmology examination, the patient stated that there were two children present in the room, that the examiner looked as though he was Haitian, and that the ground had sand and grass. In fact, there were no children present, the examiner was Caucasian, and the floor was hospital tile.
On her general neurological examination, she was found to be alert and oriented to name, day of the week, month, date, and year. She was unable to state her location, describing that she could not see the building for clues regarding her surroundings. She had minimal ability to smell scent with the right nostril but otherwise demonstrated absence of the sense of smell. Her facial sensation was intact, her facial musculature was symmetric, her hearing was intact, her palate was elevated symmetrically, and her tongue protruded in midline. She had full strength, no deficits to light touch, no dysmetria, and was able to ambulate unassisted with a normal stride and arm swing.
The MoCA for the blind was administered, with the patient’s overall score being 13/22. Her attention was excellent for numbers in both forward and backward order. Attention to a list of letters was excellent and with no errors. Her serial subtraction was poor. Repetition was largely intact, with a few minor errors (e.g., “is” instead of “were”). The patient’s fluency was fair, and she was able to name 11 words. Her abstraction was intact, but her delayed recall was very poor. For orientation, she was convinced that the location was a house. The following day, the MoCA for the blind was repeated, and again her score was 13/22. She continued to have poor serial subtraction. Her fluency was worse, as she only named seven words, although her delayed recall was slightly improved. She continued to endorse that she was located in a house.
The patient was followed throughout the resection of her brain mass and postoperative recovery. After a brief period of postoperative delirium, she recovered well. The patient, her husband, and nurses all endorsed lack of hallucinations. Although her blindness, unfortunately, was unchanged, the patient and her family were pleased that she was no longer distressed by the visual hallucinations.
As the patient recovered from the operation, her husband noted that her personality had returned to baseline. She was no longer apathetic. She had returned to her kind self and was described by nurses as pleasant. She quietly chatted with visitors and no longer called out to hallucinations or described any visual hallucinations in her room.
On the day of her discharge, her score on the MoCA for the blind had increased to 18/22. Her attention to a list of letters was excellent and with no errors. Her serial subtraction was greatly improved, and she quickly obtained four correct answers. Repetition was largely intact, though, as previously, with a few minor errors (e.g., “is” instead of “were”). Her fluency was improved, and she was able to name 15 words. Her abstraction was intact. Her delayed recall was greatly improved, as she quickly remembered four out of five words. Her orientation also improved, as she was quickly able to report that her location was the hospital.
Discussion
This case is most notable for the complete resolution of visual hallucinations with resection of a prefrontal mass, despite continued blindness.
In general, hallucinations are usually regarded within the study of psychoses but can occur in a wide range of medical and psychiatric conditions. Visual hallucinations can be seen in psychiatric disorders but are more indicative of neurological conditions. In psychodynamic therapy, Freud, in 1953, related hallucinations to dreams as thoughts transformed into visual images, while Kolb and Brodie, in 1982, postulated that hallucinations represent unconscious material coming into consciousness as a response to psychological needs, such as guilt or wishes.1 Several neurophysiologic hypotheses for hallucinations have also been described. Hughlings Jackson, in 1932, described a disinhibition model, in which the impedance of upper inhibitory influence allows middle-level activity to be released.1 Marazzi, in 1970, suggested dissociation of the primary sensory cortex and the regulatory cortical association areas.1 West, in 1975, proposed a perceptual release theory, in which the brain’s censorship mechanism, without sensory input, allows the surfacing of earlier perception or memories.1 Morrison, in 1995, suggested that hallucinations are normal intrusive thoughts misattributed to an external source and that this ego-dystonic experience can lead to anxiety or discomfort.1 Further complicating this picture is the role of cultural background and how this affects the concept of reality and the interpretation of subjective experience.
Hallucinations thus represent an interesting neuroscientific challenge and have been explored in both bottom-up and top-down approaches.2 Bottom-up approaches consider phylogenetically older sensory and limbic systems, while top-down approaches consider higher-order executive neurophysiological dysfunctions associated with hallucinations.2 A two-hit hypothesis integrates these approaches. For example, hallucinations (usually auditory-visual) have been associated with aberrant activity in the sensory cortices and high levels of dopamine in the limbic system, in the case of schizophrenia, with the utility of antipsychotic drugs to block central dopamine activity to relieve these symptoms.1 These abnormalities occur in the setting of prefrontal dysfunction. Increased serotonin may also be implicated in hallucinations, as seen with substances such as LSD and ecstasy or with the side effect of selective serotonin reuptake inhibitors. In contrast, decreased acetylcholine has also been described, such as with plants like the belladonna and Datura that contain scopolamine, atropine, or antimuscarinic agents, and with the hallucinations seen in Alzheimer’s disease and Lewy body dementia (often in the setting of cognitive decline).1 Decreased N-methyl-D-aspartate may also be involved, as glutamate inhibitors such as ketamine can induce hallucinations.1 Deep brain structure activity may generate or modulate hallucinations, and the particular neo-cortical regions in individual patients may affect their perceptual content.2 The hallucinating brain may have stronger input from thalamus and subcortical centers, reduced control and monitoring by the prefrontal cortex, and aberrant activation of the dorsal anterior cingulate involved in source monitoring and increased ventral striatal activity associated with salience.1,2 These bottom-up and top-down approaches are integrated to explore the interactions among auditory-linguistic association cortices, caudal and rostral limbic/paralimbic systems, prefrontal cortices, ventral striatum, and thalamic nuclei, as well as the glutamatergic, GABAergic, and ventral tegmental dopaminergic modulation of these systems.2
Given our patient’s initial presentation of blindness with visual hallucinations, Charles Bonnet syndrome was considered.3 Other case reports have described the cause of Charles Bonnet hallucinations as deafferentation or lack of true visual input to the brain, causing a release phenomenon similar to phantom limb syndrome.4 This was supported by a study of 13 normally sighted patients who were blindfolded for 5 consecutive days. Subsequently, 10 of these patients reported hallucinations after an average of 1 day.4 Charles Bonnet syndrome has also been described in prior research as a cortical release phenomenon, with sensory deprivation from ocular pathology releasing the visual cortex from regular stimuli by external stiumuli.3 Neuroimaging has shown this hypothesis to be plausible. Some patients with Charles Bonnet syndrome may have dysfunction in the primary and secondary visual cortices, and transient cortical activation occurs in the inferior lateral temporal cortex during the appearance of visual hallucinations.3 In Charles Bonnet syndrome, hallucinations are often small animals or people, and reality testing is typically preserved.
In our patient in the above case, the content of visual hallucinations was not trivial, and the patient did not have intact reality testing. This may be explained by the two-hit hypothesis. The two-hit hypothesis suggests that in order for perception of something not there to occur, with loss of reality testing and associated belief that the perception is true, there are two important functional components. First, excessive activity of the sensory cortices is present, underlying the perceptual content. Second, there is inadequate activity in prefrontal cortices associated with modulation of the limbic-subcortical emotional and salience processing, with higher-order monitoring of mentation, with rational executive functions, and with reality testing and violation of expectations.5 The present case report provides support for this two-hit hypothesis by demonstrating that hallucinations ceased with resolved prefrontal dysfunction while the visual dysfunction remained. The apathy and personality change and their resolution are consistent with known functions of the orbitofrontal cortex in motivation and social-emotional behavior.6
1 : Hallucinations: etiology and clinical implications. Ind Psychiatry J 2009; 18:119–126Crossref, Medline, Google Scholar
2 : Functional neuroimaging of hallucinations in schizophrenia: toward an integration of bottom-up and top-down approaches. Mol Psychiatry 1996; 1:367–375Medline, Google Scholar
3 : Neuroimaging studies in patients with Charles Bonnet syndrome. Psychogeriatrics 2009; 9:77–84Crossref, Medline, Google Scholar
4 : Cases: Charles Bonnet syndrome: visual loss and hallucinations. CMAJ 2009; 181:175–176Crossref, Medline, Google Scholar
5 : Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain 2017; 140:497–507Crossref, Medline, Google Scholar
6 : Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci 2006; 18:871–879Crossref, Medline, Google Scholar



