Mild Traumatic Brain Injury and Aggression, Impulsivity, and History of Other- and Self-Directed Aggression
Abstract
Mild traumatic brain injury (mTBI) is highly prevalent, with an estimated occurrence in the United States of more than 1.3 million per year. While one consequence of mTBI is impulsive aggressive behavior, very few studies have examined the relationship between history of mTBI and aggressive behavior in impulsively aggressive individuals. The authors examined the relationship between history of mTBI in a healthy control group (HC; N=453), a control group with psychiatric disorders (PC; N=486), and individuals with intermittent explosive disorder (IED; N=695), a disorder of primary impulsive aggression. Results demonstrated that IED study participants were significantly more likely to have a history of mTBI (with or without history of a brief loss of consciousness [LOC]) compared with both HC and PC participants. A similar observation was made with regard to self-directed aggression (i.e., suicidal or self-injurious behavior), although group differences were only among those with mTBI with LOC. For both other- and self-directed aggression variables, the authors observed a stepwise increase in dimensional aggression and impulsivity scores across participants as a function of mTBI history. Given that impulsive aggressive behavior begins very early in life, these data are consistent with the hypothesis that lifelong presence of an impulsive aggressive temperament places impulsive aggressive individuals in circumstances that put them at greater risk for mTBI compared with other individuals with and without nonimpulsive aggressive psychopathology.
According to the U.S. Department of Veterans Affairs/Department of Defense, mild traumatic brain injury (mTBI) is defined as head injury that may result in alteration of consciousness, posttraumatic amnesia (up to 24 hours postinjury), and/or possible loss of consciousness (LOC) (less than 30 minutes) that may or may not be visible on structural imaging (e.g., computerized tomography [CT] scan1). mTBI is highly prevalent, with an estimated occurrence of over 1.3 million injuries annually in the United States.2 However, this is likely a significant underestimation of true mTBI injuries, since many people who experience mild head injury do not seek medical treatment.3 Rates among military servicemen have been shown to be even higher, with an estimated rate of 15% of returning Operation Enduring Freedom/Operation Iraqi Freedom soldiers sustaining an mTBI.4 mTBI is associated with a number of cognitive, physical, and emotional sequelae that typically resolve within 3 months of injury.1 However, a growing body of research indicates that anywhere from 10% to 31% of post-mTBI patients experience symptoms, including lingering physical symptoms and mood disturbances, beyond the three-month period.5,6 Factors associated with more persistent post-mTBI symptoms include female gender, older age, pre-existing psychiatric issues, and medical complications.7,8 However, it should be noted that mTBI is a diagnostically heterogeneous condition, due to both the variability in symptom presentation and severity (whether there is LOC and/or posttraumatic amnesia uncomplicated vs. complicated injury), as well as variability in understanding and application of mTBI terminology and classification among health care providers.9,10
A growing area of interest related to postconcussive-mTBI symptoms includes changes in aggressive behavior, in both verbal and physical forms, after head injury.11 Among those with history of TBI, aggression may be due to a combination of both neuropsychological and emotional deficits, including decreased inhibition and increased frustration,12 a relationship well established in criminal populations. For example, in a study conducted by Slaughter et al.,13 58% of incarcerated persons reported having ever experienced an mTBI, and 29% reported experiencing an mTBI within the previous year. In comparison with those with remote or no history of TBI, those who reported a TBI within the past year exhibited greater neuropsychological deficits related to phonemic verbal fluency and mental flexibility, both of which are associated with frontal network dysfunction. Moreover, those who endorsed a recent history of TBI reported significantly higher levels of anger and aggression on the Brief Anger-Aggression Questionnaire than did control subjects. Importantly, the two groups did not differ in psychiatric disorder prevalence. Similarly, increased TBI is also found in domestic violence perpetrators.14,15 The relationship between aggression post-TBI has also been noted in nonincarcerated populations.16 Furthermore, those who exhibit aggressive behavior after head injury tend to also have greater difficulty with activities associated with daily living and social functioning.17
However, the directional causality between TBI and aggression remains in question. Several studies suggest that pre-existing disturbances of mood, or aggressive behavior, are predictive of future post-TBI aggressive behavior. For example, Tateno et al.18 reported predictive factors of post-TBI aggression to be preinjury substance abuse, mood, or aggressive disorders. Studies of veterans and returning military personnel further demonstrate that PTSD symptoms, mood disturbance, suicidality, substance abuse or misuse, lower education, and previous history of arrest or domestic violence are more predictive of aggression than is the presence of TBI itself.19–22 In contrast, Ramesh et al.23 reported that previous history of TBI preceding initiation of cocaine use may increase the risk of substance abuse.
Advancements in neuroimaging and biomarkers speak to structural differences among the brains of those with history of TBI and aggression. Epstein et al.24 found evidence of increased aggression, anxiety, and depression as well as cortical thinning of the right orbitofrontal cortex among those with history of mTBI,24 although similar abnormalities are observed as a function of aggression regardless of history of mTBI.25 In addition, research indicates reduction of fractional anisotropy, associated with reduced white matter functioning in frontotemporo-occipital regions, in those with history of mTBI.26 Lastly, a systematic review of the concussion-mTBI biomarker literature indicated a number of biomarkers associated with irritability and aggression in mTBI, including COMT, SLC6A4 (SERT), and MMP-9 proteins.27 A number of studies have also demonstrated correlations between the number of head injuries and aggression, with a higher number of concussions/mTBI resulting in higher levels of aggression and irritability.28 Thus, although a relationship between head injury and aggression exists, additional research is needed to more fully characterize the nature of this relationship, especially in individuals with mTBI.
The aim of this study was to explore the relationship between history of mTBI and aggression in a large clinical research sample of physically healthy individuals with and without psychiatric disorder, including intermittent explosive disorder (IED), a disorder of outwardly directed impulsive aggression.29 We hypothesized that (a) study participants with IED would be more likely to have a history of mTBI than would both healthy and psychiatric controls, (b) study participants with a history of self-directed aggression (suicidal or self-injurious behavior) would be more likely to have a history of mTBI than would those without this history, and (c) study participants with a history of mTBI would have higher trait aggression and trait impulsivity scores than those without this history. Finally, we hypothesized that measures of aggression and impulsivity would follow a stepwise increase in magnitude as a function of severity of mTBI history (e.g., no mTBI vs. mTBI without LOC vs. mTBI with LOC).
Methods
Participants
A total of 1,634 adult individuals participated in this study. All participants were physically healthy and were systematically evaluated regarding aggressive and other behaviors as part of a larger program designed to study correlates of impulsive-aggressive, and other personality-related, behaviors in human subjects. Subjects were recruited through public service announcements, newspaper, and other media—advertisements seeking out individuals who (a) reported psychosocial difficulty related to one or more psychiatric conditions or (b) had little evidence of psychopathology. All subjects provided informed consent and signed the informed consent document approved by the institutional review board.
Diagnostic Assessment
Syndromal and personality disorder diagnoses were made according to DSM-5 criteria. Diagnoses were made using information from (a) the Structured Clinical Interview for DSM Diagnoses (SCID-I)30 for syndromal (formerly Axis I) disorders and the Structured Interview for the Diagnosis of DSM Personality Disorder for Personality (formerly Axis II) Disorder31; (b) clinical interview by a research psychiatrist; and (c) review of all other available clinical data. This process resulted in good-to-excellent interrater reliabilities (mean kappa of 0.84±0.05; range=0.79–0.93) across anxiety, mood, substance use, impulse control, and personality disorders. Final diagnoses were assigned by team best-estimate consensus procedures involving research psychiatrists and clinical psychologists, as has been previously described.32 Subjects with a current history of a substance use disorder or of a life history of any bipolar disorder, schizophrenia (or other psychotic disorder), or mental retardation/intellectual disability were excluded from the study. In addition, participants with a history of mTBI with LOC greater than 30 minutes were excluded from the study (see below).
After diagnostic assignment, 453 participants had no evidence of any psychiatric diagnosis, and as such were designated healthy control (HC) subjects; 486 participants met criteria for a current/lifetime diagnosis of a syndromal psychiatric disorder or personality disorder but not IED, and as such were designated psychiatric control (PC) subjects; and 695 participants met criteria for IED. Of the 1,181 participants with history of a psychiatric disorder, a majority (67.6%) reported (a) history of formal psychiatric evaluation, treatment, or both (52.8%) or (b) history of behavioral disturbance during which the subject, or others, thought they should have sought mental health services but did not (14.7%). The means (±SD) for demographic, psychosocial functional/life satisfaction, and psychometric behavioral variables for the three groups are summarized in Table 1. The syndromal and personality disorder diagnoses for the two psychiatric groups (PC and IED) are summarized in Table 2.
| Characteristic | HC (N=453) | PC (N=486) | IED (N=695) | pb | Group Differences |
|---|---|---|---|---|---|
| Demographic | |||||
| Age (years) | 30.4±9.0 | 32.3±9.6 | 35.8±10.0 | <0.001 | HC<PC<IED |
| Gender (% male) | 56.5% | 58.6% | 54.5% | 0.373 | HC=PC=IED |
| Race (% Caucasian) | 55.8% | 56.8% | 51.7% | 0.009 | HC=PC>IED |
| Socioeconomic status score | 40.4±13.6 | 33.2±13.9 | 36.0±13.0 | <0.001 | HC>IED>PC |
| Psychosocial function | |||||
| Global Assessment of Function score | 83.3±5.4 | 64.3±11.6 | 55.8±8.0 | <0.001 | HC>PC>IED |
| Q-LES-Q score | 52.2±7.9 | 45.2±10.2 | 39.0±10.4 | <0.001 | HC>PC>IED |
| Psychometric variable | |||||
| Aggression | |||||
| LHA | 4.7±3.6 | 8.2±5.6 | 18.0±4.5 | <0.001 | HC<PC<IED |
| BPA | 27.9±10.1 | 33.7±11.7 | 46.6±11.9 | <0.001 | HC<PC<IED |
| Impulsivity | |||||
| LHIB | 22.6±15.6 | 35.8±20.2 | 52.8±20.0 | <0.001 | HC<PC<IED |
| BIS–11 | 55.4±9.2 | 63.5±10.7 | 68.6±11.5 | <0.001 | HC<PC<IED |
TABLE 1. Demographic, Functional, and Psychometric Characteristics of the Study Participantsa
| Diagnosis | PC (N=486) | IED (N=695) | p | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Current syndromal disorders | |||||
| Any depressive disorder | 80 | 16.5 | 152 | 21.9 | 0.021 |
| Any anxiety disorder | 87 | 17.9 | 161 | 23.2 | 0.030 |
| Stress and trauma disorders | 23 | 4.7 | 87 | 12.5 | <0.001* |
| OCD | 5 | 1.0 | 27 | 3.9 | 0.003 |
| Eating disorders | 13 | 2.7 | 37 | 5.3 | 0.028 |
| Somatoform disorders | 7 | 1.4 | 12 | 1.7 | 0.816 |
| Non-IEDs | 2 | 0.4 | 10 | 1.4 | 0.137 |
| Lifetime syndromal disorders | |||||
| Any depressive disorder | 218 | 44.9 | 417 | 60.0 | <0.001* |
| Any anxiety disorder | 116 | 23.9 | 206 | 29.6 | 0.029 |
| Any substance use disorder | 170 | 35.0 | 353 | 50.8 | <0.001* |
| Stress and trauma disorders | 60 | 12.8 | 145 | 20.9 | 0.001* |
| OCD | 9 | 1.9 | 36 | 5.2 | 0.003 |
| Eating disorders | 37 | 7.6 | 74 | 10.6 | 0.085 |
| Somatoform disorders | 7 | 1.4 | 13 | 1.9 | 0.652 |
| Non-IEDs | 5 | 1.0 | 24 | 3.5 | 0.007 |
| Personality disorders | |||||
| Cluster A (odd) | 42 | 8.6 | 121 | 17.4 | <0.001* |
| Cluster B (dramatic) | 99 | 20.4 | 319 | 45.9 | <0.001* |
| Cluster C (anxious) | 109 | 22.4 | 180 | 25.9 | 0.191 |
| Personality disorder, not otherwise specified | 149 | 30.7 | 231 | 33.2 | 0.376 |
TABLE 2. Syndromal and Personality Disorder Diagnoses Among Study Participantsa
Assessment for History of mTBI
History of mTBI was recorded directly from the study participants during the diagnostic assessment interview by using a structured interview of our own design that contained most, but not all, of the items in the Ohio State University TBI–Identification Method (OSU-TBI-ID).33 The OSU-TBI-ID was developed during the later stages of our data collection and therefore not available for this study. mTBI was defined as self-reported history of a blow to the head associated with mild, and brief, neurological symptoms typically associated with mTBI, including any of the following: feeling dazed or dizzy, disorientation, memory difficulties lasting less than 24 hours, and LOC less than 30 minutes. Potential participants with history of a blow to the head and a period of LOC for more than 30 minutes were not included in this study.
Assessment of Aggression, Impulsivity, and Related Behaviors
In this study, aggression was conceptualized as behavior by one individual directed toward another person or object during which either verbal force or physical force is used to injure, to coerce, or to express anger. As such, aggression was assessed with the Life History of Aggression (LHA)34 scale and the Buss-Perry Aggression (BPA)35 questionnaire. The LHA assesses history of actual aggressive behavior, and the BPA assesses aggressive tendencies as a personality trait. The LHA is a widely used five-item measure that quantitatively assesses one’s life history of overt aggressive behavior (i.e., aggressive thoughts and urges are not counted). It is conducted as a semistructured interview. Internal consistency (α=0.87), interrater reliability (r=0.94), and test-retest reliability (r=0.80) is good to excellent. BPA Aggression, also a widely used assessment of trait aggression, comprises the BPA’s Verbal Aggression and Physical Aggression subscales and has good psychometric properties. Impulsivity was assessed with the Life History of Impulsive Behavior (LHIB)36 and with the Barratt Impulsiveness Scale (BIS-11).37 The LHIB assesses history of actual impulsive behavior and is conceptually similar to the LHA. It includes 20 items regarding impulsive behavior and is scored on a 5-point ordinal scale (as is the LHA). The LHIB demonstrates good internal consistency (α=0.96) and test-retest reliability (r=0.88). Psychosocial function was assessed with the Global Assessment of Function38 scale, and satisfaction of life experience was assessed with the Quality of Life Experience and Satisfaction Questionnaire.39
Statistical Analysis and Data Reduction
For analytic purposes, mTBI subjects were divided into those without history of mTBI (N=1,356; 83.0%), those with a history of mTBI but without LOC (N=168; 10.3%), and those with history of mTBI and a brief period of LOC lasting less than 30 minutes (N=110; 6.7%). Statistical procedures included chi-square, t test, and analysis of covariance (ANCOVA), as appropriate. All reported analyses were adjusted for age, sex, ethnicity, and socioeconomic status. A two-tailed alpha value of 0.05 was used to denote statistical significance for all analyses, except in cases in which a correction for multiple comparisons was more appropriate. Data reduction involved the creation of composite variables for trait aggression and trait impulsivity. Because each of the individual variables related to these dimensions were highly correlated with each other, composite variables were created by z-transforming each individual variable and taking the mean z-score of each of the related variables. Post hoc analyses involved adjustment for comorbid syndromal disorders (lifetime, depressive, substance use, and stress-trauma disorders).
Results
Demographic and Psychometric Characteristics of the Study Sample
The three diagnostic groups differed modestly, but significantly, in age, socioeconomic score, and ethnicity distribution, but not in distribution of sex (Table 1). Accordingly, all relevant analyses factored into these demographic differences. The groups significantly differed in all psychosocial function and satisfaction variables and in all psychometric behavioral variables, as was expected. Finally, the PC and IED groups did not significantly differ in rates of syndromal comorbidity with the exception of lifetime depressive, lifetime substance use, and current and lifetime stress-trauma disorders (Table 2).
History of mTBI Among the Study Participants (N=268)
Among the mTBI without reported history of LOC (N=168), 78.1% reported only one mTBI, 13.7% reported only two, and 8.3% reported three or more mTBIs. Of the total mTBI group reporting mTBI with LOC (N=110), 84.5% reported one mTBI with LOC, 10.9% reported two mTBIs with LOC, and 4.5% reported three or more mTBIs LOC.
History of mTBI Without LOC (mTBI) and mTBI With LOC (mTBI/LOC) as a Function of Diagnostic Group
IED participants had a significantly greater history of mTBI and mTBI/LOC than did either HC or PC participants (Table 3). The two control groups did not differ in this regard, and thus these groups were combined for subsequent analysis. These results were not changed when adding disorders with a higher rate of comorbid IED (see above), added as a separate layered factor to the chi-square analysis.
| Group | No mTBI | mTBI/Without LOC | mTBI/With LOC | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| HC | 403 | 89.0 | 32 | 7.1 | 18 | 4.0 |
| PC | 428 | 88.1 | 40 | 8.2 | 18 | 3.7 |
| IED | 525 | 75.5 | 94 | 13.5 | 76 | 10.9 |
TABLE 3. Relationship Between Diagnostic Groups and History of Mild Traumatic Brain Injury (mTBI) With and Without History of Brief Loss of Consciousness (LOC)a
Relationship Between the Number of Reported Head Injuries and the Number of Reported LOC Episodes With Measures of Aggression and Impulsivity
Next, we examined the relationship between composite aggression and impulsivity scores with the number of mTBI/LOC episodes. We used four categories for this analysis: no history of mTBI, mTBI without LOC, mTBI with one LOC episode, and mTBI with two or more LOC episodes. An ANCOVA revealed a significant effect for aggression scores as a function of reported mTBI/LOC (F=17.73, df=3, 1515, p<0.001) (Figure 1). Aggression scores of those reporting a history of mTBI without LOC were higher than among those without history of mTBI but similar to those of participants reporting history of one LOC episode. However, participants reporting two or more LOC episodes had the highest aggression scores of all groups. Adding IED status to the model did not change these results (F=6.11, df=3, 1514, p<0.001). An ANCOVA with impulsivity scores revealed a similar result (F=8.80, df=3, 1058, p<0.001) but with a stepwise fashion increase in impulsivity scores from the “no mTBI” group to the “two or more mTBI/LOC episodes” group (Figure 1). Adding IED status to the model reduced this result to a trend for statistical significance (F=2.26, df=3, 1057, p=0.08).
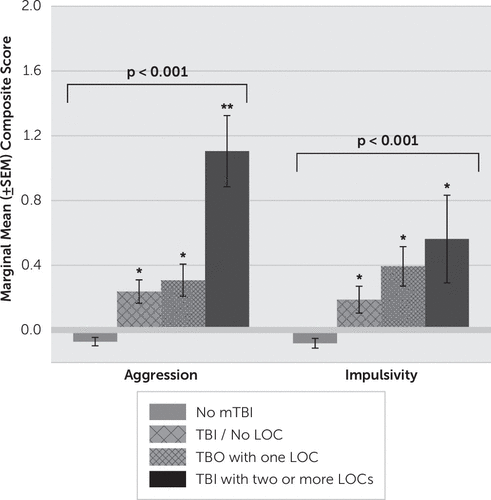
FIGURE 1. Marginal Means Composite Aggression and Impulsivity Scores for Reported History of Mild Traumatic Brain Injury (mTBI) With and Without History of One or More Brief Episodes of Loss of Consciousness (LOC)a
a A single asterisk indicates a significant difference when compared with the “no mTBI” group; a double asterisk for aggression scores (left) indicates a significant difference for two or more LOC episodes when compared with the mTBI-only group and the one-LOC group, which did not differ from each other. ±SEM=standard error of the means.
Association of mTBI/LOC and History of Self-Aggressive (S-AGG) Behavior
Study participants with a history of suicide attempt (SA), history of self-injurious behavior (SIB), and history of either (SA or SIB) had a significantly increased rate for history of mTBI with LOC but not for mTBI without LOC than did control participants (Table 4).
| Group | No mTBI | mTBI/Without LOC | mTBI/With LOC | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| No suicidal behavior | 1,205 | 84.1 | 138 | 9.6 | 90 | 6.3 |
| Suicidal behaviora | 151 | 75.1 | 22 | 10.9 | 28 | 13.9 |
| No self-injurious behavior | 1,227 | 83.7 | 147 | 10.0 | 92 | 6.3 |
| Self-injurious behaviorb | 129 | 76.8 | 13 | 7.7 | 26 | 15.5 |
| No suicidal behavior or self-injurious behavior | 1,144 | 84.7 | 131 | 9.7 | 76 | 5.6 |
| Suicidal behavior and/or self-injurious behaviorc | 212 | 74.9 | 29 | 10.2 | 42 | 14.8 |
TABLE 4. Relationship Between History of Suicidal Behavior and/or Self-Injurious Behavior and History of Mild Traumatic Brain Injury (mTBI) With and Without History of Brief Loss of Consciousness (LOC)
Relationship Between the Number of Reported mTBIs With and Without the Number of Reported LOC Episodes With Measures of Aggression and Impulsivity as a Function of S-AGG
An ANCOVA of aggression and impulsivity scores, as a function of S-AGG and mTBI with or without LOC, revealed no differences between number of mTBIs with and without LOC. Thus, these groups were combined for further analysis. A subsequent ANCOVA revealed a significant stepwise increase in aggression scores from S-AGG−/mTBI− to S-AGG+/TBI+ (F=66.72, df=3, 1515, p<0.001) (Figure 2). Although a similar ANCOVA analysis with impulsivity scores was also statistically significant (F=38.60, df=5, 1056, p<0.001), impulsivity scores displayed a different pattern of results in which S-AGG−/mTBI+ participants had higher impulsivity scores than did S-AGG−/mTBI− participants but with S-AGG+/mTBI− and S-AGG+/mTBI+ participants having similar impulsivity scores, each significantly higher than those among both groups of S-AGG− participants (Figure 2).
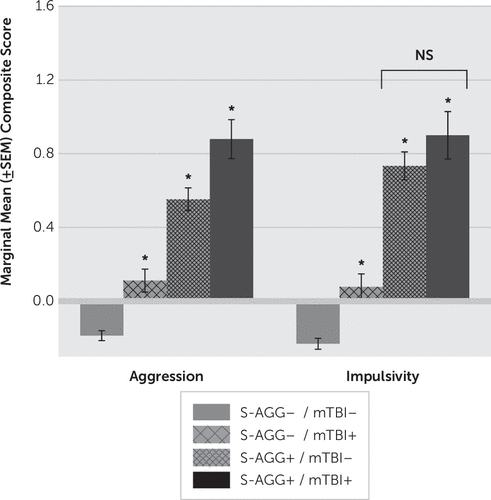
FIGURE 2. Marginal Means Composite Aggression and Impulsivity Scores for Reported History of Mild Traumatic Brain Injury (mTBI) as a Function of Self-Directed Aggressive Behavior (S-AGG)a
a A single asterisk indicates a significant difference when compared with the “no S-AGG” group and when compared with each other, except where specified. NS=not significant; ±SEM=standard error of the means.
Discussion
The key finding from this study is that individuals with IED (i.e., other-directed aggression) are significantly more likely to have a history of mTBI (with or without history of a brief LOC) than are both healthy and psychiatric controls. A similar observation was made for self-directed aggression (i.e., suicidal or self-injurious behavior), although the group difference was only among those with mTBI and LOC. For both variables, we observed a stepwise increase in aggression and in impulsivity scores going from no history of mTBI to mTBI with two or more mTBIs with LOC. At the very least, these findings support the hypothesis that individuals with IED, as well as those with a history of suicidal or self-injurious behavior or both, are at greater risk for mTBI with or without LOC than are those without IED or these self-aggressive behaviors. However, these findings also highlight the potential role of altered mental state (AMS) or LOC in contributing to additional structural impairment that may be difficult to observe on traditional neuroimaging methodology (e.g., CT) and thus may contribute to a delayed or complicated recovery. Although the role of AMS/LOC in mTBI in complicated recovery remains unclear, there have been several studies that indicate greater neurobehavioral and neuropsychological impairment, as well as structural white matter changes, among those who report mTBI with LOC than among those with mTBI with no LOC or healthy controls.40,41
Differences in rates of mTBI, with or without LOC, between IED and HC participants was expected given the fact that individuals with substantial psychopathology (i.e., IED) are more likely to differ from healthy controls on most behavior variables. However, the observation that IED participants also differed from PC participants, who also differed from HC participants on many of the variables tested, suggests that the association between IED and history of mTBI was not due simply to general psychopathology. Differences in mTBI rates were accompanied by dimensional increases in aggression (and impulsivity) scores, and thus differences in mTBI rates may be accounted for by elevations in these specific behavioral traits.
Analysis of composite scores for trait aggression and impulsivity revealed that each behavioral trait was significantly elevated as a function of mTBI (with or without LOC) in comparison with those of study participants without positive mTBI history. Controlling for the presence of IED reduced the magnitude of these relationships but did not eliminate the statistical significance of this result for either composite aggression or impulsivity scores. In actuality, our post hoc analysis likely underestimates the relationship between aggression-impulsivity scores and mTBI. Because IED participants have high scores on aggression and impulsivity, a high proportion of the variance associated with aggression-impulsivity was removed by using IED as a covariate. However, even controlling for the presence of IED indicates greater aggression and impulsivity among those with history of mTBI than among with those without history of mTBI.
On the basis of these data alone, we cannot say whether the presence of high trait impulsivity and aggression led IED participants to be in circumstances that increase risk for mTBI or whether history of mTBI altered the brains of mTBI participants, leading to an increase in aggressive and impulsive behavior post-mTBI. This is because we did not systematically collect data regarding mTBI in relationship to the onset of IED. That said, impulsive-aggressive behaviors are present from very early life,42 and individuals with this temperament are likely to place themselves in circumstances associated with bodily injury, including mTBI. Additionally, these findings are in general agreement with the results of studies examining individuals soon after mTBI,18–22 which report that premorbid aggression better predicts the occurrence of post-TBI aggression than does recent history of mTBI itself. Although it is known that TBI, and certainly severe TBI, can be associated with changes in brain structure24 and with changes in personality features, including aggression,24 we have found that brain structure changes in IED participants were not influenced by history of mTBI.25 This is not to suggest that changes in brain structure or function, and aggressive behavior, associated with mTBI do not occur. We suggest, simply, that presence of mTBI in these study participants was likely due to the dimensional presence of impulsive-aggressive behavior, across individuals, rather than to a primary effect of mTBI on measures of aggression and impulsivity.
This study has several strengths and limitations. First, our large participant sample provided extensive diagnostic and phenomenologic information and data from reliable and valid measures of aggression and impulsivity. Its second strength is its inclusion of a nonhealthy control group with substantial psychopathology and significantly lower scores on measures of aggression and impulsivity in comparison with IED study participants. This psychiatric control group is useful, as it helps account for the effect of general, nonaggressive, psychopathology on the history of mTBI. Third, although the psychiatric subjects in the study were not primarily recruited from treatment settings, most (67.6%) had a history of formal treatment for a psychiatric disorder (52.8%) or of behavioral disturbance that should have been assessed by mental health professionals (14.7%). Accordingly, most of the psychiatric subjects in this study are likely to be similar to those drawn from a treatment setting. Limitations include the self-report nature of the history of mTBI as well as the nonsystematic collection of timing data and the nature of the mTBIs. Although most reported that these mTBIs occurred during a sport activity or during a motor-vehicle accident, we did not record the exact context in many cases. However, it was known that none of the participants experienced more than a mild TBI, since our study inclusion criteria required that any history of traumatic brain injury be limited to a head injury associated with less than 30 minutes of LOC; on average, current study participants with a history of mTBI reported less than 5 minutes of LOC.
Conclusions
Individuals with IED, as well as persons with a history of suicidal or self-injurious behavior, are significantly more likely to have a history of mTBI than are those in appropriate comparison groups. In both cases of other- and self-directed aggression, a stepwise increase in aggression and in impulsivity scores was observed as a function of type and number of mTBI episodes. These findings support the hypothesis that individuals with IED, as well as those with a history of suicidal or self-injurious behavior, are at greater risk for mTBI with or without LOC than are those without IED or these self-aggressive behaviors. Although it is likely that impulsive and aggressive traits, which are evident very early in life, place impulsive- aggressive individuals in circumstances in which physical, and head, injury will occur, our data do not allow us to reach this firm conclusion. Evaluation of those with IED, self-directed aggression, or both should include questions about the history of head injury with or without LOC.
1 VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. Washington, DC, US Department of Defense, Office of Veteran Affairs, 2009Google Scholar
2
3 : Update on the epidemiology of concussion/mild traumatic brain injury. Curr Pain Headache Rep 2015; 19:32Crossref, Medline, Google Scholar
4 : Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med 2008; 358:453–463Crossref, Medline, Google Scholar
5 : Post-concussion syndrome: prevalence after mild traumatic brain injury in comparison with a sample without head injury. Brain Inj 2012; 26:14–26Crossref, Medline, Google Scholar
6 : Postconcussion syndrome after mild traumatic brain injury in western Greece. J Trauma 2010; 69:789–794Crossref, Medline, Google Scholar
7 : Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on mild traumatic brain injury. J Rehabil Med 2004; 43(Suppl):84–105Crossref, Google Scholar
8 : Cognitive functioning and postconcussive symptoms in trauma patients with and without mild TBI. Arch Clin Neuropsychol 2006; 21:255–273Crossref, Medline, Google Scholar
9 : Methodological issues and research recommendations for prognosis after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil 2014; 95(Suppl):S265–S277Crossref, Medline, Google Scholar
10 : Will neuroimaging reveal a severe intracranial injury in this adult with minor head trauma? the Rational Clinical Examination Systematic Review. JAMA 2015; 314:2672–2681Crossref, Medline, Google Scholar
11 : Correlates and prevalence of aggression at six months and one year after first-time traumatic brain injury. J Neuropsychiatry Clin Neurosci 2017; 29:334–342Link, Google Scholar
12 : Contemporary approaches to the management of irritability and aggression following traumatic brain injury. Neuropsychol Rehabil 2003; 13:211–240Crossref, Medline, Google Scholar
13 : Traumatic brain injury in a county jail population: prevalence, neuropsychological functioning and psychiatric disorders. Brain Inj 2003; 17:731–741Crossref, Medline, Google Scholar
14 : Prevalence of traumatic brain injury in intimate partner violence offenders compared to the general population: a meta-analysis. Trauma Violence Abuse 2012; 13:77–82Crossref, Medline, Google Scholar
15 : Prevalence of traumatic brain injury in juvenile offenders: a meta-analysis. Child Neuropsychol 2013; 19:225–234Crossref, Medline, Google Scholar
16 : Aggression after traumatic brain injury: analysing socially desirable responses and the nature of aggressive traits. Brain Inj 2006; 20:1163–1173Crossref, Medline, Google Scholar
17 : Aggression after traumatic brain injury: prevalence and correlates. J Neuropsychiatry Clin Neurosci 2009; 21:420–429Link, Google Scholar
18 : Clinical correlates of aggressive behavior after traumatic brain injury. J Neuropsychiatry Clin Neurosci 2003; 15:155–160Link, Google Scholar
19 : Protective factors and risk modification of violence in Iraq and Afghanistan War veterans. J Clin Psychiatry 2012; 73:e767–e773Crossref, Medline, Google Scholar
20 : Factors associated with physical aggression among US Army soldiers. Aggress Behav 2012; 38:357–367Crossref, Medline, Google Scholar
21 : Violent behaviour in U.K. military personnel returning home after deployment. Psychol Med 2012; 42:1663–1673Crossref, Medline, Google Scholar
22 : Predicting non-familial major physical violent crime perpetration in the US Army from administrative data. Psychol Med 2016; 46:303–316Crossref, Medline, Google Scholar
23 : Prevalence of traumatic brain injury in cocaine-dependent research volunteers. Am J Addict 2015; 24:341–347Crossref, Medline, Google Scholar
24 : Orbitofrontal cortical thinning and aggression in mild traumatic brain injury patients. Brain Behav 2016; 6:e00581Crossref, Medline, Google Scholar
25 : Frontolimbic morphometric abnormalities in intermittent explosive disorder and aggression. Biol Psychiatry CNNI 2016; 1:32–38Google Scholar
26 : White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. AJNR Am J Neuroradiol 2008; 29:514–519Crossref, Medline, Google Scholar
27 : Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J Neurotrauma 2013; 30:657–670Crossref, Medline, Google Scholar
28 : Association between concussion and mental health in former collegiate athletes. Inj Epidemiol 2014; 1:28Crossref, Medline, Google Scholar
29 : Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psychiatry 2012; 169:577–588Crossref, Medline, Google Scholar
30 : Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, Psychiatric Institute, Biometrics Research, 1997Google Scholar
31 : Structured Interview for DSM-IV Personality Disorder: SIDP. Washington, DC, American Psychiatric Publishing, 1997Google Scholar
32 : Personality disorder—not otherwise specified evidence of validity and consideration for DSM-5. Compr Psychiatry 2012; 53:907–914Crossref, Medline, Google Scholar
33 : Initial reliability and validity of the Ohio State University TBI identification method. J Head Trauma Rehabil 2007; 22:318–329Crossref, Medline, Google Scholar
34 : Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Res 1997; 73:147–157Crossref, Medline, Google Scholar
35 : The Aggression Questionnaire. J Pers Soc Psychol 1992; 63:452–459Crossref, Medline, Google Scholar
36 : Life history of impulsive behavior: development and validation of a new questionnaire. J Psychiatr Res 2012; 46:346–352Crossref, Medline, Google Scholar
37 : Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol 1995; 51:768–774Crossref, Medline, Google Scholar
38 Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Publishing, 1994Google Scholar
39 : Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull 1993; 29:321–326Medline, Google Scholar
40 : White matter integrity in veterans with mild traumatic brain injury: associations with executive function and loss of consciousness. J Head Trauma Rehabil 2014; 29:21–32Crossref, Medline, Google Scholar
41 : Loss of consciousness is related to white matter injury in mild traumatic brain injury. J Neurotrauma 2016; 33:2000–2010Crossref, Medline, Google Scholar
42 : Early development of physical aggression and early risk factors for chronic physical aggression in humans. Curr Top Behav Neurosci 2014; 17:315–327Crossref, Medline, Google Scholar



