Characterizing the Link Between Glial Activation and Changed Functional Connectivity in National Football League Players Using Multimodal Neuroimaging
Abstract
Objective:
The primary objective of this preliminary study was to examine the impact of NFL play on interregional functional connectivity between two brain regions, the supramarginal gyrus (SMG) and the thalamus, identified as having higher binding of [11C]DPA-713 in NFL players. The authors’ secondary objective was to examine the effect of years since play on the interregional connectivity.
Methods:
Resting-state functional MRI was used to examine functional brain changes between regions with evidence of past injury in active or recently retired NFL players (defined as ≤12 years since NFL play) and distantly retired players (defined as >12 years since NFL play). Age-comparable individuals without a history of concussion or participation in collegiate or professional collision sports were included as a control group.
Results:
Compared with healthy control subjects, NFL players showed a loss of anticorrelation between the left SMG and bilateral thalami (mean z score=−2.434, p=0.015). No difference was observed when examining right SMG connectivity. The pattern of connectivity in active and recently retired players mimicked the pattern observed in distantly retired players and older control subjects.
Conclusions:
Further study of the clinical significance of this altered pattern of interregional connectivity in active and recently retired NFL players is needed.
There is growing concern that a subset of National Football League (NFL) players experience onset of cognitive and other neuropsychiatric symptoms that are linked to a history of sports-related concussion. Development of in vivo biomarkers to examine the neuropathological underpinnings of this linkage is paramount. The 2017 Berlin Concussion in Sport Group Consensus Statement (1, 2) indicates that multiple concussive blows may be a risk factor for cognitive impairment and mental health problems (e.g., depression) in some individuals and that more research is needed. In line with this, there is increased recognition of the neuropathological sequelae associated with recurrent concussive injuries (3). Currently, diagnosis of chronic traumatic encephalopathy, a tauopathy associated with a history of sports-related concussive events, is made at autopsy (4). There is strong interest in identifying in vivo biomarkers, which are crucial for longitudinal study of the pathological response to head trauma, prognostication efforts, and therapeutic monitoring. In the present study, we used the novel approach of obtaining the results from prior [11C]DPA-713 positron emission tomography (PET) analyses to guide a focused study of resting-state functional MRI (rsfMRI) data acquired from NFL players and healthy control subjects.
In two previous independent studies, our research group used [11C]DPA-713 with PET to image a marker of injury or repair in the brains of active and retired NFL players (5, 6). [11C]DPA-713 binds specifically to the translocator protein 18 kDa that is increased in its expression by activated microglia and reactive astrocytes after brain injury (7). The binding of [11C]DPA-713 was higher in bilateral supramarginal gyri (SMG) and bilateral thalami in NFL players compared with healthy control subjects. The purpose of the present analysis was to link evidence of brain injury and repair (i.e., [11C]DPA-713 findings) to altered brain function (i.e., rsfMRI results) by using the paradigm that prolonged glial activation may be linked to the pathophysiology of neurodegeneration after concussive injuries. Previous studies of altered cerebral functional connectivity using rsfMRI (8–10) and task-based functional MRI (fMRI) (11–15) in athletes with a history of concussive events have yielded inconsistent results and have not undertaken a focused analysis based on the results of PET imaging. In the present preliminary analysis, our primary objective was to examine the impact of NFL play on interregional connectivity between the SMG and the thalamus by using rsfMRI. We hypothesized that evidence of disrupted functional connectivity between these two regions would be found in NFL players compared with healthy control subjects. Our secondary objective was to examine the effect of years since play on the interregional connectivity.
Methods
Participants
This cross-sectional case-control study was approved at two different time points by the institutional review board at Johns Hopkins University, because the study populations from our two previous studies (5, 6) were combined for the present study. The first protocol involved recruitment of older, former NFL players, whereas the second protocol involved recruitment of younger, active or recently retired players. All methods pertaining to the present analyses were identical and previously described in both prior studies (5, 6). It is noteworthy that prior reported results from this study population included results for participants with PET imaging data regardless of rsfMRI data collection. In the present study, only individuals with both PET and rsfMRI data were included. In addition to NFL players and alumni, age-comparable healthy control subjects were recruited. Details about NFL play and past concussion, as defined by the Quality Standards Subcommittee of the American Academy of Neurology (16), were obtained via self-report. Importantly, all control subjects denied a history of concussion and participation in collegiate or professional collision sports. Written informed consent was obtained from all participants.
Image Acquisition
Participants underwent brain MRI on a Philips Achieva 3-Tesla scanner (Andover, Mass.), with a 32-channel head matrix coil, and [11C]DPA-713 PET imaging at a single time point. Details of the PET imaging have been described previously (5, 6). For structural T1-weighted imaging, three-dimensional magnetization-prepared rapid gradient-echo (MPRAGE) sequence was used (TR=6.73 ms, TE=3.1 ms, inversion time=842 ms, flip angle=8°, resolution=1.0×1.0×1.2 mm3, SENSitivity Encoding [SENSE] acceleration factor=2). The T2*-weighted blood-oxygen-level-dependent functional images for rsfMRI were acquired using two-dimensional gradient-echo echo-planar imaging (TR=2,000 ms, TE=30 ms, flip angle=75°, acquisition matrix=80×80×37, slice thickness=3 mm, slice gap=1 mm, acquisition=ascending, SENSE acceleration factor=2, volumes acquired=210). Participants were given the following instructions: “keep still,” “keep eyes closed,” and “do not fall asleep.”
Image Processing
Imaging data were processed with Statistical Parametric Mapping version 12 (Wellcome Department of Imaging Neuroscience, University College London) and custom MATLAB (MathWorks, Natick, Mass.) scripts. After slice timing and motion correction, the ArtRepair toolbox (https://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html) was used to tag outlier fMRI volumes with excessive noise related to motion and global signal changes. Images were coregistered to MPRAGE images and normalized to a Montreal Neurological Institute template. CSF and white matter masks were generated during the unified segmentation process with normalization. An anatomical component-based noise-correction method (CompCor) (17) was utilized for nuisance regression, with 95% of the signal variance within each mask used as regressors. Bandpass filtering was performed at 0.1–0.01 Hz, and motion volume censoring was conducted to remove volumes tagged by ArtRepair as outlier volumes. Finally, spatial smoothing was performed with a Gaussian kernel of 6-mm full width at half maximum.
Image Analysis
For each study subject, 6-mm spherical regions of interest were placed at the SMG and thalami bilaterally. As described above, these regions were chosen on the basis of results of [11C]DPA-713 PET imaging. To minimize multiple comparison concerns, only the SMG-thalami connectivity was examined. The eigenvariate of the time course was extracted, and Pearson’s correlation coefficients were calculated between time courses for each side of the SMG and the mean time course for bilateral thalami. Because the range and mean of connectivity measured by Pearson’s correlations may vary across individuals on the basis of nonphysiological factors, the data were normalized through z-score transformation to allow between-subject comparison.
Statistical Analysis
Demographic characteristics were compared with regard to descriptive variables by using Fisher’s exact (proportions) and Mann-Whitney U (continuous) tests to assess for statistical significance. For our primary outcome, the Mann-Whitney U test was used to compare the mean z-score value calculated for each participant between bilateral thalami and the left and right SMG in the NFL group compared with the healthy control group. For the subgroup analysis examining the influence of years since play, NFL players were divided into active or recently retired players (defined as <12 years since NFL play) and distantly retired players (defined as >12 years since NFL play). This categorization was derived empirically on the basis of histograms, which showed an unnatural gap in years since NFL play created by the original participant recruitment (one study of older, former players and the other study of younger, active or recently retired players). Healthy control subjects were divided into equivalent groupings on the basis of age. By using the Mann-Whitney U test, each subgroup was compared with age-matched control subjects, and then the two NFL subgroups were compared with each other. Statistical significance was set at a p value <0.05.
Results
rsfMRI data were successfully obtained for 24 NFL players (one study subject was excluded as a result of positive urine toxicology) and 21 healthy control subjects (three study subjects were unable to tolerate the MRI scan, and one required lorazepam before scanning) from the pool of 25 NFL players and 25 healthy control subjects who completed [11C]DPA-713 PET scanning. The median age of NFL players and control subjects was 37.5 years (interquartile range, 30–63) and 29.0 years (interquartile range, 26–55), respectively, with no difference between the two study groups (U test=−1.914, p=0.06). There was no difference in race (Fisher’s exact test=0.0086, p=1.000), with 25% (N=6/24) of study subjects in the NFL group and 24% (N=5/21) in the control group self-reporting as African American and the remaining participants self-reporting as Caucasian. The median number of concussions reported among NFL players was 4.0 (interquartile range, 3–12). Excluding three active players, the median number of years since NFL play was 9.0 (interquartile range, 1.9–32).
Mean correlation coefficients for left and right SMG-bilateral thalami connectivity are shown in Figures 1 and 2, respectively. Statistical results of primary and subgroup analyses are summarized in Table 1. When comparing all NFL players and all control subjects, there was a significant difference in left SMG-bilateral thalami functional connectivity (mean z score=−2.434, p=0.015). Even when restricting the analysis to active and recently retired NFL players (N=14) and respective control subjects (N=15), this statistical significance remained (z=−2.226, p=0.026). There was no difference in interregional connectivity between distantly retired NFL players (N=10) and respective control subjects (N=6) or between the two NFL subgroups. The interconnectivity between the right SMG and bilateral thalami showed no difference between the NFL and control groups in any of the analyses.
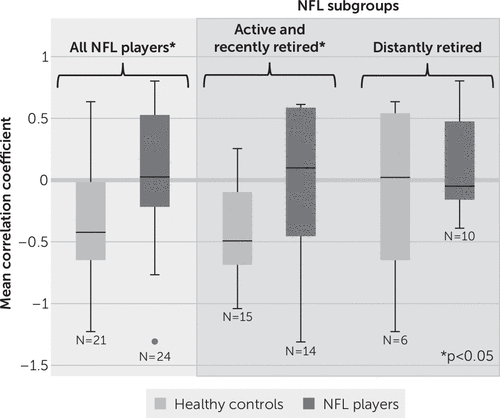
FIGURE 1. Mean correlation coefficients in the left supramarginal gyri and bilateral thalami for all participants and each subgroupa
a Analyses are presented for all participants and for subgroups on the basis of years since National Football League (NFL) play (active and recently retired subgroup, ≤12 years since play; distantly retired subgroup, ≥12 years since play). Healthy control subjects had no history of concussion or participation in collegiate or professional collision sports. An asterisk denotes statistical significance (p<0.05).
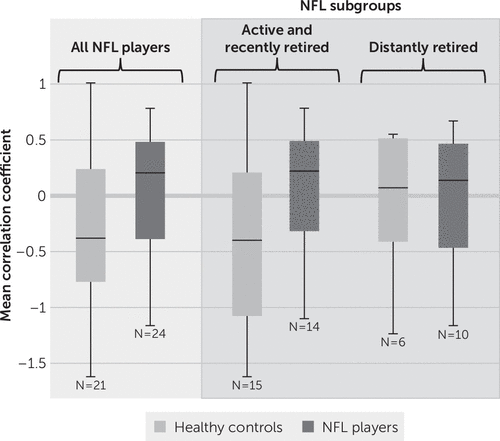
FIGURE 2. Mean correlation coefficients in the right supramarginal gyri and bilateral thalami for all participants and each subgroupa
a Analyses are presented for all participants and for subgroups on the basis of years since National Football League (NFL) play (active and recently retired subgroup, ≤12 years since play; distantly retired subgroup, ≥12 years since play). Healthy control subjects had no history of concussion or participation in collegiate or professional collision sports.
| Left SMG-bilateral thalami | Right SMG-bilateral thalami | |||
|---|---|---|---|---|
| Variable | z Score | p | z Score | p |
| All players versus healthy control subjects | –2.434 | 0.015* | –1.638 | 0.101 |
| Active and recently retired players versus healthy control subjects | –2.226 | 0.026* | –1.746 | 0.081 |
| Distantly retired players versus healthy control subjects | –0.217 | 0.828 | –0.325 | 0.745 |
| Distantly retired players versus Active and recently retired players | 0.176 | 0.861 | 0.234 | 0.815 |
TABLE 1. Statistical results for analyses of the supramarginal gyri (SMG) and bilateral thalami in all participants and each subgroupa
Discussion
We used results from [11C]DPA-713 PET to guide rsfMRI-based testing of functional brain changes between regions with evidence of past injury. This focused, multimodal approach (i.e., using PET imaging to guide a targeted rsfMRI analysis) mitigated multiplicity with associated risk of type I error and was aimed to link a relevant biomarker for injury to functional brain changes. We found a loss of anticorrelation between the left SMG and bilateral thalami in NFL players compared with healthy control subjects (Figure 1). This altered pattern of interregional connectivity was related to time since play, with a subgroup analysis showing differences between control subjects and active and recently retired players but not distantly retired players. The connectivity between the right SMG and bilateral thalami showed a similar loss of anticorrelation. However, the results did not reach statistical significance (Figure 2). Loss of either correlation or anticorrelation may be construed as weakening of the functional relationship between the regions interrogated, possibly as a result of neurological injury (18, 19). Interestingly, the pattern of connectivity in active and recently retired NFL players more closely resembled the pattern observed in older control subjects and distantly retired players (Figure 1) than that observed in younger control subjects.
Our results should be interpreted with several limitations. First, because the data were derived from a study using expensive PET imaging, the sample size was modest, preventing analyses such as examination of the independent influence of active NFL play and the number of years of NFL play. Second, as a result of the nature of the study recruitment (originally two studies), there was an unconventional gap in years since play and in age between the two player groups (active or recently retired and distantly retired). Additionally, although not statistically significant, there was a noticeable difference in the median age and distribution between NFL players and control subjects. Third, the finding of functional disruption was only significant within the left SMG and bilateral thalami. This may reflect divergence of function between the homotopic SMG regions and left side-specific higher-cognitive regions, such as that of language (e.g., Geschwind’s region). In addition, although we assumed that the anticorrelation observed in younger healthy control subjects was the nonpathological condition, our interpretation would be informed best by further studies of healthy individuals. Lastly, the cross-sectional design did not allow for testing of the causal influence of concussion on these differences in functional connectivity between NFL players and control subjects or the influence of multiple concussions.
Our preliminary findings provide further evidence of fMRI changes following concussive injuries in American football players (11–13). The different functional connectivity pattern between groups suggests that this methodological approach may be useful in studying the relationship between injury and altered functional connectivity in the brains of collision-sport athletes. Unfortunately, the specific nature of the functional role of the SMG-thalamus connection is lacking in the literature. Further investigation, including study of the clinical significance (e.g., influence on cognitive and other neuropsychiatric symptoms) of the altered connectivity pattern between the left SMG and bilateral thalami observed in active and recently retired NFL players, is needed.
1 : 5th International Conference on Concussion in Sport (Berlin). Br J Sports Med 2017; 51:837Crossref, Medline, Google Scholar
2 : A systematic review of potential long-term effects of sport-related concussion. Br J Sports Med 2017; 51:969–977Crossref, Medline, Google Scholar
3 : Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 2017; 318:360–370Crossref, Medline, Google Scholar
4 : The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016; 131:75–86Crossref, Medline, Google Scholar
5 : Neuroinflammation and brain atrophy in former NFL players: an in vivo multimodal imaging pilot study. Neurobiol Dis 2015; 74:58–65Crossref, Medline, Google Scholar
6 : Imaging of glial cell activation and white matter integrity in brains of active and recently retired national football league players. JAMA Neurol 2017; 74:67–74Crossref, Medline, Google Scholar
7 : 11C-DPA-713: a novel peripheral benzodiazepine receptor PET ligand for in vivo imaging of neuroinflammation. J Nucl Med 2007; 48:573–581Crossref, Medline, Google Scholar
8 : Changes in functional connectivity of the brain associated with a history of sport concussion: a preliminary investigation. Brain Inj 2017; 31:39–48Crossref, Medline, Google Scholar
9 : Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage 2012; 59:511–518Crossref, Medline, Google Scholar
10 : A potential biomarker in sports-related concussion: brain functional connectivity alteration of the default-mode network measured with longitudinal resting-state fMRI over thirty days. J Neurotrauma 2015; 32:327–341Crossref, Medline, Google Scholar
11 : Hypoconnectivity and hyperfrontality in retired American football players. Sci Rep 2013; 3:2972Crossref, Medline, Google Scholar
12 : The role of location of subconcussive head impacts in fMRI brain activation change. Dev Neuropsychol 2015; 40:74–79Crossref, Medline, Google Scholar
13 : Effects of career duration, concussion history, and playing position on white matter microstructure and functional neural recruitment in former college and professional football athletes. Radiology 2018; 286:967–977Crossref, Medline, Google Scholar
14 : Do brain activation changes persist in athletes with a history of multiple concussions who are asymptomatic? Brain Inj 2012; 26:1217–1225Crossref, Medline, Google Scholar
15 : Lack of long-term fMRI differences after multiple sports-related concussions. Brain Inj 2012; 26:1684–1696Crossref, Medline, Google Scholar
16 : Practice parameter: the management of concussion in sports (summary statement): Report of the Quality Standards Subcommittee. Neurology 1997; 48:581–585Crossref, Medline, Google Scholar
17 : A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007; 37:90–101Crossref, Medline, Google Scholar
18 : Early functional connectome integrity and 1-year recovery in comatose survivors of cardiac arrest. Radiology 2018; 287:247–255Crossref, Medline, Google Scholar
19 : Hyperconnectivity is a fundamental response to neurological disruption. Neuropsychology 2015; 29:59–75Crossref, Medline, Google Scholar



