Clinical Correlates of Dream Enactment Behaviors in Previously Deployed OEF/OIF/OND Veterans: An Exploratory Analysis
Abstract
Objective:
Veterans with posttraumatic stress disorder (PTSD) frequently report dream enactment behavior (DEB). Although DEBs are associated with PTSD symptoms, their relationship with other sleep disorders, including REM behavior disorder, warrants reexamination of their clinical correlates.
Methods:
The investigators used a cross-sectional, exploratory analysis to compare demographic and clinical characteristics of veterans endorsing regularly occurring DEB compared with those endorsing no or infrequent DEB. The participants comprised a convenience sample of servicemembers who were previously deployed to Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn (OEF/OIF/OND) and enrolled in an ongoing cohort study.
Results:
Of the 78 eligible participants, 19 (24.4%) endorsed DEBs occurring at least once per week in the past month. Compared with participants who reported no or infrequent DEBs, participants with regularly occurring DEBs had poorer sleep quality, greater PTSD severity, a higher number of reported mild traumatic brain injuries (mTBI) with loss of consciousness, and a higher likelihood of being diagnosed with sleep disorders. After adjustment for global sleep quality, a significant association persisted between DEBs and the number of mTBI with loss of consciousness but not between DEBs and the severity of PTSD symptoms.
Conclusions:
These results suggest that mTBI may disrupt neural circuits regulating sleep among OIF/OEF/OND veterans. Prospective, polysomnographic assessment of muscle tone and behavioral events during REM sleep is needed to characterize the physiology of DEBs in this population.
Nightmares and insomnia are the only sleep symptoms included in current diagnostic criteria for posttraumatic stress disorder (PTSD) (1); however, patients with PTSD report a variety of sleep complaints beyond these core symptoms (2). Other disruptive sleep abnormalities include dream enactment behaviors (DEBs), which are movements or vocalizations, such as kicking, thrashing, or yelling, that are presumed to occur in response to dream content during sleep (2, 3). DEBs were previously described as a manifestation of PTSD-related phenomena (3–5). However, the differential diagnosis of DEB is broader. Associated conditions include REM sleep behavior disorder (RBD), non-REM parasomnias, nocturnal epilepsy, and secondary manifestations of sleep disorders, such as severe obstructive sleep apnea (OSA) or periodic limb movement disorder (PLMD) (6–8). From a clinical standpoint, attribution of DEB phenomena solely to the PTSD syndrome in a given patient may lead to missed diagnoses of concurrent sleep disorders that have substantial clinical implications if left untreated or unrecognized.
RBD is one of the most important diagnostic considerations in a patient with DEB. In part, this is because the presence of RBD can herald the onset of neurodegeneration, most commonly synucleinopathies such as Parkinson’s disease (9). The relevance of whether DEB in a patient with PTSD represents RBD is underscored in OIF/OEF/OND veterans, because mild traumatic brain injury (mTBI) (10, 11) and PTSD itself (12) are reported to contribute to future neurodegeneration risk. In addition, clinical conditions that may be present in individuals with PTSD may predispose to RBD (e.g., antidepressant use and alcohol withdrawal) (9) or are associated with future neurodegeneration (i.e., depressive disorders) (13). As these recently deployed veterans age, there is a critical need to address risk assessment, including early identification of neurodegenerative biomarkers.
Examination of clinical correlates between PTSD, DEB, and mTBI in post-9/11 U.S. veterans is an appropriate first step to understanding their potential clinicopathological relationships and the utility of incorporating standardized, polysomnographic assessments in future study designs. To examine the hypothesis that DEBs may be associated with clinical factors beyond PTSD symptoms, including other sleep disorders, traumatic brain injury (TBI) exposures, and antidepressant use, we performed an exploratory analysis using data from the Houston branch of the Translational Research Center for TBI and Stress Disorders (TRACTS), an ongoing national cohort study of post-9/11 veterans with high rates of TBI exposures, PTSD, and sleep disturbances (14, 15).
Methods
Participants
Participants comprised the first 100 individuals enrolled in the Houston TRACTS cohort from July 2015 to October 2017. Eligible participants were male and female OIF/OEF/OND veterans, ages 18–65 years, with at least one deployment (Figure 1). Exclusionary criteria were diagnosed neurological illnesses other than TBI (e.g., multiple sclerosis, stroke, and history of seizures not related to TBI), schizophrenia, schizoaffective or bipolar disorder, and active suicidal or homicidal ideation requiring crisis intervention. This analysis excluded veterans with a history of moderate to severe TBI, given the differences in functional and cognitive outcomes between mTBI and moderate or severe TBI and the relatively small number of veterans with moderate to severe TBI in the Houston cohort. Participants were required to complete the clinical interview described below and to have a history of exposure to at least one lifetime emotionally traumatic event. Recruitment sources included clinician referral from the post-deployment, primary care, and mental health clinics at the Michael E. DeBakey Veterans Affairs (VA) Medical Center (MEDVAMC), advertisements at MEDVAMC and veteran community organizations, and letters to prescreened OIF/OEF/OND veterans seeking care at MEDVAMC. The institutional review board at Baylor College of Medicine and the research and development program of MEDVAMC approved the study protocol.
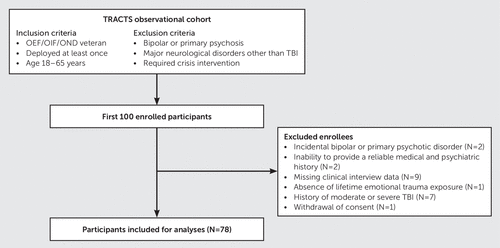
FIGURE 1. Flowchart of the study participantsa
a OEF/OIF/OND=Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn, TBI=traumatic brain injury, TRACTS=Translational Research Center for Traumatic Brain Injury and Stress Disorders.
Assessments
After providing informed consent, participants completed a psychiatric diagnostic interview, as detailed previously (15). Mood, anxiety, alcohol, and substance use disorders were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders Non-Patient Edition (SCID-IV) (16). The Clinician-Administered PTSD Scale for DSM-IV (CAPS-IV) (17) was used to assess the presence or absence of current PTSD and its severity. Lifetime TBI exposure was measured with the Boston Assessment of TBI-Lifetime (BAT-L), a semistructured interview designed to assess blast and TBI exposures before, during, and after military service (18). The presence and severity of TBI and results of the CAPS-IV and SCID-IV assessments were reviewed in consensus meetings of senior TRACTS investigators to ensure uniformity in assessment administration. The Pittsburgh Sleep Quality Index (PSQI) (19) was used to assess sleep quality in the past month, with a global score >5 indicating poor sleep quality.
Evidence for DEB events was identified with the PTSD Addendum of the PSQI (PSQI-A). The PSQI-A, a seven-item scale originally developed as a sleep-related measure of PTSD symptoms, assesses the frequency of disruptive nocturnal behaviors frequently reported by patients with PTSD (3). In a sample of military veterans, a total score ≥4 has a sensitivity of 71% and a specificity of 82% for a clinical diagnosis of PTSD (4). Participants are asked to identify the frequency of symptoms interfering with their sleep in the past month, including hot flashes, general nervousness, trauma-related memories or nightmares, trauma-unrelated anxiety, and DEBs.
To identify DEB specifically, PSQI-A item 1g asks whether participants had “episodes of ‘acting out’ their dreams, such as kicking, punching, running, or screaming.” Responses are scored on an ordinal scale: 0 (not in the past month), 1 (less than once a week), 2 (once or twice a week), or 3 (three or more times a week). Because DEBs occur occasionally in healthy individuals (20), Item 1g scores were dichotomized such that scores of 0 or 1 (DEB−) versus 2 or 3 (DEB+) distinguished participants with regularly occurring, frequent, and more likely pathological DEB events.
For each participant, the presence of sleep disorder diagnoses was assessed via a systematic review of sleep medicine consults, sleep study reports, problem lists, and progress notes in VA and non-VA records. When available, the location, type, date, and diagnostic impression included in polysomnographic reports were recorded.
Currently prescribed medications were obtained from the medication list in electronic medical records and confirmed by participant self-report. Participants also completed standardized questionnaires to obtain demographic information and deployment history (15).
Statistical Analysis
The primary outcome was the frequency of DEB, dichotomized as events occurring at least once per week (DEB+) versus no or infrequent events (DEB−) in the past month, as described above. Sociodemographic characteristics (age, sex, marital status, etc.), mTBI exposure, current PTSD status, known sleep disorder history, antidepressant use, and additional psychiatric predictor variables were compared between patients with DEBs occurring at least once per week (DEB+) and those with no or infrequent DEBs (DEB−), using the Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables (Table 1). Next, variables with a p value <0.1 in the previous step were included for stepwise model selection in a multivariable logistic regression model, with DEB frequency (DEB+ versus DEB−) as the binary outcome. The entry and removal threshold were both set at a p value of 0.1 in the stepwise selection procedure. Because the total PSQI score is expected to be highly correlated with the outcome and may overshadow other important covariates, we conducted two sets of models by including or excluding the PSQI total score within the independent variables, respectively. We examined the multicollinearity among the variables and dropped the ones that were highly correlated with others. A p value <0.05 was considered statistically significant. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, N.C.).
| Characteristic | Overall | DEB+ (N=19)b | DEB– (N=59)c | pd | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Gender | |||||||
| Male | 72 | 92.31 | 19 | 100.00 | 53 | 89.83 | 0.556 |
| Female | 6 | 7.69 | 0 | 0.00 | 6 | 10.17 | |
| Race/ethnicity | |||||||
| White | 28 | 35.90 | 6 | 31.58 | 22 | 37.29 | 0.773 |
| Black | 23 | 29.49 | 5 | 26.32 | 18 | 30.51 | |
| Hispanic or Latino | 19 | 24.36 | 5 | 26.32 | 14 | 23.73 | |
| Other | 8 | 10.26 | 3 | 15.79 | 5 | 8.47 | |
| Married | 37 | 47.44 | 10 | 52.63 | 27 | 45.76 | 0.792 |
| Education | |||||||
| 12 years or GED | 17 | 21.79 | 4 | 21.05 | 13 | 22.03 | 0.657 |
| Some college | 40 | 51.28 | 8 | 42.11 | 32 | 52.24 | |
| College degree | 14 | 17.95 | 5 | 26.32 | 9 | 15.25 | |
| Postgraduate degree | 7 | 8.97 | 2 | 10.53 | 5 | 8.47 | |
| Employed full-time or in school | 60 | 76.92 | 12 | 63.16 | 48 | 81.36 | 0.123 |
| Served in the Army | 49 | 62.82 | 13 | 68.42 | 36 | 61.02 | 0.599 |
| Military service affiliated | 75 | 96.15 | 19 | 100.00 | 56 | 94.92 | 1.000 |
| Lifetime mTBI | |||||||
| None | 8 | 10.26 | 1 | 5.26 | 7 | 11.86 | 0.500 |
| Single event | 26 | 33.33 | 5 | 26.32 | 21 | 35.59 | |
| >1 event | 44 | 56.41 | 13 | 68.42 | 31 | 52.54 | |
| Sleep disorder | 30 | 38.46 | 12 | 63.16 | 18 | 30.51 | 0.015 |
| Current PTSD | |||||||
| None | 37 | 47.44 | 4 | 21.05 | 33 | 55.93 | 0.009 |
| Yes | 34 | 43.59 | 11 | 57.89 | 23 | 38.98 | |
| Partiale | 7 | 8.97 | 4 | 21.05 | 3 | 5.08 | |
| Current mood disorder | 28 | 35.90 | 9 | 47.37 | 19 | 32.20 | 0.276 |
| Current alcohol abuse or dependence | 10 | 12.82 | 3 | 15.79 | 7 | 11.86 | 0.699 |
| Current cannabis abuse or dependence | 7 | 8.97 | 1 | 5.26 | 6 | 10.17 | 1.000 |
| Current tobacco use | 25 | 32.05 | 7 | 36.84 | 18 | 30.51 | 0.778 |
| Antidepressant usef | 37 | 48.68 | 12 | 66.67 | 25 | 43.10 | 0.107 |
| Selective serotonin reuptake inhibitor | 14 | 17.95 | 5 | 26.32 | 9 | 15.25 | 0.310 |
| Serotonin norepinephrine reuptake inhibitor | 6 | 7.69 | 3 | 15.79 | 3 | 5.08 | 0.151 |
| Mirtazapine, trazodone, or bupropion | 8 | 10.26 | 1 | 5.26 | 7 | 11.86 | 0.671 |
| More than one class of antidepressants | 9 | 11.54 | 3 | 15.79 | 6 | 10.17 | 0.680 |
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 34.71 | 6.40 | 33.42 | 4.27 | 35.12 | 6.93 | 0.556 |
| Body mass indexf | 32.04 | 5.55 | 34.18 | 4.60 | 31.38 | 5.69 | 0.075 |
| Time since last deployment (months)g | 82.83 | 34.86 | 76.79 | 32.34 | 84.81 | 35.69 | 0.471 |
| CAPS-IV total score | 47.06 | 26.39 | 62.63 | 28.38 | 42.05 | 23.87 | 0.005 |
| PSQI total score | 12.64 | 3.92 | 15.68 | 2.89 | 11.66 | 3.71 | <0.001 |
| Number of blast exposures ≤10 metersg | 2.62 | 4.25 | 4.05 | 5.56 | 2.16 | 3.66 | 0.27 |
| Number of mTBIs with LOC | 1.14 | 1.12 | 1.68 | 1.20 | 0.97 | 1.05 | 0.017 |
TABLE 1. Demographic and clinical characteristics of study participants, overall and by dream enactment behavior (DEB) frequency (N=78)a
Results
Of the first 100 enrolled participants in the Houston TRACTS cohort, 78 were included in the planned analyses (Figure 1). Twenty-two enrollees were excluded for the following reasons: diagnoses of bipolar disorder (N=1) and primary psychosis (N=1), inability to provide a reliable medical and psychiatric history (N=2), missing data from the clinical interview (N=9), history of moderate or severe TBI (N=7), absence of lifetime emotional trauma exposure (N=1), and withdrawal of consent (N=1).
Demographic and clinical characteristics of the analyzed sample are summarized in Table 1. Overall, 30.7% (N=24) of participants had OSA. In addition, two participants had OSA comorbid with PLMD or central alveolar hypoventilation, respectively; two had PLMD alone; one had bruxism causing arousals; and one had narcolepsy with cataplexy. These diagnoses were identified from one or more diagnostic laboratory-based sleep studies available for 27 participants (34.6%) or from continuous positive airway pressure titration studies, home studies, or other records available for 11 participants (14.1%).
In the analyzed sample, 24.4% (N=19) endorsed DEBs occurring at least once per week in the past month (DEB+), and 76.6% (N=59) were categorized as having no or infrequent DEBs (DEB−). The DEB+ group had higher CAPS-IV scores (62.63 [SD=28.38] versus 42.05 [SD=23.87], p=0.005), higher PSQI total scores (15.68 [SD=2.89] versus 11.66 [SD=3.71], p<0.001), a higher number of mTBIs with loss of consciousness (LOC) (1.68 [SD=1.20] versus 0.97 [SD=1.05], p=0.015), and a greater likelihood of having a sleep disorder diagnosis (63.2% versus 30.5%, p=0.015) compared with the DEB− group. There was not a statistically significant difference in the time since last deployment between groups (Table 1).
In the first set of model selection that excluded the PSQI total score, we selected a multivariable model (model 1) that included the CAPS-IV total score (odds ratio=1.028, 95% CI=1.006−1.051, p=0.013) and the number of mTBIs with LOC (p=0.065) (Table 2). However, when the PSQI total score was included in the first step of the model selection, the identified model (model 2) no longer included the CAPS-IV score (Table 2). The selected model suggested that regularly occurring DEB was positively associated with the number of mTBIs with LOC (odds ratio=1.879, 95% CI=1.100–3.210, p=0.021), as well as the PSQI total score (odds ratio=1.588, 95% CI=1.219–2.068, p<0.001).
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient estimate | SE | Odds ratio | 95% CI | Coefficient estimate | SE | Odds ratio | 95% CI | Coefficient estimate | SE | Odds ratio | 95% CI |
| mTBI LOC | 0.449 | 0.243 | 1.567 | 0.973, 2.522 | 0.631* | 0.273 | 1.879 | 1.100, 3.210 | 0.605* | 0.279 | 1.83 | 1.059, 3.164 |
| CAPS-IV total score | 0.028* | 0.011 | 1.028 | 1.006, 1.051 | – | – | – | – | 0.005 | 0.013 | 1.005 | 0.979, 1.031 |
| PSQI total score | – | – | – | – | 0.462*** | 0.135 | 1.588 | 1.219, 2.068 | 0.438** | 0.146 | 1.55 | 1.163, 2.066 |
TABLE 2. Multivariable logistic regression models for regularly occurring dream enactment behaviorsa
Indeed, when incorporating the CAPS-IV total score into the model that adjusted for the PSQI total score and the number of mTBIs with LOC (model 3), the CAPS-IV total score became nonsignificant (p=0.702) with reduced effect size. Considering the criteria for establishing mediation (21), as well as the positive correlation between the CAPS-IV and PSQI total scores (Spearman’s rho=0.48, p<0.001), the results collectively suggest that the PSQI total score largely explained and mediated the relationship between CAPS-IV scores and DEBs.
Discussion
To gain insights into the potential pathophysiology of the previously reported association of DEBs with PTSD symptom severity (3–5), we examined relationships between DEB, PTSD, sleep disorder diagnoses, and mTBI in a sample of previously deployed OEF/OIF/OND veterans. We found regularly occurring and disruptive DEBs to be most strongly associated with the number of mTBIs with LOC, the PSQI total score (i.e., the severity of global sleep impairment), and the CAPS-IV total score (i.e., increased PTSD symptom severity). After adjusting for the PSQI total score, the number of mTBIs with LOC remained a significant predictor of regularly occurring DEBs (DEB+). In contrast, after adjusting for the PSQI total score, the association with PTSD severity became statistically nonsignificant, suggesting that the relationship between PTSD severity and regularly occurring DEB is mediated by the severity of global sleep impairment (i.e., PSQI total score).
Our data indicate that the relationship between PTSD severity and DEB frequency may be explained by the severity of global sleep impairment as opposed to a specific impact of the PTSD syndrome, for example, to the pathophysiology of intrusive symptoms. Global sleep impairment can be related to multiple etiological factors, including the presence of other sleep disorders (e.g., OSA), mTBI, and other measures of psychological distress.
Our finding of an association between DEBs and the number of mTBIs with LOC highlights the need for standardized polysomnographic assessment with additional upper-limb electrodes to investigate whether DEB represents REM sleep alterations in veterans. Classically, REM parasomnias present with recall of abnormal behaviors during sleep (22). DEBs are considered surrogate markers of altered REM architecture, namely the loss of REM atonia that accompanies RBD; as such, clinical and screening assessments for this REM parasomnia include self-reports or bed-partner reports of DEBs (23, 24).
While it is unknown whether repeated mTBIs directly injure or initiate a neurodegenerative process affecting the neural structures regulating REM atonia or their connections, several lines of evidence suggest that the association between TBI and DEB requires further examination. For example, in a sample of 54 participants with TBIs of differing severities who underwent polysomnography, seven (13%) of the participants were diagnosed with RBD (25). In a case-control study comprising 694 patients, TBI with LOC was associated with higher odds of RBD (11). Furthermore, animal models have demonstrated that blast-induced injury affects brainstem white matter (26, 27), but it is unknown whether these injuries affect the connections of neural circuits regulating REM sleep.
Although none of the participants in the present study were diagnosed with REM parasomnias, we cannot exclude the presence of these parasomnias in this sample, because these diagnoses were not the focus of clinical assessments identified via medical record reviews. In clinical practice, the common attribution of PTSD as a basis for DEBs among veterans with PTSD may preclude or distract from proactive, reliable, and valid assessments of other sleep disorders in veterans with PTSD, who may, for example, otherwise demonstrate evidence for REM atonia loss, complex or simple movements, vocalizations, or other REM behavioral events (28) on sleep disorder screening instruments or polysomnogram recordings. Furthermore, in the absence of focused testing for REM parasomnias, polysomnographic evaluations often are biased toward assessments for sleep-disordered breathing, fail to include upper-limb electromyography (EMG), and lack standardized approaches to polysomnographic interpretation of muscle tone during REM sleep. Our finding of an association between DEBs and the number of mTBIs with LOC provides support for incorporation of optimal EMG derivations and scoring methods to further characterize the polysomnographic correlates of DEB in OEF/OIF/OND veterans.
As an alternative explanation, a recently proposed parasomnia, trauma-associated sleep disorder, describes nocturnal disruptive behaviors in the context of trauma exposure, with more severe symptoms occurring in individuals most recently exposed (29). However, in our study sample, we did not find a statistically significant association between time since deployment and DEBs. Although antidepressants may increase muscle tone during REM sleep and possibly unveil RBD (30), antidepressant use was not a statistically significant predictor of DEB in our sample, again highlighting the need for more accurate polysomnographic and medication assessments in future studies of these veterans
The purpose of this exploratory analysis was to examine correlates of DEB that would form the basis for future hypothesis-driven analyses in the overall TRACTs cohort and design of prospective studies. As such, we recognize the limitations of this investigation.
Retrospective assessment limits the accuracy of self-reported TBI exposures. We surmise that DEBs are specifically associated with the number of mTBIs with LOC as opposed to other TBI indices (e.g., the number of blast exposures) because the number of TBIs with LOC has been reported to be associated with cognitive and behavioral outcomes among individuals who self-report TBI (31), as well as due to the challenges of eliciting an accurate history of mTBI on a retrospective basis. For example, distinguishing anxiety or surprise in a combat situation from the experience of an altered mental status is especially difficult. Although the BAT-L was designed to assist in discriminating psychological shock from an alteration of consciousness (18), the validity of self-report years after the event is not well established.
Migraines have been associated with DEBs (32); however, standardized headache histories were not available to replicate this association in our study sample. Because a detailed assessment of medication compliance was not performed, the actual rates of antidepressant exposure remain unclear. Our measurement of DEBs relied on a single question from the PSQI-A, and the psychometric properties of this single question are unknown; a validated assessment of DEBs in this population requires systematic investigation of sleep physiology.
Our sample size of the first 100 enrolled individuals was determined arbitrarily for the purposes of conducting a hypothesis-generating exploratory analysis. The total number of participants classified as DEB+ (N=19), while relatively small, comprises nearly a quarter of the participants included for analysis. However, the relatively small sample size allows for detection of medium to large effect sizes only. For example, the detectable standardized mean difference between the two DEB groups was a Cohen’s d of 0.7 with an alpha of 0.05 and 80% power. Therefore, it is possible that we did not identify predictors of DEBs with small to medium effect sizes. Additionally, this convenience sample of mostly male volunteer research subjects receiving VA medical care may not represent the entire OEF/OIF/OND veteran population.
We attempted to identify sleep disorder diagnoses as extensively as possible via thorough chart reviews. We found that the most prevalent sleep disorder diagnosis, among those evaluated for such, was OSA. Although other sleep disorders were not associated with DEB in our multivariate model, the lack of prospective polysomnography and positive airway pressure usage data limits determination of whether sleep-disordered breathing or other sleep-related movement disorders predict regularly occurring DEBs. Because obstructive events occur more frequently in REM sleep, movements associated with microarousals may have been interpreted as dream enactment. Potential relationships between nightmares and DEBs also require further study. The high rates of self-reported DEBs and poor sleep quality among the participants in our study underscore the need for research that clarifies polysomnographic correlates of these behaviors. We hypothesize that a subgroup of patients may harbor abnormal REM sleep mechanisms.
The extent to which DEBs, other sleep disorders, TBI, and emotional distress in OEF/OIF/OND veterans are related to the pathophysiology of RBD has implications for diagnostic and treatment algorithms in veterans presenting with PTSD syndromes.
Accordingly, future studies of OEF/OIF/OND veterans should incorporate measures of sleep- related complaints, clinical sleep histories, neurological examinations, bed-partner reports, polysomnographic findings, and positive airway pressure device adherence data. Prospective video polysomnography using upper-limb EMG should be performed and analyzed in a standardized manner to assess whether the high rates of self-reported DEBs are indeed related to increased muscle tone during REM sleep. Neuroimaging studies would further inform whether veterans with DEB have structural changes in brain stem regions implicated in RBD.
In summary, this exploratory analysis found that the number of mTBIs with LOC and the PSQI total score predicted the presence of regularly occurring DEBs in a sample of previously deployed OEF/OIF/OND veterans after adjusting for PTSD severity. Further characterization of this sample that includes standardized, polysomnographic assessment of muscle tone and behavioral events during REM sleep is a logical next step to determine whether repeated mTBI is associated with an increased risk of RBD in this patient population.
1 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Publishing, 2013Google Scholar
2 : Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry 2013; 170:372–382Crossref, Medline, Google Scholar
3 : A brief sleep scale for posttraumatic stress disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. J Anxiety Disord 2005; 19:233–244Crossref, Medline, Google Scholar
4 : Validation of the Pittsburgh Sleep Quality Index Addendum for posttraumatic stress disorder (PSQI-A) in US male military veterans. J Trauma Stress 2013; 26:192–200Crossref, Medline, Google Scholar
5 : Principles and Practice of Sleep Medicine, 6th ed. Philadelphia, Elsevier, 2017Google Scholar
6 : Idiopathic REM sleep behavior disorder and neurodegenerative risk: To tell or not to tell to the patient? How to minimize the risk? Sleep Med Rev 2017; 36:82–95Crossref, Medline, Google Scholar
7 : Periodic limb movements during sleep mimicking REM sleep behavior disorder: a new form of periodic limb movement disorder. Sleep (Basel) (Epub ahead of print, March 1, 2017) doi: 10.1093/sleep/zsw063Google Scholar
8 : Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep 2005; 28:203–206Crossref, Medline, Google Scholar
9 : REM sleep behavior disorder: diagnosis, clinical implications, and future directions. Mayo Clin Proc 2017; 92:1723–1736Crossref, Medline, Google Scholar
10 : Mild TBI and risk of Parkinson disease: a chronic effects of neurotrauma consortium study. Neurology 2018; 90:e1771–e1779Crossref, Medline, Google Scholar
11 : Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology 2012; 79:428–434Crossref, Medline, Google Scholar
12 : Post-traumatic stress disorder and risk of Parkinson disease: a nationwide longitudinal study. Am J Geriatr Psychiatry 2017; 25:917–923Crossref, Medline, Google Scholar
13 : Depression and subsequent risk of Parkinson disease: a nationwide cohort study. Neurology 2015; 84:2422–2429Crossref, Medline, Google Scholar
14 : Trauma sequelae are uniquely associated with components of self-reported sleep dysfunction in OEF/OIF/OND veterans. Behav Sleep Med 2018; 16:38–63Crossref, Medline, Google Scholar
15 : A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: the TRACTS longitudinal prospective cohort study. Int J Methods Psychiatr Res (Epub ahead of print, February 17, 2017) doi: 10.1002/mpr.1556 Google Scholar
16 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP). New York, New York State Psychiatric Institute, 2002Google Scholar
17 : The development of a clinician-administered PTSD scale. J Trauma Stress 1995; 8:75–90Crossref, Medline, Google Scholar
18 : The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) semistructured interview: evidence of research utility and validity. J Head Trauma Rehabil 2014; 29:89–98Crossref, Medline, Google Scholar
19 : The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28:193–213Crossref, Medline, Google Scholar
20 : Dream-enacting behaviors in a normal population. Sleep 2009; 32:1629–1636Crossref, Medline, Google Scholar
21 : The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986; 51:1173–1182Crossref, Medline, Google Scholar
22 : Parasomnias. CMAJ 2014; 186:E273–E280Crossref, Medline, Google Scholar
23 : Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK). Sleep Med 2010; 11:43–48Crossref, Medline, Google Scholar
24 : A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord 2012; 27:913–916Crossref, Medline, Google Scholar
25 : Sleep disorders in chronic traumatic brain injury. J Clin Sleep Med 2007; 3:357–362Crossref, Medline, Google Scholar
26 : Mild traumatic brain injury and diffuse axonal injury in swine. J Neurotrauma 2011; 28:1747–1755Crossref, Medline, Google Scholar
27 : Establishment of a novel rat model of blast-related diffuse axonal injury. Exp Ther Med 2018; 16:93–102Medline, Google Scholar
28 : Rapid eye movement sleep behavioral events: a new marker for neurodegeneration in early Parkinson disease? Sleep (Basel) 2014; 37:431–438Crossref, Medline, Google Scholar
29 : Trauma associated sleep disorder: A parasomnia induced by trauma. Sleep Med Rev 2018; 37:94–104Crossref, Medline, Google Scholar
30 : Antidepressants increase REM sleep muscle tone in patients with and without REM sleep behavior disorder. Sleep (Basel) 2015; 38:907–917Medline, Google Scholar
31 : Reliability and predictive validity of the Ohio State University TBI identification method with prisoners. J Head Trauma Rehabil 2009; 24:279–291Crossref, Medline, Google Scholar
32 : Dream-enacting behaviour is associated with impaired sleep and severe headache-related disability in migraine patients. Cephalalgia 2013; 33:868–878Crossref, Medline, Google Scholar



