Cotard’s Delusion From Subacute Encephalopathy With Seizures in Alcoholism
Cotard’s delusion, or the presence of nihilistic beliefs that may extend to the conclusion that one is dead, is associated with a range of neurological and psychiatric disorders (1, 2). Although the mechanism underlying this delusion remains speculative, some reports have proposed misinterpretation of an impaired sensory input from a right-hemisphere localization as an underlying mechanism (3). This is a case report on postictal Cotard’s delusion in a patient with subacute encephalopathy with seizures in alcoholism (SESA). SESA is a rare neurological syndrome with focal neurological deficits. The clinical course of this patient and the imaging findings support and clarify the proposed mechanism for this unusual delusional belief.
Case Report
A 69-year-old right-handed man with severe alcohol use disorder and thiamine deficiency was hospitalized with recurrent seizures followed by alterations in mental status and left-sided sensory loss with neglect. On admission, the patient vehemently complained that his left side had died and could not be used. Past medical history included a small left parasagittal frontal meningioma, laryngeal cancer (treated 17 years previously), pulmonary emboli, hepatitis C, and cervical and lumbar spondylosis. His social history included a 17-year incarceration followed by 8 years of homelessness and alcohol dependence.
The man had four prior hospitalizations with similar episodes (i.e., recurrent seizures, alterations in mental status, and sensory loss with neglect) dating back 14 months. Each of these episodes began with apparent seizures described as shaking or clonic movements on his left side, followed by postictal confusion with an inability to complete sentences, left-sided visual loss, and left-sided weakness. Admission and nursing records from the most recent hospitalization also indicated initial agitation with complaints that his left side was dead. He had an extensive evaluation for vascular, infectious, and autoimmune etiologies that included unremarkable cerebrospinal fluid results. Magnetic resonance imaging (MRI) scans showed T2/fluid-attenuated inversion recovery (FLAIR) signal hyperintensity involving the right-hemisphere parietal, occipital, posterior frontal, and posterior temporal lobes but not in typical vascular territory (see Figure 1). Corresponding fluorodeoxyglucose positron emission tomography (FDG-PET) scans showed hypermetabolic activity involving the same right-hemisphere regions (see Figure 1). Electroencephalography (EEG) revealed that the patient had frequent periodic lateralized epileptiform discharges (PLEDs) that were maximal at O2 and occurred every 1 to 2 seconds. This was confirmed with continuous EEG with video showing intermittently recurring PLEDs every 1.5 to 3 seconds over the right occipital region. Repeating the continuous EEG with video captured two seizures that began with lateral head movements to the left that were followed by left arm clonic movements and onset of rhythmic spike-wave discharges on EEG from the bilateral temporo-occipital regions. Neuropsychological testing noted prominent verbal perseverations. He was impaired in verbal expression, repetition, and naming; however, his performance greatly improved when he was allowed to write his responses. Over the course of several days, as the patient’s symptoms mostly resolved, there were neuroimaging changes in the right parietal lobe; the MRI hyperintensity revealed volume loss, and the FDG-PET scans showed hypometabolism (see Figure 1).
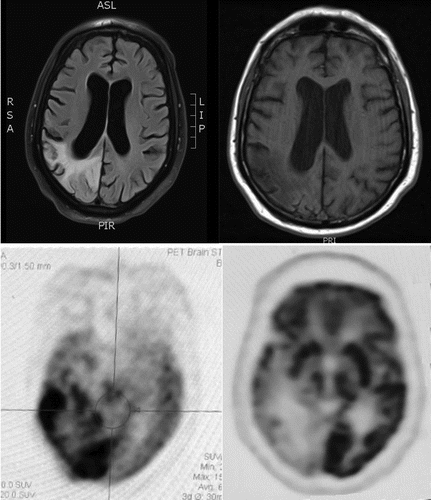
FIGURE 1. Neuroimaging findings in a 69-year-old patient with Cotard’s delusiona
aTop: The MRI image on the left shows an area of T2 signal hyperintensity in the right hemisphere involving the parietal, occipital, posterior frontal, and posterior temporal lobes with restricted diffusion that is not in a typical vascular distribution. The T1 image on the right shows volume loss in the parietal lobe. Serial MRI scans showed decreases in size, suggesting resolution of seizure-related changes and revealing volume loss and gliosis in the right parietal lobe. Bottom: The fluorodeoxyglucose positron emission tomography (FDG-PET) image on the left shows hypermetabolic activity in a large area of the right hemisphere predominantly involving the parietal, occipital, and posterior temporal lobes that suggests ongoing seizure activity. The image on the right is from a scan performed 1 month later. This image shows resolution of the hypermetabolism and a residual region of hypometabolism that suggests a focal postictal state. MRI orientation abbreviations: A=anterior; P=posterior; S=superior; I=inferior; L=left; R=right.
On examination, the patient was a disheveled, thin male who was inattentive and easily distractable, but he was oriented to person, place, and written date. He appeared agitated and complained throughout the examination that his left side was no longer living. Examination of the patient showed that his language was characterized by short phrases or answers with repetition and word-finding difficulty, yet he could speak grammatically correct sentences. Comprehension testing was compromised by his marked perseveration and intrusions from previous tasks. Naming was impaired; he could not name most objects that were presented visually, although he indicated recognition by describing them or mimicking their use. The patient was unable to read words that were spelled aloud, yet he could write intelligibly. His memory of the nature or sounds of the tested words was intact. The rest of the neurological examination included cranial nerve testing that showed a left hemianopia field loss, a reflex examination that was normal, and testing of motor function that was intact in the right-side upper and lower extremities. He did not follow commands for motor testing of the left side of his body, claiming that his limbs were dead while simultaneously moving them and lifting them up against gravity. Perception of light touch and pain was decreased in his left arm and leg, and he showed extinction to double simultaneous stimulation on the left side. Similar to previous admissions, his MRI scans showed an area of T2/FLAIR hyperintensity within the right parietal, occipital, and temporal lobes.
The overall assessment was that prolonged postictal Cotard’s delusion affected the left side of his body and that this was due to recurrent seizures consequent to antiepileptic medication nonadherence and SESA syndrome. The Cotard’s delusion of death of his left side gradually resolved along with improvements in his mental status and neuroimaging results. His seizures were initially controlled with levetiracetam and later with brivaracetam, and he was eventually discharged to a rehabilitation facility.
Discussion
This case report describes a patient with left-sided Cotard’s delusion due to SESA, which is a rare neurological syndrome that was originally reported in 1981 (4). The patient’s right temporoparietal-occipital region had PLEDs on EEG, an increased T2/FLAIR signal on MRI scans, and hypermetabolism on FDG-PET scans. His complaint of left-sided Cotard’s delusion gradually subsided with stabilization and antiepileptic therapy. Right-hemisphere lesions, possibly affecting the superior parietal cortex, may be implicated in the pathophysiology of Cotard’s delusion through the impaired integration of sensory input and affective recognition of body ownership (3, 5). During the postictal period, he also had perseverative verbal behavior with word-finding difficulty and possible optic aphasia, in that he had difficulty naming single letters when they were presented visually.
Cotard’s delusion, or the false belief of self-negation, is the misbelief that either part of one’s body or the entire person is deteriorating, destroyed, or dead. Although the most common cause of Cotard’s delusion is psychotic depression, it may result from a number of neurological disorders, including neurosyphilis, herpetic viral encephalitis, arteriovenous malformation, multiple sclerosis, Parkinson’s disease, brain neoplasm, migraine, traumatic brain injury, stroke, and epilepsy (1, 2). Investigators have reported Cotard’s delusion of death in a patient with a unilateral right-hemisphere lesion that involved both depersonalization/derealization and delusional misidentifications of people and places (3). They suggested that Cotard’s delusion may involve a two-step process of alterations in self-view or perception followed by the fixed, negative conclusion of deterioration or death.
This patient had Cotard’s delusion due to SESA, a distinct disorder that can occur among individuals with chronic alcohol dependence. SESA manifests with acute or subacute transient neurological deficits, focal seizures, and PLEDs on EEG on the side contralateral to the focal seizures (6–9). This condition differs from other complications of chronic alcohol use in that it is not clearly related to withdrawal and tends to recur with noncompliance with antiepileptic medications (10, 11). Focal nonepileptic status epilepticus may account for the encephalopathy, focal neurological deficits, reversible diffusion-weighted MRI abnormalities, and focal hyperperfusion or FDG-PET hypermetabolism (6); however, these changes may also be associated with intermittent postictal PLEDs (9, 12–14). Essentially, SESA involves a failure to return to baseline for a time after focal seizures that is associated with ongoing EEG changes along an ictal-interictal continuum (6). The underlying mechanism of SESA and PLEDs is unclear but may be related to the mechanism underlying posterior reversible encephalopathy syndrome (8).
Cotard’s delusion is often linked to right parietal, and possibly frontal and temporal, cortico-subcortical strokes or ischemia with left-sided sensory deficits including hemisensory loss and spatial neglect (2, 3, 15–17). Most neuroimaging studies of Cotard’s syndrome have noted abnormalities in the nondominant frontal and temporal lobes, and occasionally, the parietal lobes (18). Some investigators, however, have reported Cotard’s delusion from left parietal strokes and cerebral ischemia localized in the left temporoparietal region (2, 19). One review found that most neurological cases of Cotard’s delusion were accompanied by epilepsy (18), probably due to an irritative focus in the right frontal and temporal lobes (20), and postictal Cotard’s delusion has been reported in focal epilepsy patients (21). Cotard’s delusion is related to other predominantly right parietal syndromes involving lack of familiarity or acceptance of part or all of one’s body, such as asomatognosia (i.e., loss of knowledge or awareness of body parts), somatoparaphrenia (i.e., the denial of ownership of a limb or one-half of the body) (22), and depersonalization or derealization (3).
The case reported here suggests that Cotard’s delusion can result when epilepsy-related impairments in somatic sensations fail to elicit the familiarity of ownership of a part of or the entire body. There is an overlap between nihilistic delusions of one’s body and one’s existence, and patients with Cotard’s delusion may misinterpret the meaning or source of impaired somatic or internal sensations (23). The affective recognition of body ownership appears to depend on feedback sensations concerning one’s body or one’s internal body states and their integration into a body schema in the right superior parietal cortex (24). This case enhances understanding of the mechanism underlying Cotard’s delusion and its neurological causes and may serve to elicit more research into a phenomenon that transcends neurology and psychiatry.
1. : Cotard syndrome in neurological and psychiatric patients. J Neuropsychiatry Clin Neurosci 2010; 22:409–416Link, Google Scholar
2. : The Cotard syndrome. Report of two patients: with a review of the extended spectrum of ‘délire des négations’. Eur J Neurol 2004; 11:563–566Crossref, Medline, Google Scholar
3. : Delusions of death in a patient with right hemisphere infarction. Cogn Behav Neurol 2012; 25:216–223Crossref, Medline, Google Scholar
4. : Subacute encephalopathy with seizures in alcoholics: a clinical-electroencephalographic study. Clin Electroencephalogr 1981; 12:113–129Crossref, Medline, Google Scholar
5. : A neuropsychiatric analysis of the Cotard delusion. J Neuropsychiatry Clin Neurosci 2018; 30:58–65Link, Google Scholar
6. : Subacute encephalopathy with seizures in alcoholics (SESA) syndrome: further evidence supporting that may lie on the ictal-interictal continuum. J Neurol 2018; 265:2448–2450Crossref, Medline, Google Scholar
7. : Subacute encephalopathy with seizures in chronic alcoholism (SESA syndrome). Clin Electroencephalogr 2001; 32:186–190Crossref, Medline, Google Scholar
8. : A pathophysiologic approach for subacute encephalopathy with seizures in alcoholics (SESA) syndrome. J Clin Neurosci 2014; 21:1649–1652Crossref, Medline, Google Scholar
9. : Development of permanent brain damage after subacute encephalopathy with seizures in alcoholics. J Neurol Sci 2019; 396:12–17Crossref, Medline, Google Scholar
10. : Subacute encephalopathy with epileptic seizures in alcoholism (SESA): case report. Clin Electroencephalogr 2001; 32:184–185Crossref, Medline, Google Scholar
11. : Subacute encephalopathy with seizures in alcoholics syndrome: a subtype of nonconvulsive status epilepticus. Epilepsy Curr 2019; 19:77–82Crossref, Medline, Google Scholar
12. : Subacute encephalopathy and seizures in alcoholics (SESA) presenting with non-convulsive status epilepticus. Seizure 2011; 20:505–508Crossref, Medline, Google Scholar
13. : Subacute encephalopathy with seizures in alcoholics (SESA) presenting as focal nonconvulsive status epilepticus. Clin EEG Neurosci 2018; 49:414–416Crossref, Medline, Google Scholar
14. : Neuroimaging features in subacute encephalopathy with seizures in alcoholics (SESA syndrome). Seizure 2016; 40:102–107Crossref, Medline, Google Scholar
15. : Cotard delusion after stroke. Eur J Neurol 2013; 20:e98–e99Crossref, Medline, Google Scholar
16. : Poststroke delusions: what about the neuroanatomical and neurofunctional basis? Appl Neuropsychol Adult 2019; 26:392–396Crossref, Medline, Google Scholar
17. : Dysmetropsia and Cotard’s syndrome due to migrainous infarction— or not? Cephalalgia 2014; 34:717–720Crossref, Medline, Google Scholar
18. : An overview of the neurological correlates of Cotard syndrome. Eur J Psychiat 2007; 21:99–116Google Scholar
19. : Cotard and Capgras syndrome after ischemic stroke. J Stroke Cerebrovasc Dis 2015; 24:e103–e104Crossref, Medline, Google Scholar
20. : Cotard’s syndrome and temporal lobe epilepsy. Psychiatr J Univ Ott 1988; 13:36–39Medline, Google Scholar
21. : Post-ictal Cotard delusion in focal epilepsy patients. Seizure 2019; 71:80–82Crossref, Medline, Google Scholar
22. : The neuroanatomy of asomatognosia and somatoparaphrenia. J Neurol Neurosurg Psychiatry 2010; 81:276–281Crossref, Medline, Google Scholar
23. : Cotard syndrome in semantic dementia. Psychosomatics 2011; 52:571–574Crossref, Medline, Google Scholar
24. : Feeling unreal: a PET study of depersonalization disorder. Am J Psychiatry 2000; 157:1782–1788Crossref, Medline, Google Scholar



