The Lactic Acid Response to Alkalosis in Panic Disorder
Abstract
Panic patients consistently show exaggerated lactic acid response to alkalosis, whether produced by hyperventilation or by sodium lactate infusion. Understanding why this occurs may provide important clues to the pathogenesis of panic disorder. Although brain hypoxia from excessive hypocapnia-induced cerebral vasoconstriction is often cited as the mechanism of elevated brain lactic acid in panic disorder, studies of brain metabolism show that hypocapnia rarely leads to brain hypoxia. Increased lactic acid production is a normal response to intracellular alkalosis and to intracellular cyclic AMP. Thus, other possible mechanisms of the exaggerated lactic acid response in panic disorder include a disturbance of mechanisms regulating intracellular pH and factors increasing intracellular cyclic AMP. Both mechanisms are consistent with the suffocation false alarm theory of panic disorder. This review suggests a theoretical framework for future magnetic resonance spectroscopy studies that can test some of the predictions of these competing models.
Panic disorder is a condition characterized by recurrent, unprovoked attacks of extreme anxiety. Despite considerable research suggesting a neurobiological component in the etiology of panic disorder, the mechanisms of its pathogenesis remain uncertain. The exaggerated lactic acid response to alkalosis, which has been consistently observed in panic disorder, may provide an important clue to the pathogenesis of this illness. This article examines the possible mechanisms of this exaggerated lactic acid response and how they may contribute to the underlying pathophysiology of panic disorder.
Dyspnea, reactive hyperventilation, and other signs of respiratory dysregulation are among the most consistent abnormal findings in panic disorder.1–7 Patients with panic disorder also have an exaggerated lactic acid response to alkalosis produced by either hyperventilation8–10 or sodium lactate infusion.11–15 This abnormal lactic acid response to alkalosis may reflect an underlying disturbance in a metabolic process closely associated with the regulation of respiration, such as brain energy metabolism, brain pH regulation, or brain blood flow.
This article describes the normal pathways and regulation of lactic acid metabolism and reviews the literature demonstrating an exaggerated lactic acid response to respiratory alkalosis in panic disorder. The article also reviews current concepts of brain energy metabolism, pH regulation, and blood flow. In addition, it examines the possible roles of brain hypoxia due to cerebral vasoconstriction, disturbances in pH regulation, and effects mediated by adenosine 3′,5′-cyclic monophosphate (cyclic AMP) in causing this abnormal lactic acid response. Brain phosphorus-31 magnetic resonance spectroscopy (MRS) can noninvasively measure the concentration of high-energy phosphates and their breakdown products in brain parenchyma. Thus, phosphorus-31 MRS studies have the potential to test some of the predictions of these competing models of the exaggerated lactic acid response to alkalosis in panic disorder. Hydrogen ion concentration (pH) can also be measured by phosphorus-31 MRS, but with less sensitivity and reliability.16 As new methods are developed, these limitations may be overcome. This article provides a theoretical framework for future phosphorus-31 MRS studies of these questions. Understanding the mechanism of the exaggerated lactate response to alkalosis in panic disorder may advance our understanding of the pathophysiology of this condition.
The mechanism by which intravenous sodium lactate causes panic attacks in panic disorder is not fully understood and may or may not be related to the exaggerated endogenous lactic acid response to alkalosis. The mechanism of lactate-induced panic is beyond the scope of this review.
LACTATE METABOLISM
Lactate Production
An overview of lactate production and its regulation is diagrammed in Figure 1. Lactate and pyruvate are the endproducts of glycolysis. Glycolytic flux is regulated primarily by factors reflecting the energy status and the acid–base balance of the cell. In general, lactate production is increased when the cell is alkalotic or when oxygen or high-energy phosphate supplies are low. Intracellular cyclic AMP can also increase lactate production.
Lactate is produced during the glycolytic phase of glucose metabolism. Adenosine triphosphate (ATP) is generated from glucose metabolism in two phases: 1) glycolysis in the cytoplasmic compartment metabolizes glucose to pyruvate and lactate, and 2) oxidative phosphorylation within mitochondria (the citric acid cycle) metabolizes pyruvate to CO2 and H2O.
Oxidative phosphorylation produces 18 times more ATP per mole of glucose than glycolysis, but it requires adequate oxygen. Glycolysis can proceed with or without abundant oxygen and is the only physiologically significant mechanism of lactic acid production in humans. Through glycolysis, a molecule of glucose can be metabolized to two molecules of lactate and two H+ ions, producing two molecules of ATP (Figure 1). The equilibrium of the reaction catalyzed by lactate dehydrogenase causes lactate production to increase with increased glycolytic flux, even under normoxic conditions in which pyruvate is removed by oxidative phosphorylation. Under hypoxic conditions, the persistence of pyruvate and the shift in redox state further favors the production of lactate.17
Regulation of Lactate Production
Energy Metabolism:
In the presence of adequate substrate, the rate-limiting step in glycolysis is the reaction catalyzed by phosphofructokinase (PFK).18,19 PFK is the primary site of action of several mechanisms regulating the rate of glycolysis. PFK is allosterically inhibited by the high-energy phosphates ATP and phosphocreatine (PCr) and disinhibited by adenosine monophosphate (AMP), adenosine diphosphate (ADP), and inorganic phosphate (Pi). These processes coordinate glycolysis with the energy needs of the cell. PFK is also inhibited by citrate, an intermediate in the citric acid cycle. This links glycolytic production of pyruvate to the needs of the citric acid cycle. Together, these regulatory factors link the rate of glycolysis inversely to the cytoplasmic concentration of high-energy phosphates and citric acid cycle intermediates.18,19 This linkage constitutes the primary mechanism by which hypoxia increases lactic acid production. Hypoxia impedes oxidative phosphorylation, thus decreasing ATP, PCr, and citrate, while increasing ADP, AMP, and Pi. These mechanisms decreased inhibition of PFK and increased glycolytic production of lactic acid.
Acid-Base Status:
The activity of PFK is also regulated by the concentration of H+ ions. Lactic acid production decreases when intracellular pH is low (acidosis) and increases when intracellular pH is high (alkalosis).20 This effect of pH on lactic acid production provides a homeostatic mechanism for generating H+ ions to rapidly counteract a state of intracellular alkalosis.21,22 Because CO2 is a source of acid that rapidly crosses cell membranes, the respiratory alkalosis resulting from hyperventilation-induced hypocapnia causes an immediate intracellular alkalosis. Metabolic alkalosis can cause a delayed intracellular alkalosis. In their review of experimentally induced changes in acid-base status, Hood and Tannen23 found that 12 of 12 studies of respiratory alkalosis and 12 of 15 studies of metabolic alkalosis observed an increase in net lactate production. In contrast, they found that 3 of 3 studies of respiratory acidosis and 7 of 9 studies of metabolic acidosis reported a decrease in net lactate production. The regulatory effect of pH on lactate production is modulated by the concentration of high-energy phosphates and the availability of glucose as a substrate for glycolysis.20,24,25 Brautbar et al.24 showed that a combination of hyperventilation-induced respiratory alkalosis and a continuous glucose infusion led to a five-fold increase in serum lactate after 60 minutes in dogs. This increase in lactate was attributed to the disinhibiting effect of alkalosis on glycolysis, as there was no evidence for intracellular hypoxia or high-energy phosphate depletion.
These studies demonstrate that the homeostatic response to alkalosis can lead to substantial increases in lactate levels in the absence of any shortfall in oxidative metabolism.
Other Regulatory Influences:
Intracellular cyclic AMP also increases glycolytic flux and lactic acid production. These increases may be a direct result of disinhibition of PFK or an indirect result of increasing production of fructose-2-6-biphosphate, which in turn disinhibits PFK.19,26 The glycolytic effect of cyclic AMP links lactate production directly to the activity of metabotropic hormones and neurotransmitters.27
Lactate Clearance
Most lactate is cleared by reconversion to pyruvate followed by decarboxylation to acetyl coenzyme A and oxidative phosphorylation.28 This process consumes both lactate anions and H+ ions. All tissues that produce lactate also clear it by this pathway, except red blood cells (which lack mitochondria). Lactate can also be produced in one organ (e.g., muscle or brain) but consumed in another (most often the liver). In some tissues, lactate can be reconverted to glucose via gluconeogenesis, which also consumes H+ ions. Gluconeogenesis occurs primarily in the liver and accounts for approximately one-third of overall lactate clearance.28 Substrate availability, enzyme activity, and hormonal effects all influence the rate of gluconeogenesis. Acidosis generally decreases lactate clearance, whereas alkalosis has more variable effects.23 Lactate can be cleared by renal filtration when plasma concentrations exceed 6 mM/l.
Glycolysis and Lactate Metabolism in the Brain
Several distinctive features characterize glycolytic regulation in the brain. The endothelial cells of brain capillaries constitute the blood–brain barrier, which limits the diffusion of nutrients, hormones, and most other large or nonlipophilic molecules from the bloodstream into the extracellular fluid of the brain. With few exceptions, glucose is the only substrate for energy metabolism that passes from the blood into the brain. Brain capillaries are surrounded by the end-feet of astrocytes. Much of the glucose entering the brain appears to be transported directly into these astrocytes, which contain the most significant brain stores of glycogen. Recent evidence suggests that approximately half of the glucose entering astrocytes is metabolized via glycolysis to lactate, which is then shuttled to adjacent neurons to be metabolized aerobically.29
These observations suggest that energy metabolism in the brain is partially compartmentalized. Glycolysis occurs primarily in astrocytes, which release lactate to adjacent neurons for subsequent oxidative phosphorylation. Recent studies have suggested that this compartmentalized brain glucose metabolism is tightly coupled to glutaminergic neuronal activity. Most glutamate released synaptically by neurons is taken up into astrocytes, where it is converted to glutamine and then shuttled back to neurons. The relatively small ATP requirements associated with glutamate uptake and cycling within astrocytes are met by glycolytic metabolism, whereas the larger ATP requirements associated with restoring neuronal membrane electrochemical gradients are met by the oxidative metabolism of lactate within neurons.30,31
STUDIES IN PANIC DISORDER
Early Studies of Lactate Levels in Anxious Patients
The observation of exercise intolerance in patients with “anxiety neurosis” or “neurocirculatory asthenia” led to studies of the lactate response to exercise in these patients. These patients (who had many of the clinical features of panic disorder) were often found to have higher than normal lactate levels during exercise.32,33 Although Pitts and McClure34 based their original lactate infusion experiments on these observations, these early exercise studies were flawed by inadequate controls for differences in fitness between the patient and control groups. The higher lactate levels during exercise in the patients may have simply reflected a lower level of fitness. Because of inherent difficulties matching patients and control subjects for fitness and the confounding effects of motivation and other psychological factors on physiological measures of fitness (such as maximal oxygen consumption), studies of the lactate response to exercise in panic disorder are likely to be inconclusive.
Hyperventilation and Elevated Lactate in Panic Disorder
Maddock and Mateo-Bermudez8 conducted the first study designed specifically to investigate the effects of respiratory alkalosis on glycolytic metabolism and net lactate production in panic disorder. In this study, panic patients and control subjects hyperventilated to an end-tidal pCO2 of 20 mm Hg for 8 minutes. All subjects hyperventilated twice, once during an intravenous infusion of 10% glucose and once during an infusion of normal saline. Hyperventilation was intended to increase the activity of PFK by inducing an intracellular alkalosis, and thus to increase lactate production. Infusion of 10% glucose was intended to supply abundant substrate, which maximizes the alkalosis-induced increase in glycolytic flux. Patients and control subjects showed similar changes in pCO2 during and after hyperventilation. In both patients and control subjects, serum lactate increased significantly after hyperventilation during both glucose and saline infusions, with a significantly greater increase occurring during the glucose infusion. As predicted, the panic patients had a significantly greater increase in serum lactate in response to hyperventilation during the glucose infusion than the control subjects (Figure 2).
Subsequently, Maddock et al.4 used oral glucose loading combined with hyperventilation to stimulate net lactate production in 12 panic patients and 12 control subjects. Again, both groups demonstrated significantly increased serum lactate, with larger increases seen in the panic patients. However, only the subgroup of 7 patients who experienced a panic attack during hyperventilation had a significantly greater increase in serum lactate than the control group. A meta-analysis of all 20 patients and 17 control subjects from the two studies combined revealed a significantly greater increase in serum lactate following hyperventilation and glucose loading in the panic disorder subjects compared with the control subjects (Figure 3). When the total of 11 patients who panicked during hyperventilation, the 9 who did not panic, and the 17 control subjects were compared, only the patients who panicked had a significantly greater increase in serum lactate than the control subjects. The lactate levels in nonpanicking patients were intermediate between panicking patients and control subjects and did not differ significantly from either.
In contrast to the above findings, Gorman et al.3 and Dager et al.10 measured whole blood lactate following hyperventilation and found no differences between panic disorder patients and normal volunteers. However, whole blood lactate primarily reflects lactate levels in erythrocytes, which lack mitochondria and derive all their energy from glycolysis. For this reason, the dynamics of lactate metabolism in erythrocytes are dissimilar to those in all other tissues. Thus, whole blood lactate, unlike serum lactate, is an unreliable measure of systemic lactate.
Overall, these studies demonstrate greater increases in serum lactate in panic patients than control subjects following hyperventilation in the presence of abundant glucose. This suggests a greater systemic production of lactic acid under these conditions. However, it is not clear whether the exaggerated lactate response is a trait characteristic of panic disorder or simply a manifestation of acute anxiety or panic. Furthermore, measures of serum lactate reflect the net result of many factors, including the combined lactate production of all organs, the rate of efflux of lactate into the vascular compartment, and the total clearance of lactate. Organ-specific measures of lactate can provide more specific information about the metabolic basis of this abnormality in panic disorder.
In 1995, Dager et al.10 reported the results of a proton magnetic resonance spectroscopy (proton MRS) study of the brain lactate response to hyperventilation, which addressed some of these questions. This study compared asymptomatic, treatment-responsive panic disorder patients and healthy comparison subjects. There was no difference in the rate of anxiety or panic induced by hyperventilation to an end-tidal pCO2 of 20 mm Hg for 20 minutes in the two groups. Although both groups showed a significant increase in brain lactate in response to hyperventilation, brain lactate was significantly higher in the remitted panic patients (Figure 4). These results suggest that the exaggerated lactate response to respiratory alkalosis reflects a trait characteristic of patients with panic disorder and is not just an expression of acute panic. However, it is unclear what effect the antipanic medications taken by many of these patients may have had on their lactate responses. This study also demonstrated that the exaggerated lactate response could be observed within the brain and that proton MRS provides a sensitive measure of the brain lactate response to respiratory alkalosis.
A delayed recovery from hypocapnia to normocapnia following hyperventilation has been observed in panic patients9 and could account for a greater overall lactic acid response to hyperventilation in these patients. This effect was noted in the panic patients in the study by Maddock et al.4 and could have contributed to their greater overall lactic acid response. However, delayed recovery of normocapnia cannot account for the results of Maddock and Mateo-Bermudez8 and of Dager et al.10 Delayed recovery of normocapnia did not occur in the panic patients in the study of Maddock and Mateo-Bermudez.8 Dager et al.10 found an exaggerated increase in brain lactate during controlled hyperventilation when pCO2 was the same in patients and control subjects. In these studies, the panic patients had a greater lactic acid response to hyperventilation while having the same overall exposure to hypocapnia.
Lactate Infusion and Elevated Lactate in Panic Disorder
The infusion of 10 ml/kg of 0.5 molar racemic sodium lactate produces a significant metabolic alkalosis and, paradoxically, a secondary respiratory alkalosis.11 The latter may result from lactate acting as a respiratory stimulant. This combined metabolic and respiratory alkalosis could lead to increased endogenous lactic acid production, via the effect of intracellular pH on PFK. Most lactate infusion studies stop the infusion when the patient has a panic attack but continue the infusion for a full 20 minutes in subjects who do not panic. Since 70% to 90% of panic patients panic during the infusion compared with only 10% to 20% of normal subjects, the patients consistently receive less exogenous lactate than normal subjects. This difference confounds postinfusion measures of total serum or blood lactate. However, Liebowitz et al.11 measured blood lactate frequently during the infusion and found significantly higher levels in the patients compared with the control subjects after an equal volume of infusion. Similarly, Rainey et al.12 observed a more rapid increase in plasma lactate in panic patients than in comparison subjects at the same point in the infusion.
Several recent studies have used proton MRS to estimate brain lactate levels in response to sodium lactate infusions in panic patients. These studies, which administered full 20-minute infusions in all subjects, have consistently observed significantly greater increases in brain lactate in the panic patients than in the comparison group.13–15 The disproportionate increase in brain lactate is most marked among patients who panicked in response to lactate infusion. In these lactate-sensitive subjects, brain lactate levels rise more rapidly during the infusion, reach higher levels, and remain elevated longer after the infusion compared with levels in control subjects (Figure 5). In a second lactate infusion in a small subsample of these patients during clinical remission on fluoxetine, the brain lactate response remained disproportionately greater even when no lactate-induced panic attack occurred.14
Thus, the findings from lactate infusions parallel those from hyperventilation studies. Panic patients show an exaggerated lactate response to both alkalotic challenges. The effect is most evident in patients who are sensitive to the anxiety-inducing effects of the alkalotic challenge. However, there is evidence that the exaggerated lactate response may be a trait feature of panic disorder, since it is also observed in remitted patients who do not panic during the procedure. Proton MRS consistently provides a sensitive measure of the exaggerated brain lactate response to alkalosis in panic patients. Importantly, none of these studies have investigated whether the exaggerated lactate response to alkalosis is specific to panic disorder.
POSSIBLE MECHANISMS
A variety of physiological or pathophysiological mechanisms may contribute to the exaggerated lactate response to alkalosis observed in panic disorder. Identifying the specific mechanism of this abnormal response may increase our understanding of the underlying pathophysiology of panic disorder. Three possible mechanisms suggested by investigators demonstrating this effect are 1) hypoxia secondary to cerebral vasoconstriction;10,12,13,35 2) a disturbance in the regulation of intracellular pH;8,10 and 3) increased adrenergic activity.8 The mechanisms are summarized in Table 1. The following discussion examines the possible contributions of each of these mechanisms to the exaggerated lactate response to alkalosis observed in patients with panic disorder.
Cerebral Vasoconstriction and Brain Hypoxia
Cerebral blood flow changes rapidly in direct relationship to changes in arterial pCO2. One function served by this response is to counteract changes in brain pH, such as those occurring during hyperventilation-induced alkalosis.22 Hyperventilation reduces cerebral blood flow (CBF) by 40% to 50%.36 However, in both humans and animals, this decrease in CBF does not usually cause metabolic changes indicating brain hypoxia. The increase in brain lactate caused by hyperventilation appears to result from the direct effects of alkalosis on glycolytic flux.19,23,36–38 However, it is possible that panic disorder patients have an abnormal vasoconstrictor response to alkalosis that leads to brain hypoxia.
During hyperventilation, CBF reaches its minimum at an arterial pCO2 of approximately 25 to 30 mm Hg.36 Although lower arterial pCO2 levels may not further reduce CBF, they impede oxygen delivery by the Bohr effect (the shift in the oxyhemoglobin dissociation curve to the left with increasing pH). Clinically, hyperventilation can lead to diminished mental function, slowing of the EEG, paresthesias, muscle contractions, and, in the extreme case, loss of consciousness. These effects are often interpreted as signs of progressive brain hypoxia. However, as summarized by Edvinsson et al.,36 “the clinical and experimental evidence for brain hypoxia is somewhat scant.” Reviews of experimental studies of brain metabolism during hyperventilation have concluded that hyperventilation leading to a pCO2 as low as 14 mm Hg does not produce brain hypoxia.19,37,38 The subjective effects of hyperventilation may be the result of altered function of the numerous receptors, channels, transporters, and enzymes in neurons that are highly sensitive to increases in pH, rather than the result of hypoxia.39
In attempting to determine the cause of increased lactate production in response to respiratory alkalosis, Siesjo19 noted that it is difficult to distinguish between lactate production that occurs as a result of increased intracellular pH activating PFK and lactate production that occurs as a result of decreased oxygen delivery and cellular hypoxia. He concluded that “the most direct way of testing whether or not hypoxia is present is to measure concentrations of labile phosphates in the tissue.” van Rijen et al.40 measured brain lactate with proton MRS and additionally measured ATP, ADP, AMP, Pi, PCr, and pH with phosphorus-31 MRS in human volunteers who hyperventilated to an average pCO2 of 16 mm Hg. Concurrent transcranial Doppler sonography showed a 42% reduction in left middle cerebral artery flow. Brain lactate increased significantly during and after hyperventilation, but no change was observed in the phosphate compounds, suggesting that hypoxia did not occur.
Hyperventilation causes a large increase in arterial pH but only a small increase in brain pH.40,41 The relative stability of brain pH results from homeostatic processes, which serve to counteract changes in intracellular pH. One of the most important mechanisms of this defense against intracellular alkalosis in the brain is the increased production of lactic acid via glycolysis.19,37 This mechanism can operate in the presence of fully adequate oxygen tension. Numerous biochemical studies42–47 and more recent spectroscopic studies40,41 have shown that high-energy phosphate levels (ATP and PCr) remain unchanged during hyperventilation that produces pCO2 levels of approximately 14 mm Hg. Only one study, which involved severe hypocapnia in rats, (pCO2=10 mm Hg) found evidence for decreased PCr and increased ADP, indicating that metabolically significant hypoxia had occurred.48 Studies of cerebral oxygen consumption (CMRO2) during hyperventilation also typically show no reduction in CMRO2.47,49,50 Overall, the evidence suggests that respiratory alkalosis leading to a pCO2 of ≥14 mm Hg produces a rise in brain lactate as a result of increased glycolysis in the service of regulating intracellular pH, and that brain hypoxia does not occur.
However, it is possible that an abnormal cerebrovascular response to alkalosis in panic disorder could render such patients distinctly vulnerable to brain hypoxia during hyperventilation. Recent studies have produced preliminary evidence for an abnormal vasoconstrictor response to respiratory alkalosis in patients with panic disorder. Two studies using transcranial Doppler ultrasonography of basilar artery blood flow found that panic patients have a greater than normal reduction in basilar artery flow during moderate hyperventilation (pCO2=25 mm Hg).51,52 However, there is not yet any evidence that metabolically significant hypoxia occurs in panic patients during hyperventilation. Nonetheless, a vulnerability to cerebral hypoxia during mild to moderate respiratory alkalosis could contribute to many of the characteristic manifestations of panic disorder.
Phosphorus-31 MRS studies of high-energy phosphates could provide an experimental test of the hypothesis that respiratory alkalosis leads to metabolically significant brain hypoxia in panic patients and not in normal subjects. Using this technique in normal volunteers, Van Rijen et al.40 have shown that ATP, ADP, AMP, Pi, and PCr do not change with hyperventilation (to a pCO2 of 15.8 mm Hg). If ATP and PCr decreased while ADP, AMP and Pi increased in response to a similar degree of hyperventilation in panic disorder patients but not in normal subjects, this would demonstrate an abnormal vulnerability to brain hypoxia in response to respiratory alkalosis in panic disorder.
A Disturbance in the Regulation of Intracellular pH
Intracellular pH is one of the most tightly regulated parameters in the mammalian nervous system. The maximum change in H+ concentration that can be tolerated is approximately 0.00005 mM.22 H+ concentration changes beyond this range can disrupt the tertiary structure, and thus the function, of cellular proteins. Intracellular pH is controlled by a variety of regulatory mechanisms, including the increased production of lactic acid in response to alkalosis. The exaggerated lactic acid response to alkalosis in panic disorder could result from a metabolic disturbance that increases the activity of this pH regulating mechanism. For many illnesses, including panic disorder, experimentally demonstrated physiological disturbances often represent compensatory and adaptive responses to underlying, and perhaps less easily observed, abnormalities in function.53 From this perspective, the increased lactic acid production could represent a compensatory response to a deficiency in one of the other homeostatic mechanisms protecting intracellular pH.
A variety of different mechanisms could cause a compensatory increase in lactic acid production during alkalosis. Intracellular pH is regulated by a combination of three mechanisms: 1) physicochemical buffering, 2) transport of H+ (acid) or HCO3– (base) across the cell membrane, and 3) metabolic processes generating or consuming H+ ions. Increased glycolytic production of lactic acid is the most important example of the last mechanism.22,39,54 A relative deficiency in one of the buffering or transport mechanisms protecting intracellular pH could lead to a wide range of abnormalities in the response of panic patients to disturbances of intracellular pH. These could include altered responses to acidotic as well as alkalotic challenges, such as the increased sensitivity to hypercapnia often noted in panic disorder.1,35 The presence of a pH regulating deficiency of this type in panic disorder could, in principle, be detected by a phosphorus-31 MRS study of the intracellular pH response to hyperventilation. In this general model, the increased lactic acid production results not from hypoxia, but from a deficiency in one of the mechanisms protecting against alkalosis. The model predicts that during hyperventilation, panic patients compared with normal subjects would not show a decrease in high-energy phosphates and would have a slower or less complete correction of hyperventilation-induced intracellular alkalosis. This effect might be most apparent under conditions that limit lactic acid production, such as substrate depletion by prolonged hyperventilation in fasting subjects. This depletion would limit the extent to which increased lactic acid production could compensate for the hypothesized deficiency in intracellular pH regulation.
This model posits a deficiency in one of the buffering or transport mechanisms that regulate intracellular pH. The most important intracellular physicochemical buffer is the CO2/HCO3– system. Additional intracellular buffering capacity results from the imidazole groups on histidine residues of proteins and the free and bound forms of phosphate (including inorganic phosphate, AMP, ADP, and ATP).55 A deficiency in one of these buffers could lead to a greater reliance on lactic acid production in the homeostatic response to intracellular alkalosis. At present, however, there is little evidence of deficiencies in these intracellular buffers in panic patients. In a phosphorus-31 MRS study of frontal cortex in treated patients with panic disorder and control subjects under “resting” conditions (without any controlled psychological or physiological manipulations), Shioiri et al.56 found no significant differences in levels of any phosphate compounds.
Multiple active and passive mechanisms for transporting H+ or HCO3– across the cell membrane have been identified in brain parenchyma. These mechanisms modify intracellular and interstitial pH in a reciprocal manner. In neurons and glia, passive entry of H+ through the glutamate-gated cation channel and passive efflux of HCO3– through the GABA-gated chloride channel may slightly acidify the neuron and alkalinize the interstitial fluid.57 Multiple active transport mechanisms that help regulate intracellular pH have been demonstrated in neurons and glia.58,59 Four types of Na+/H+ exchanger (NHE) have been found in the brain.58 The NHEs alkalinize the neuron by exchanging intracellular H+ for extracellular Na+. The extrusion of H+ by NHEs is activated by agonist binding to both beta and alpha adrenoreceptors.60–62 Another pH regulating transport mechanism, the Cl–/HCO3– exchanger, is particularly important in the recovery of cortical neurons from intracellular alkalosis.63 This anion exchanger also helps regulate the smooth muscle vasoconstrictor response to alkalosis64 and the respiratory response to hypercapnia.65 Intracellular pH is also regulated by a H+/ lactate cotransporter, which influences pH in both neurons and glia,58,59 and by an Na+/HCO3– cotransporter, which is specific to glia. The latter mechanism may have an important role in linking glutamate uptake to glial alkalinization and increased production of lactic acid. All of these active transport mechanisms can operate in both directions, to either acidify or alkalinize neurons.58 Dysregulation of one of the pH regulating transport mechanisms in patients with panic disorder could lead to an exaggerated, compensatory lactate response to alkalosis, as well as other abnormal responses to changes in pCO2 and intracellular pH.
It is important to note that an exaggerated cerebral vasoconstrictor response to hyperventilation could itself result from a disturbance in pH regulation. Intracellular and perivascular pH of the smooth muscle cells lining the cerebral arterioles are principal determinants of the cerebral vasoconstrictor response to hyperventilation.36 An exaggeration of this vasoconstrictor response, which preliminary evidence suggests occurs in panic disorder, could result from a dysregulation of one of the homeostatic mechanisms, such as the Cl–/HCO3– anion exchanger, which normally limit the hyperventilation-induced increase in pH within arteriolar smooth muscle cells.
Cyclic AMP–Mediated Effects
Cyclic AMP stimulates lactate production via either a direct or an indirect stimulation of PFK. Thus, the many hormones, neurotransmitters, and drugs that activate the cyclic AMP second messenger system, including the catecholamines, could increase lactate production by this mechanism. The blood–brain barrier, however, prevents circulating epinephrine and peptide hormones from increasing intracellular cyclic AMP levels within brain parenchyma. Most glycolysis in the brain appears to occur in astrocytes, and these cells are influenced by the synaptic release of norepinephrine (NE) and vasoactive intestinal polypeptide (VIP). These neurotransmitters increase intracellular cyclic AMP via their metabotropic receptors on astrocytes, and thus increase glycolysis and lactate production. The arborization patterns of VIP-containing and NE-containing neurons in the cortex suggest that the former regulate glycolytic metabolism locally while the latter have more global effects on glial glycolytic metabolism.29
Heightened activity of this noradrenergic system could contribute to the globally increased brain lactate response to alkalosis observed in panic disorder.15 In this scenario, a phosphorus-31 MRS study would be expected to show no decrease in high-energy phosphates and, if sufficiently sensitive methods were available, a more rapid or more complete normalization of intracellular pH during hyperventilation-induced alkalosis in panic patients. These effects would occur because enhanced lactic acid production would supplement other pH regulating responses. Furthermore, in this model, the exaggerated lactate response to alkalosis in panic disorder could be blocked by centrally active beta-blockers such as propranolol. Finally, this model predicts that alkalosis is not essential for excess lactate production to occur in panic disorder and that elevated lactate levels would be expected in response to a variety of anxiogenic challenges. Consistent with these ideas, Uhde and Boulenger66 showed that the ingestion of 480 mg of caffeine (which increases intracellular cyclic AMP levels and crosses the blood–brain barrier) led to a greater increase in plasma lactate in panic patients compared with control subjects. Tancer et al.67 subsequently showed that panic patients had a greater lactic acid response to caffeine than patients with social phobia, using a similar challenge procedure. However, these findings are difficult to interpret because the panic patients and the control subjects in these studies were not matched for usual caffeine consumption. Panic patients frequently consume little or no caffeine.68 Dager et al.69 have shown that nonclinical subjects have a greater brain lactate response to caffeine during prolonged caffeine abstinence than during regular use. Thus the exaggerated lactate increase in the panic patients may have resulted from a lesser degree of tolerance to caffeine. Further studies of lactate levels in response to anxiogenic challenges that do not affect pH would help to define the conditions under which panic patients show an exaggerated lactic acid response.
Implications for Pathogenesis
Any comprehensive theory of the pathogenesis of panic disorder must incorporate a biopsychosocial perspective because elements from each domain can influence onset, continuation, and recovery in this illness. However, abundant evidence from biological, family, and twin studies suggests that an underlying neurobiological dysfunction is present in panic disorder. The exaggerated lactate response to alkalosis in panic disorder may be one manifestation of such an underlying abnormality.
Each of the possible mechanisms discussed above could have a causal or contributory role in the pathogenesis of panic disorder. Alternatively, the exaggerated lactate response to alkalosis in panic disorder may simply be an epiphenomenon of the illness, one of the many physiological changes consequent upon recurrent panic attacks. This latter possibility is less likely in view of the replicated finding that the exaggerated lactate response also occurs in panic patients during clinical remission.10,14 Cerebral hypoxia in response to mild respiratory alkalosis could have a contributory role in panic attacks: mild hyperventilation triggered by anxiety could lead to symptoms of hypoxia and thus exacerbate the “vicious cycle” of panic. This mechanism is unlikely to be a primary cause of panic disorder, since it is clear that hypocapnia does not consistently precede or trigger panic attacks.70
Similarly, dysregulation of intracellular pH would be expected to have only a contributory, not a causal, role if the dysfunction is limited to mechanisms that counteract alkalosis, since alkalosis does not consistently precede or trigger panic attacks. However, many brain pH regulating mechanisms function across the entire range of physiological pH, including alkalotic, normal, and acidotic conditions.58 An abnormality that affects the regulation of intracellular pH across its entire physiological range could have a causal role in panic attacks and would be consistent with the suffocation false alarm theory of panic disorder.1 This theory argues that 1) detection of imminent suffocation triggers an evolved response that includes breathlessness, panic, and the urge to flee, and that bears many similarities to a spontaneous panic attack, and 2) these symptoms and other features of panic disorder, such as respiratory dysregulation, lactate and carbon dioxide hypersensitivity, and sleep panic attacks, support the proposition that panic attacks result from a physiological misinterpretation by a “suffocation monitor” that inappropriately sets off the suffocation alarm response. The neural system that monitors the adequacy of ventilation is primarily influenced by the intracellular pH within chemosensitive neurons in the brainstem.65 A disturbance in the regulation of intracellular pH could lead to misinterpretations of the adequacy of ventilation and thus trigger recurrent false suffocation alarms, even though compensatory mechanisms ultimately prevent any metabolically harmful deviations from normal intracellular pH.
Heightened activity of the beta-adrenoreceptor system affecting brain cell glycolytic metabolism could be one expression of a more general disturbance of central noradrenergic systems in panic disorder. Altered noradrenergic function could be an epiphenomenon of the illness, or it could play a role in its pathogenesis. Central noradrenergic systems appear to play a key role in fear and arousal, as well as in cardiopulmonary regulation.71 In addition, increased glycolytic production of lactic acid due to heightened beta-adrenoreceptor activation could lead to transiently decreased intracellular pH, which in chemosensitive regions could influence respiratory regulation and trigger false suffocation alarms. Arguing against an important role for beta-adrenergic mechanisms in the pathogenesis of panic disorder is the inconsistent evidence for efficacy of centrally active beta-blockers in the treatment of this condition.72,73 In addition, peripheral beta-adrenoreceptor systems have consistently been shown to be downregulated and desensitized in panic disorder,53 although it is not known if similar dynamic changes occur in the central noradrenergic system. Nonetheless, there are many interactions between pH and the noradrenergic system. Alkalosis increases beta-receptor agonist binding affinity,74 and decreased vesicular pH resensitizes beta receptors.75 In turn, both alpha and beta receptors can activate the Na+/H+ exchangers, which regulate intracellular pH. A dysregulation of the central noradrenergic system or a disturbance of the complex interaction between the noradrenergic system and the regulation of intracellular pH could have either a contributory or a causal role in the pathogenesis of panic disorder.
SUMMARY AND CONCLUSIONS
Lactic acid production in humans can increase 1) as part of the normal homeostatic response to intracellular alkalosis, 2) in response to metabolically significant hypoxia, and 3) in response to agents such as norepinephrine, which act to increase intracellular cyclic AMP. Patients with panic disorder consistently demonstrate an exaggerated increase in lactate level in response to respiratory alkalosis. This effect has been observed in both serum and brain lactate levels in response to the respiratory alkalosis produced by hyperventilation and the mixed metabolic and respiratory alkalosis produced by a sodium lactate infusion. Three distinct mechanisms have been proposed to account for this abnormality in panic disorder: 1) hypoxia caused by an abnormally severe cerebral vasoconstrictor response to hypocapnia; 2) heightened noradrenergic activity; and 3) a compensatory response to a dysregulation or deficiency of one of the components of intracellular pH regulation. The latter model may have more explanatory power, since it can account for a wider range of abnormal responses to changes in pH—including the exaggerated cerebrovascular response to hyperventilation that been reported in panic disorder. A disturbance in the regulation of intracellular pH could give rise to the characteristic dysregulation of respiration in panic disorder and is consistent with the suffocation false alarm theory of panic disorder. Especially as more sensitive techniques become available, it may be possible to test these hypothesized mechanisms by experiments that use phosphorus-31 MRS during hyperventilation to measure brain high-energy phosphates and pH in patients with panic disorder. Understanding the physiological mechanism of this exaggerated lactate response to alkalosis may contribute to our understanding of the pathophysiology of panic disorder. However, the question of whether an exaggerated lactate response to alkalosis is specific to panic disorder remains to be answered. Thus, future studies should also examine this phenomenon in patients with other psychiatric disorders, such as major depression and social phobia.
ACKNOWLEDGMENTS
The author is grateful to Drs. Walton T. Roth, Stephen Dager, Tom Jue, Paul Mole, and Amy Garrett and to Nora Mealy for their very helpful comments on earlier versions of this manuscript, and to Trish Foley for assistance with manuscript preparation. A preliminary version of this paper was presented at the Anxiety Disorders Association of America annual meeting, San Diego, CA, March 26–28, 1999.
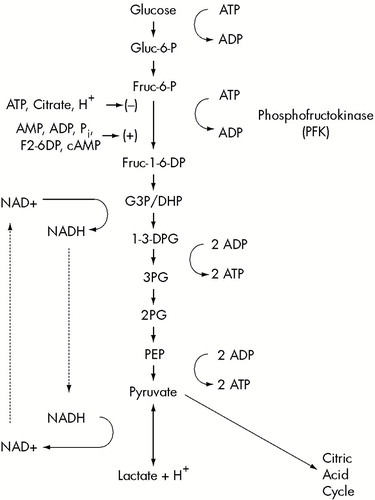
FIGURE 1. The glycolytic pathway from glucose to pyruvate and lactateFactors that inhibit (–) or disinhibit (+) phosphofructokinase are indicated. Also shown is the cycle by which NAD+ is consumed during glycolysis but regenerated by the reduction of pyruvate to lactate.
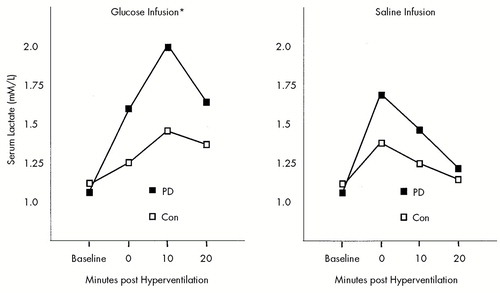
FIGURE 2. Serum lactate response following 8 minutes of hyperventilation during infusion of 10% glucose or normal salinePD=panic disorder patients (n=8), Con=control subjects (n=5). *Significantly greater serum lactate response to hyperventilation during the glucose infusion in PD than Con (t=1.88, df=11, P=0.04, one-tailed). Adapted from Maddock and Mateo-Bermudez.8
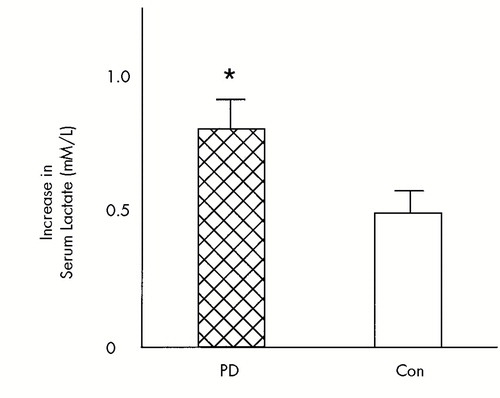
FIGURE 3. The increases in serum lactate following glucose loading and hyperventilation from a meta-analysis of two studies (means and standard errors)PD=panic disorder patients (n=20), Con=control subjects (n=17). *Significantly greater increase in lactate following hyperventilation in PD than Con (t=2.1, df=35, P=0.02, one-tailed). Adapted from Maddock and Mateo-Bermudez8 and Maddock et al.4
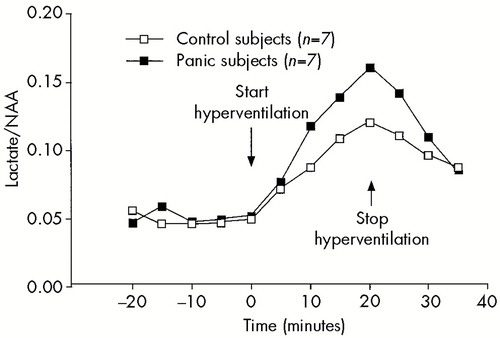
FIGURE 4. Brain lactate response to 20 minutes of hyperventilation in 7 panic patients and 7 comparison subjectsBrain lactate concentration is estimated from lactate/N-acetyl aspartate (NAA) ratio as measured by H+ MRS. A significant group by time interaction was observed (F=2.5, df=9,108, P=0.01). From Dager et al.,10 reprinted with permission.
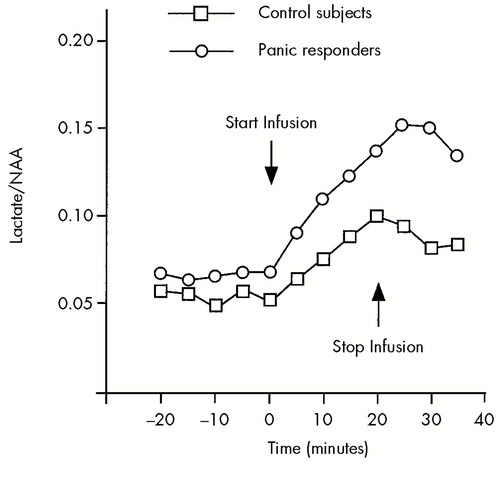
FIGURE 5. Brain lactate response to a 20-minute infusion of sodium lactate in 10 panic patients who panicked during lactate infusion and 10 control subjectsMean (and standard deviation) brain lactate concentration is estimated from lactate/N-acetyl aspartate (NAA) ratio as measured by H+ MRS. A significant group by time interaction was observed (F=5.8, df=9,171, P=0.001). From Dager et al.,14 reprinted with permission.
 |
1 Klein DF: False suffocation alarms, spontaneous panics, and related conditions: an integrative hypothesis. Arch Gen Psychiatry 1993; 50:306–317Crossref, Medline, Google Scholar
2 Gorman JM, Fyer MR, Goetz R, et al: Blood gas changes and hypophosphatemia in lactate-induced panic. Arch Gen Psychiatry 1986; 43:1067–1071Google Scholar
3 Gorman JM, Fyer MR, Goetz R, et al: Ventilatory physiology of patients with panic disorder. Arch Gen Psychiatry 1988; 45:31–39Crossref, Medline, Google Scholar
4 Maddock RJ, Carter CS, Geitzen DW: Elevated serum lactate associated with panic attacks induced by hyperventilation. Psychiatry Res 1991; 38:301–311Crossref, Medline, Google Scholar
5 Papp LA, Martinez JM, Klein DF, et al: Respiratory psychophysiology of panic disorder: three respiratory challenges in 98 subjects. Am J Psychiatry 1997; 154:1557–1565Google Scholar
6 Maddock RJ, Carter CS, Tavano-Hall L, et al: Hypocapnia during cardiac stress scintigraphy in chest pain patients with panic disorder. Psychosomatic Med 1998; 60:52–55Crossref, Medline, Google Scholar
7 van Zijderveld GA, Veltman DJ, van Dyck R, et al: Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res 1999; 33:73–78Crossref, Medline, Google Scholar
8 Maddock RJ, Mateo-Bermudez J: Elevated serum lactate following hyperventilation during glucose infusion in panic disorder. Biol Psychiatry 1990; 27:411–418Crossref, Medline, Google Scholar
9 Maddock RJ, Carter CS: Hyperventilation-induced panic attacks in panic disorder with agoraphobia. Biol Psychiatry 1991; 29:843–854 Crossref, Medline, Google Scholar
10 Dager SR, Strauss WL, Marro KI, et al: Proton magnetic resonance spectroscopy investigation of hyperventilation in subjects with panic disorder and comparison subjects. Am J Psychiatry 1995; 152:666–672Crossref, Medline, Google Scholar
11 Liebowitz MR, Gorman JM, Fyer AJ, et al: Lactate provocation of panic attacks, II: biochemical and physiological findings. Arch Gen Psychiatry 1985; 42:709–719Crossref, Medline, Google Scholar
12 Rainey JM, Frohman CE, Warner K, et al: Panic anxiety and lactate metabolism. Psychopharmacol Bull 1985; 21:434–437Medline, Google Scholar
13 Dager SR, Marro KI, Richards TL, et al: Preliminary application of magnetic resonance spectroscopy to investigate lactate-induced panic. Am J Psychiatry 1994; 151:57–63Crossref, Medline, Google Scholar
14 Dager SR, Richards T, Strauss WL, et al: Single voxel 1H MRS investigation of brain metabolic changes during lactate-induced panic. Psychiatry Res: Neuroimaging 1997; 76:89–99Crossref, Medline, Google Scholar
15 Dager SR, Friedman SD, Heide A, et al: Two-dimensional proton echo-planar spectroscopic imaging of brain metabolic changes during lactate-induced panic. Arch Gen Psychiatry 1999; 56:70– 77Crossref, Medline, Google Scholar
16 Dager SR, Steen RG: Applications of magnetic resonance spectroscopy to the investigation of neuropsychiatric disorders. Neuropsychopharmacology 1992; 6:249–266Medline, Google Scholar
17 Mellergard P, Siesjo BK: Cerebral energy metabolism and pH, in pH and Brain Function, edited by Kaila K, Ransom BR. New York, Wiley-Liss, 1998, pp 67–91Google Scholar
18 Coleman JE: Metabolic interrelationships between carbohydrates, lipids, and proteins, in Metabolic Control and Disease, 8th edition, edited by Bondy PK, Rosenberg LE. Philadelphia, WB Saunders, 1980, pp 161–232Google Scholar
19 Siesjo BK: Brain Energy Metabolism. New York, Wiley, 1978 Google Scholar
20 Trivedi B, Danforth WH: Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem 1966; 241:4110–4112Google Scholar
21 Kjallquist A, Nardini M, Siesjo BK: The regulation of extra- and intracellular acid-base parameters in the rat brain during hyper- and hypocapnia. Acta Physiol Scand 1969; 76:485–494Crossref, Medline, Google Scholar
22 Kuschinsky W: Role of hydrogen ions in regulation of cerebral blood flow and other regional flows. Advances in Microcirculation 1982; 11:1–19Google Scholar
23 Hood VL, Tannen RL: pH control of lactic acid and keto acid production: a mechanism of acid-base regulation. Mineral Electrolyte Metabolism 1983; 9:317–325Medline, Google Scholar
24 Brautbar N, Leibovici H, Massry SG: On the mechanism of hypophosphatemia during acute hyperventilation: Evidence for increased muscle glycolysis. Mineral Electrolyte Metabolism 1983; 9:45–50Medline, Google Scholar
25 Erecinska M, Deas J, Silver IA: The effect of pH on glycolysis and phosphofructokinase activity in cultured cells and synaptosomes. J Neurochem 1995; 65:2765–2772Google Scholar
26 Depre C, Ponchaut S, Deprez J, et al: Cyclic AMP suppresses the inhibition of glycolysis by alternative oxidizable substrates in the heart. J Clin Invest 1998; 101:390–397Crossref, Medline, Google Scholar
27 Massara F, Camanni F: Propranalol block of adrenaline-induced hypophosphatemia in man. Clin Sci 1970; 38:245–250Crossref, Medline, Google Scholar
28 Frommer JP: Lactic acidosis. Med Clin North Am 1983; 67:815–829Crossref, Medline, Google Scholar
29 Magistretti PJ, Pellerin L, Martin JL: Brain energy metabolism: an integrated cellular perspective, in Psychopharmacology: The Fourth Generation of Progress, edited by Bloom FE, Kupfer DJ. New York, Raven, 1995, pp 657–670Google Scholar
30 Sibson NR, Dhankhar A, Mason GF, et al: Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci 1998; 95:316–321Crossref, Medline, Google Scholar
31 Pellerin L, Magestretti PJ: Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci 1994; 91:10625– 10629Google Scholar
32 Jones M, Scarisbrick R: The effect of exercise on soldiers with neurocirculatory asthenia. Psychosomatic Med 1946; 8:188–194Crossref, Medline, Google Scholar
33 Cohen ME, White P: Life Situations, emotions and neurocirculatory asthenia (anxiety neurosis, neurasthenia, effort syndrome). Proceedings of the Association for Research in Nervous and Mental Disease 1950; 29:832–869Google Scholar
34 Pitts FN, McClure JN: Lactate metabolism in anxiety neurosis. N Engl J Med 1967; 277:1329–1336Google Scholar
35 Carr DB, Sheehan DV: Panic anxiety: a new biological model. J Clin Psychiatry 1984; 45:323–330Medline, Google Scholar
36 Edvinsson L, MacKenzie ET, McCulloch J: Changes in arterial gas tensions, in Cerebral Blood Flow and Metabolism, edited by Edvinsson L, MacKenzie ET, McCulloch J. New York, Raven, 1993, pp 524–552Google Scholar
37 Leusen I, Weyne J: Metabolic processes in the brain during respiratory and non-respiratory alkalosis and acidosis, in Acid-Base Homeostasis of the Brain Extracellular Fluid and the Respiratory Control System, edited by Loeschcke HH. Stuttgart, Thieme, 1976, pp 27–44Google Scholar
38 Kauppinen RA, Williams SR: Use of NMR spectroscopy in monitoring cerebral pH and metabolism during systemic and focal acid-base disturbances, in pH and Brain Function, edited by Kaila K, Ransom BR. New York, Wiley-Liss, 1998, pp 605–619Google Scholar
39 Kaila K, Ransom BR: Concept of pH and its importance in neurobiology, in pH and Brain Function, edited by Kaila K, Ransom BR. New York, Wiley-Liss, 1998, pp 3–10Google Scholar
40 van Rijen PC, Luyten PR, van Der Sprenkel JW, et al: 1H and 31P NMR measurement of cerebral lactate, high-energy phosphate levels, and pH in humans during voluntary hyperventilation: associated EEG, capnographic, and Doppler findings. Magn Reson Med 1989; 10:182–193Crossref, Medline, Google Scholar
41 Petroff OA, Prichard JW, Behar KL, et al: Cerebral metabolism in hyper- and hypocarbia: 31P and 1H nuclear magnetic resonance studies. Neurology 1985; 35:1681–1688Google Scholar
42 Granholm L, Lukjanova L, Siesjo BK: The effect of marked hyperventilation upon tissue levels of NADH, lactate, pyruvate, phosphocreatine and adenosine phosphates of rat brain. Acta Physiol Scand 1969; 77:179–180Crossref, Medline, Google Scholar
43 Granholm L, Siesjo BK: The effect of combined respiratory and nonrespiratory alkalosis on energy metabolites and acid-base parameters in the rat brain. Acta Physiol Scand 1971; 81:307–314Crossref, Medline, Google Scholar
44 Nilsson L, Busto R: Controlled hyperventilation and its effects on brain energy and acid-base parameters. Acta Anaesthesiol Scand 1973; 17:243–252Crossref, Medline, Google Scholar
45 Carlsson C, Nilsson L, Siesjo BK: Cerebral metabolic changes in arterial hypocapnia of short duration. Acta Anaesthesiol Scand 1974; 18:104–113Crossref, Medline, Google Scholar
46 Kogure K, Busto R, Matsumoto A, et al: Effect of hyperventilation on dynamics of cerebral energy metabolism. Am J Physiol 1975; 228:1862–1867Google Scholar
47 Young RS, Yagel SK: Cerebral physiological and metabolic effects of hyperventilation in the neonatal dog. Ann Neurol 1984; 16:337–342Crossref, Medline, Google Scholar
48 Macmillan V, Siesjo BK: The influence of hypocapnia upon intracellular pH and upon some carbohydrate substrates, amino acids and organic phosphates in the brain. J Neurochem 1973; 21:1283–1299Google Scholar
49 Wasserman AJ, Patterson JL: The cerebral vascular response to reduction in arterial carbon dioxide tension. J Clin Invest 1961; 40:1297–1303Google Scholar
50 McHenry LC, Slocum HC, Bivens HE, et al: Hyperventilation in awake and anesthetized man: effects on cerebral blood flow and cerebral metabolism. Arch Neurol 1965; 12:270–277Crossref, Medline, Google Scholar
51 Ball S, Shekhar A: Basilar artery response to hyperventilation in panic disorder. Am J Psychiatry 1997; 154:1603–1604Google Scholar
52 Gibbs DM: Hyperventilation-induced cerebral ischemia in panic disorder and effect of nimodipine. Am J Psychiatry 1992; 149:1589–1591Google Scholar
53 Maddock RJ, Carter CS, Magliozzi JR, et al: Evidence that decreased function of lymphocyte B adrenoreceptors reflects regulatory and adaptive processes in panic disorder with agoraphobia. Am J Psychiatry 1993; 150:1219–1225Google Scholar
54 Davson H, Segal MB: Physiology of the CSF and Blood-Brain Barriers. Boca Raton, FL, CRC, 1996, pp 459–488Google Scholar
55 Chesler M: Principles and practical aspects of pH buffering, in pH and Brain Function, edited by Kaila K, Ransom BR. New York, Wiley-Liss, 1998, pp 11–20Google Scholar
56 Shioiri T, Kato T, Murashita J, et al: High-energy phosphate metabolism in the frontal lobes of patients with panic disorder detected by phase-encoded 31P MRS. Biol Psychiatry 1996; 40:785– 93Crossref, Medline, Google Scholar
57 Bevensee MO, Boron WF: Thermodynamics and physiology of cellular pH regulation, in pH and Brain Function, edited by Kaila K, Ransom BR. New York, Wiley-Liss, 1998, pp 173–194Google Scholar
58 Bevensee MO, Boron WF: pH regulation in mammalian neurons, in pH and Brain Function, edited by Kaila K, Ransom BR. New York, Wiley-Liss, 1998, pp 211–231Google Scholar
59 Rose CR, Ransom BR: pH regulation in mammalian glia, in pH and Brain Function, edited by Kaila K, Ransom BR. New York, Wiley-Liss, 1998, pp 253–275Google Scholar
60 Hall RA, Premont RT, Chow CW, et al: The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature 1998; 392:626–630Crossref, Medline, Google Scholar
61 Smith GA, Brett CL, Church J: Effects of noradrenaline on intracellular pH in acutely dissociated adult rat hippocampal CA1 neurones. J Physiology 1998; 512(pt 2):487–505Google Scholar
62 Schlüter KD, Schäfer M, Balser C, et al: Influence of pHi and creatine phosphate on alpha-adrenoceptor-mediated cardiac hypertrophy. J Mol Cell Cardiol 1998; 30:763–771Crossref, Medline, Google Scholar
63 Ou-yang Y, Mellergard P, Siesjo B: Regulation of intracellular pH in single rat cortical neurons in vitro: a microspectrofluorometric study. J Cereb Blood Flow Metab 1993; 13:827–840Crossref, Medline, Google Scholar
64 Brosius FC 3rd, Pisoni RL, et al: AE anion exchanger mRNA and protein expression in vascular smooth muscle cells, aorta, and renal microvessels. Am J Physiol 1997; 273(6, pt 2):F1039–F1047Google Scholar
65 Nattie E: Control and disturbances of cerebrospinal fluid pH, in pH and Brain Function, edited by Kaila K, Ransom BR. New York, Wiley-Liss, 1998, pp 629–650Google Scholar
66 Uhde TW, Boulenger JP: Caffeine model of panic, in New Directions in Affective Disorders, edited by Lerer B, Gershon S. New York, Springer-Verlag, 1989, pp 410–413Google Scholar
67 Tancer ME, Stein MB, Uhde TW: Lactic acid response to caffeine in panic disorder: comparison to social phobics and normal controls. Anxiety 1994; 1:138–140Crossref, Medline, Google Scholar
68 Lee MA, Cameron OG, Greden JF: Anxiety and caffeine consumption in people with anxiety disorders. Psychiatry Res 1985; 15:211–217Crossref, Medline, Google Scholar
69 Dager SR, Layton ME, Strauss W, et al: Human brain metabolic response to caffeine and the effects of tolerance. Am J Psychiatry 1999; 156:229–237Medline, Google Scholar
70 Garssen B, Buikhuisen M, van Dyck R: Hyperventilation and panic attacks. Am J Psychiatry 1996; 153:513–518Crossref, Medline, Google Scholar
71 Redmond DE Jr: Studies of the nucleus locus coeruleus in monkeys and hypotheses for neuropsychopharmacology, in Psychopharmacology: The Third Generation of Progress, edited by Melzer HY. New York, Raven, 1987, pp 967–975Google Scholar
72 Munjack DJ, Crocker B, Cabe D, et al: Alprazolam, propranolol, and placebo in the treatment of panic disorder and agoraphobia with panic attacks. J Clin Psychopharmacol 1989; 9:22–27Medline, Google Scholar
73 Ravaris CL, Friedman MJ, Hauri PJ, et al: A controlled study of alprazolam and propranolol in panic-disordered and agoraphobic outpatients. J Clin Psychopharmacol 1991; 11:344–350Crossref, Medline, Google Scholar
74 Modest VE, Butterworth JF 4th: Effect of pH and lidocaine on beta-adrenergic receptor binding: interaction during resuscitation? Chest 1995; 108:1373–1379Google Scholar
75 Krueger KM, Daaka Y, Pitcher JA, et al: The role of sequestration in G protein-coupled receptor resensitization. Regulation of beta2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem 1997; 272:5–8Crossref, Medline, Google Scholar



