Self-Administered Screening for Mild Cognitive Impairment: Initial Validation of a Computerized Test Battery
Abstract
The Computer-Administered Neuropsychological Screen for Mild Cognitive Impairment (CANS-MCI), a computer administered, scored, and interpreted touch screen battery was evaluated for its ability to detect mild cognitive impairment. Subjects were three hundred ten community-dwelling elders who enrolled in an National Institute on Aging (NIA)-funded study. One-month test-retest reliability correlations were all significant (p<0.05–p<0.001). Concurrent validity correlations were all significant (p<0.001). A high level of diagnostic validity was attained relative to the Weschler Memory Scale-Revised (WMS-R) LMS-II test (p<0.001). Confirmatory factor analysis supported a three-factor model indicating the tests measure the intended cognitive dimensions of memory, language/spatial fluency, and executive function/mental control. Goodness-of-fit indicators were strong (Bentler Comparative Fit Index = 0.99; Root Mean Square Error of Approximation=0.055). Initial validation analyses indicate that the CANS-MCI shows promise of being a reliable, valid screening tool in determining whether more intensive testing for early cognitive impairment is warranted.
Since most new research and treatments for dementia focus on slowing the progression of Alzheimer’s disease (AD),1,2 it is critical to identify the need for intensive diagnostic evaluation so that early treatment can delay its progression.3,4 People with mild cognitive impairment (MCI), characterized by marked memory impairment without disorientation, confusion, or abnormal general cognitive functioning appear to develop AD at a rate of 10%–15% a year.5–7 Research concerning MCI, both as a distinct diagnostic entity and as a precursor to AD,6–11 suggests that instruments focused upon MCI measurement would provide useful screening information for decisions concerning full diagnostic evaluations for AD. Brief or automated neuropsychological tests may be the preliminary step most suited to determine the need for evaluations, which require costly neuropsychological, biochemical, or neuroimaging techniques.12
While memory deficits have been found to be the most reliable single predictor,5,10,13–15 studies indicate that tests sampling different cognitive domains, when combined, significantly enhance the predictive validity of a test battery because of variations in the initial cognitive deficits associated with AD.15–19 Current methods of detection are costly and often deferred until later in the disease process when interventions to delay AD are likely to be less effective. Therefore, an effective screening device for MCI would be cost efficient and incorporate tests that assess multiple cognitive domains. In this article, we assess the reliability and validity of a touch screen test battery that accomplishes these goals and is administered, scored, and interpreted by computer: The Computer-Administered Neuropsychological Screen for Mild Cognitive Impairment (CANS-MCI).
METHODS
Subjects
A community sample of 310 elderly people was recruited through senior centers, American Legion halls, and retirement homes in four counties of Washington State. Exclusionary criteria were non-English language, significant hand tremor, inability to sustain a seated position for 45 minutes, recent surgery, cognitive side effects of drugs, indications of recent alcohol abuse, or inadequacies in visual acuity, reading ability, hearing, or dominant hand agility. The subjects were predominantly Caucasian (86%), female (65%), and had at least some college education (76%). Subject age ranged from 51–93 years, with the majority between 60–80 years of age (63%).
Neuropsychological Measures
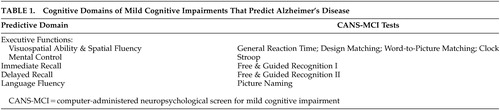
The CANS-MCI tests were based upon the cognitive domains found to be predictive of AD in previous neuropsychological research: visuospatial ability and spatial fluency;4,15,17,20–22 executive mental control;1,23,24 immediate and delayed recall;4,5,13–15,17,18,20–27 and language fluency 4,5,17,18,20–22,25,27 (Table 1). The usability of the CANS-MCI has been previously evaluated.28 It can be fully self-administered, regardless of computer experience, even by elderly people with MCI. It does not cause significant anxiety-based cognitive interference during testing. After a researcher enters a subject identification and adjusts the volume, the tests, including any assistance needed, are administered by a computer with external speakers and a touch screen.
The CANS-MCI was developed to include tests designed to measure executive function, language fluency, and memory (Table 1). The executive function tests sample two cognitive abilities: mental control and spatial abilities. Mental control is measured with a Stroop test (on which the user matches the ink color of word names rather than the name itself). Spatial abilities were tested with: a general reaction time test with minimal cognitive complexity (on which the user touches ascending numbers presented on a jumbled display; design-letter matching; word-to-picture matching; and a clock hand placement test (touching the hour and minute positions for a series of digital times). Memory for 20 object names was measured with an immediate and a delayed free and guided recognition test. Language fluency is tested with a picture naming test (on which pictures are each presented with four 2-letter word beginnings).
Unless specifically described as latency measures, the CANS-MCI and conventional neuropsychological tests in this study measure response accuracy. All latency and accuracy measures on the CANS-MCI are scalable scores.
The following conventional neuropsychological tests were used to assess the validity of the CANS-MCI tests: Weschler Memory Scale-Revised (WMS-R) Logical Memory Immediate and Delayed (LMS-I and LMS-II);29 Mattis Dementia Rating Scale (DRS);30 and Weschler Adult Intelligence Scale, Digit Symbol test.31 LMS-II scores were used to classify participants as having normal cognitive functioning or MCI. The LMS-II has previously been found to differentiate normal from impaired cognitive functioning.4,5,17
Procedures
Each testing session lasted approximately 1 hour. The progression of tests was designed to minimize intertest interference. The order was: Digit Symbol, DRS, LMS-I, CANS-MCI, LMS-II. The CANS-MCI tests lasted approximately 1/2 hour. During the first session, test procedures were started after the testing procedure was explained and informed written consent obtained. Subjects were tested at Time 1, one month later (Time 2), and six months later (Time 3). This study was approved by the University of Washington’s Human Subjects Committee and the Western Institutional Review Board.
Data Analyses
Alpha coefficient reliabilities were performed to analyze the internal consistency of the scale items. Pearson correlations and paired sample t tests were used to evaluate the test-retest reliability of the CANS-MCI tests. Concurrent validity was evaluated by running Pearson correlations to compare the CANS-MCI tests with scores on conventional neuropsychological tests administered during the same test sessions. To assess diagnostic validity, independent sample t tests were used to analyze differences between those subjects in the lowest 10th percentile of cognitive functioning and subjects in the highest 90th percentile based on their LMS-II scores. We conducted exploratory and confirmatory factor analyses on different subsets of data to establish whether our tests measure the expected cognitive domains.
RESULTS
Reliability
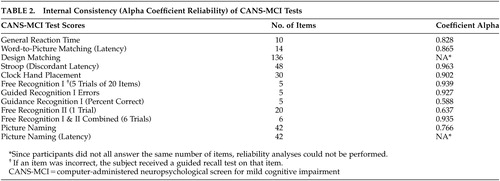
Internal consistency was examined (Table 2) using Cronbach's Coefficient alpha reliabilities to determine the degree to which individual test items correlate with one another. Only two test scores (Free Recognition II and Guidance Recognition I Percent Correct) did not meet the predetermined standard for internal consistency (α >.70). When the Free Recognition II score was combined with the Free Recognition I score, the combination had very high reliability (α=0.93).
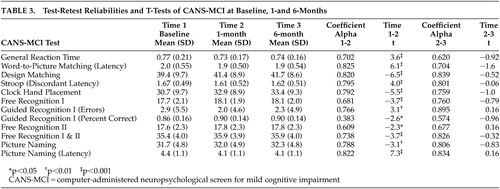
Correlations measuring the stability of the CANS-MCI over a 1-month period ranged from 0.61–0.85 (Table 3). The Free Recognition I and II tests had coefficient alphas below 0.70. When these tests were combined to form a more global memory measure the alpha was acceptable (α=0.74), therefore, they were included in further analyses. Six-month test-retest reliabilities ranged from 0.62–0.89; 9 of the 12 scores demonstrated higher 6-month reliabilities than 1-month reliabilities. One of the two measures of guidance effectiveness (Guidance Percent Correct) had low internal consistency and test-retest alphas (1-month and 6-month) and therefore was not included in further analyses. Separate analyses indicated little relationship to education level on either internal consistency or test-retest reliability of the measures. All Time 2 test scores were significantly better than Time 1 scores but scores remained stable from times 2 to 3. When subjects were already familiar with the testing procedures, test scores on the CANS-MCI demonstrated markedly higher score stability over 6 months than they did over the initial 1-month period. This was confirmed by t test analyses that showed significant improvements in all scores from Time 1 to Time 2 but no significant differences from Time 2 to Time 3.
Validity
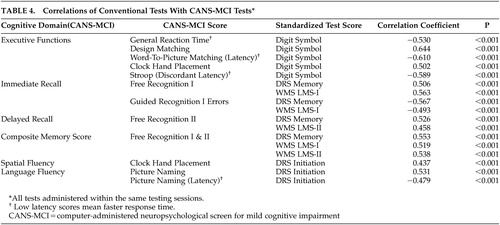
The concurrent validity of the CANS-MCI was evaluated by examining the correlations of the CANS-MCI component tests with the scores on the Digit Symbol, LMS-I and II, and the DRS. The correlations between each CANS-MCI test and its domain-specific conventional neuropsychological tests are listed in Table 4. The coefficients ranged from 0.44 to 0.64, all highly significant (p<0.001). For 10 of the 11 CANS-MCI tests, the correlation was the strongest with the conventional neuropsychological tests designed to measure the comparable cognitive domains. Immediate memory on the CANS-MCI (Free Recognition I) was more highly correlated with the delayed memory score on the LMS-II (r=0.560; data not shown) than with the hypothesized immediate memory scale on the DRS (r=0.506).
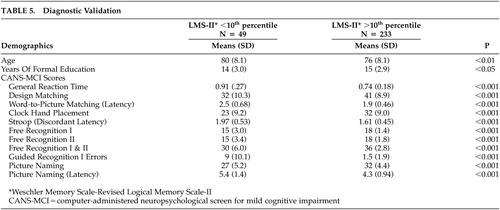
Diagnostic Validation
T tests were performed to analyze differences between memory-impaired and unimpaired groups. Though Free Recognition I and II scales individually have moderate reliabilities, they were separated in the t tests because we hypothesized that they might measure different aspects of a cognitive function. Highly significant differences were observed between the memory intact group and the memory-impaired group on all CANS-MCI tests. A significant difference in age and education between groups was also observed (Table 5).
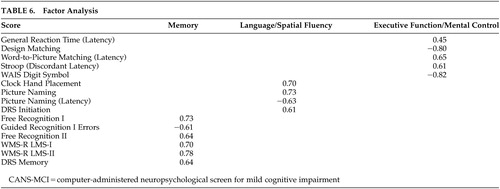
Factor Analysis
Confirmatory factor analysis supported the highly correlated three-factor model (memory, language/spatial fluency, and executive function/mental control) found in the exploratory analysis (data not shown) indicating the tests measure the intended cognitive dimensions (Table 6). The factor loadings in the confirmatory analysis were all significant and ranged from 0.45–0.82. The Free and Guided Recognition, Guided Recognition Errors, LMS, and DRS Memory tests comprised the memory factor. Four tests scores made up the language/spatial fluency factor: Clock Hand Placement, Picture naming, Picture Naming (latency), and DRS Initiation. The Executive Function/Mental Control factor consisted of General Reaction Time, Design Matching, Word-to-Picture Matching (Latency), Stroop (Discordant Latency), and WAIS Digit Symbol. Goodness of fit indicators were strong (χ2 70= 155.65, p=0.001.; Bentler Comparative Fit Index =0.99; Root Mean Square Error of Approximation=0.055). The three factors were highly correlated (0.91, 0.75, 0.97). Because the correlation between language/spatial fluency and executive function/mental control factors was so high (.97) we combined them into one factor and tested the two-factor model against the three-factor model. The two-factor solution was not as strong as the three-factor model (χ2 2= 15.96, p<0.001).
Discussion
The results of these initial analyses indicate the CANS-MCI is a user-friendly, reliable, and valid instrument that measures several cognitive domains. The CANS-MCI computer interactions were designed to provide a high degree of self-administration usability. Our results indicate high internal reliability and high test-retest reliability comparable to other validated instruments.30–33 One score, Guidance Percent Correct, was not highly reliable and therefore was not included in further analyses. The test scores are all more consistent from Time 2 to Time 3 than they were from Time 1 to Time 2, as demonstrated by the t-scores. Even memory tests are more stable once subjects are familiar with the tests, despite the much longer time interval. All CANS-MCI test scores improve with one previous exposure, which indicates that familiarity with the testing procedures appears to make test results more dependable. It would be advisable to have a patient take the test once for practice and use the second testing as the baseline measure. Recommendations for more extensive evaluation should be based upon multiple testing sessions, at least several months apart.
The CANS-MCI subtests correlate with the LMS-I & II, Digit Symbol and DRS highly enough to demonstrate concurrent validity. However, the moderate correlations indicate some differences, which might be attributed to the different ranges of ability the tests were designed to measure. For example, the DRS Initiation Scale is less discriminating at higher ability levels30 than are the CANS-MCI Clock Hand Placement and Picture Naming tests with which is it best correlated; 42% of the subjects obtained a perfect DRS Initiation score, while 4.8% and .5% received perfect scores on the Clock Hand Placement and Naming Accuracy tests, respectively. The LMS-II is less discriminating than the CANS-MCI Free Recognition II score at the lower levels of functioning; 20 (7%) subjects received a LMS-II score of 0 and 1 (0.4%) of the subjects received the lowest score on the Free Recognition II. On the other hand, the LMS-II appears to be more discriminating at the higher levels of functioning. No subjects received a perfect LMS-II score, while 19% of subjects got the highest score possible on Free Recognition II. The CANS-MCI Free Recognition II test is substantially different from the LMS-II free recall test because free recall without prompting (used in the LMS-II) is more difficult than guided recall. Like other tests that guide learning,34 the CANS-MCI Free Recognition test may enhance “deep semantic processing,”13 allowing trace memories to improve its scores while not improving test scores on unprompted recall.35
Diagnostic validity is an important criterion for cognitive screening effectiveness. T tests based upon the LMS-II diagnostic criterion show that the CANS-MCI tests differentiate between the memory-impaired and unimpaired elderly. Although the reference measure (LMS-II) is a limited criterion of diagnostic validity, the corresponding differences on all the CANS-MCI tests suggest that the CANS-MCI has enough diagnostic validity to pursue more extensive analyses. The CANS-MCI also measures changes over time, a feature that seems likely to enhance its validity when assessing highly educated people who have greater cognitive reserve. Given that reserve capacity can mask the progression of AD,36 comparisons of highly educated persons against their own previous scores might detect the insidious progression of predementia changes long before the diagnosis of MCI or AD would otherwise be reached. The extent to which sensitivity/specificity and longitudinal analyses confirm this dimension of validity is currently being studied, using full neuropsychological evaluations as the criterion standard.
The factor analyses indicated that CANS-MCI tests load onto three main factors: memory, language/spatial fluency, and executive function/mental control. These factors are similar to the three aspects of cognitive performance measured by the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) test batteries.37 The CANS-MCI, LMS and DRS tests that measure the immediate acquisition or delayed retention abilities load onto Memory. Language/spatial fluency has many tests that measure the retrieval from semantic memory of words (or in the case of the Clock Hand Placement Test, spatial representation of numbers). The General Reaction Time, Design Matching, Word-to-Picture Matching, Stroop (Discordant Latency), and the Digit Symbol tests make up the Executive Function/Mental Control factor which appears to represent rapid attention switching and mental control. The memory, language/spatial fluency, and executive function/mental control factors were highly correlated, as would be expected of cognitive functions all associated with MCI and/or AD. Even though highly correlated, they appear to be distinct factors.
The Clock Hand Placement test was not part of the executive function/mental control factor as hypothesized, though in pairwise analyses (Table 4) the test had the highest correlation with the Digit Symbol Test--a conventional measure of several executive functioning abilities that is highly predictive of AD.21 However, the Clock Hand Placement test also correlated moderately with the DRS Initiation Scale (r=0.437), a scale that involves the naming of items in a grocery store. Both the Clock Hand Placement and DRS Initiation tests involve mental visualization translated into a response, and both load on the language/spatial fluency factor. The test that most heavily loads on language/spatial fluency (picture naming) also requires visualization of an answer before making a response.
Computerized tests have several advantages. By consistent administration and scoring, they have the ability to reduce examiner administration and scoring error,12,38 as well as reduce the influence of the examiner on patient response.33 Because such tests are self-administered and no training is required to administer them, they take little staff time. Other cognitive screening tests, both brief and computerized, still take significant amounts of staff time to administer and score. Medications for stalling memory decay are being widely used and are expected to be most effective when started early in the course of dementia. Therefore, it will become increasingly important to identify cognitive impairment early in the process of decline.3,4,39 The CANS-MCI is also amenable to translation with automated replacement of text, pictures, and audio segments based upon choice of a language and a specific location (e.g., English/England) at the beginning of the testing program. The CANS-MCI was created for U.S. English speakers and versions have not yet been validated for other populations.
There are several limitations to the study. A community sample increases the chance of false positives because of the low incidence of impairment in the population as compared to a clinical sample.40 There may also be a selection bias in our sample since participants were volunteers with high levels of education.
The ability of the CANS-MCI to discriminate between individuals with and without MCI has not yet been adequately compared with findings of a criterion standard such as an independent neuropsychological battery. We do not yet know what combination of tests and scores have the most sensitivity and specificity in predicting the presence of MCI. The next step in our research is to determine this using a full neuropsychological battery as the criterion standard. Further, we will look at what amount of change over time is significant enough to warrant a recommendation for comprehensive professional testing.
The CANS-MCI is a promising tool for low-cost, objective clinical screening. Further validation research is under way to determine if its longitudinal use may indicate the presence or absence of cognitive impairment well enough to guide clinician decisions about the need for a complete diagnostic evaluation.
ACKNOWLEDGMENTS
The authors wish to thank Bruce Center, Ph.D. for statistical assistance.
Research for this study was supported by the National Institute on Aging, the National Institutes of Health (1R43AG1865801 and 2R44AG18658-02), and an earlier test development grant from the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (LIP 61-501).
A partial version of these data was presented at the 8th International Conference on Alzheimer’s Disease and Related Disorders, Stockholm, Sweden 2002.
 |
 |
 |
 |
 |
 |
1 Bookheimer SA, Strojwas BS, Cohen MS, et al: Patterns of brain activation in people at risk for developing Alzheimer's disease. N Engl J Med 2000; 343(7):450–456Crossref, Medline, Google Scholar
2 Petersen RC, Stevens JC, Ganguli M, et al: Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurol 2001; 56:1133–1142Crossref, Medline, Google Scholar
3 Brookmeyer R, Gray S, Kawas C: Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health 1998; 88:1337–1342Crossref, Medline, Google Scholar
4 Howieson DB, Dame A, Camicioli R, et al: Cognitive markers preceding Alzheimer’s dementia in the healthy oldest old. J Am Geriatr Soc 1997; 45:584–589Crossref, Medline, Google Scholar
5 Petersen RC, Smith GE, Waring SC, et al: Mild cognitive impairment: clinical characterization and outcome. Arch Neuro 1999; 56:303–308Crossref, Medline, Google Scholar
6 Shah S, Tangalos EG, Petersen RC: Mild cognitive impairment: when is it a precursor to Alzheimer’s disease? Geriatrics 2000; 55:62–68Medline, Google Scholar
7 Petersen RC, Doody R, Kurz A, et al: Current concepts in mild cognitive impairment. Arch Neurol 2001; 58:1985–1992Crossref, Medline, Google Scholar
8 Ritchie K, Touchon J: Mild cognitive impairment: conceptual basis and current nosological status. Lancet2000; 355:225–228Google Scholar
9 Chertkow H: Mild cognitive impairment. Curr Opin Neurol 2002; 15:401–407Crossref, Medline, Google Scholar
10 Morris JC, Storandt M, Miller JP, et al: Mild cognitive impairment represents early-stage Alzheimer Disease. Arch Neurol 2001; 58:397–405Medline, Google Scholar
11 Reischies FM, Hellweg R: Prediction of deterioration in mild cognitive disorder in old age neuropsychological and neurochemical parameters of dementia diseases. Compr Psychiatry 2000; 41:Suppl 2:66–75Google Scholar
12 Green RC, Green J, Harrison JM, et al: Screening for cognitive impairment in older individuals. Arch Neurol 1994; 51:779–786Crossref, Medline, Google Scholar
13 Grober E, Kawas C: Learning and retention in preclinical and early Alzheimer's disease. Psychol Aging 1997; 12(1):183–188Crossref, Medline, Google Scholar
14 Fuld PA, Masur DM, Blau AD, et al: Object-memory evaluation for prospective detection of dementia in normal functioning elderly: predictive and normative data. J Clin Exp Neuropsychol 1990; 12:520–528Crossref, Medline, Google Scholar
15 Kluger A, Ferris SH, Golomb J, et al: Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psychiatry Neurol 1999; 12(4):168–179Crossref, Medline, Google Scholar
16 Jacobs DM, Sano M, Dooneief G, et al: Neuropsychological detection and characterization of preclinical Alzheimer's disease. Neurol 1995; 45:957–962Crossref, Medline, Google Scholar
17 Chen P, Ratcliff G, Belle SH, et al: Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurol 2000; 55:1847–1853Crossref, Medline, Google Scholar
18 Hänninen T, Hallikainen M, Koivisto K, et al: A follow-up study of age-associated memory impairment neuropsychological predictors of dementia. J Am Geriatr Soc 1995; 43:1007–1015Crossref, Medline, Google Scholar
19 Bozoki A, Giordani B. Heidebrink JL, et al:. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol 2001; 58:411–416Crossref, Medline, Google Scholar
20 Masur DM, Sliwinski M, Lipton RB, et al: Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurol 1994; 44:1427–1432Crossref, Medline, Google Scholar
21 Flicker C, Ferris SH, Reisberg B: A two-year longitudinal study of cognitive function in normal aging and Alzheimer's disease. J Geriatr Psychiatry Neurol 1993; 6:84–96Crossref, Medline, Google Scholar
22 Welsh KA, Butters N, Hughes JP, et al: Detection and staging of dementia in Alzheimer's disease: use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Arch Neurol 1992; 49(5):448–452Crossref, Medline, Google Scholar
23 Tierney MC, Szalai JP, Snow WG, et al: Prediction of probable Alzheimer's disease in memory-impaired patients: a prospective longitudinal study. Neurol1996; 46(3):661–665Google Scholar
24 Berg L, Danziger WL, Storandt M, et al: Predictive features in mild senile dementia of the Alzheimer type. Neurol1984; 34:563–569Google Scholar
25 Bondi MW, Monsch AU, Galasko D, et al: Preclinical cognitive markers of dementia of the Alzheimer's type. Neuropsychol1994; 8(3):374–384Google Scholar
26 Tuokko H, Vernon-Wilkinson R, Weir J, et al: Cued recall and early identification of dementia. J Clin Exp Neuropsychol 1991; 13:871–879Crossref, Medline, Google Scholar
27 Jacobs DM, Sano M, Dooneief G, et al: Neuropsychological detection and characterization of preclinical Alzheimer's disease. Neurol 1995; 45:957–962Crossref, Medline, Google Scholar
28 Hill E, Hammond K: The usability of multimedia automated psychological tests to screen for Alzheimer's disease. Proc AMIA Symposium 2000:1030Google Scholar
29 Wechsler, D: Wechsler Memory Scale-Revised. New York, NY: Psychological Corporation; 1987Google Scholar
30 Jurica PJ, Leitten CL, Mattis, S: DRS-2 Dementia Rating Scale -2: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc.; 2001Google Scholar
31 Wechsler, D: Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: The Psychological Corporation; 1981Google Scholar
32 Ruchinskas RA, Singer HK, Repetz NK: Clock drawing, clock copying, and physical abilities in geriatric rehabilitation. Arch Phys Med Rehabil 2001; 82:920–924Crossref, Medline, Google Scholar
33 Campbell KA, Rohlman DS, Storzbach D, et al: Test-retest reliability of psychological and neurobehavioral tests self-administered by computer. Assessment 1999; 6(1):21–32Crossref, Medline, Google Scholar
34 Buschke H: Cued recall in amnesia. J of Clinical Neuropsychol 1984; 6(4):433–440Crossref, Medline, Google Scholar
35 Tuokko H, Crockett D: Cued recall and memory disorders in dementia. J Clin Exp Neuropsychol 1989; 11(2):278–294Crossref, Medline, Google Scholar
36 Snowdon DA, Kemper SJ, Mortimer JA, et al: Linguistic ability in early life and cognitive function and Alzheimer's disease in late life. JAMA 1996; 275(7):528–532Crossref, Medline, Google Scholar
37 Morris JC, Heyman A, Mohs RC, et al: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurol 1989; 39:1159–1165Crossref, Medline, Google Scholar
38 Letz R, Green RC, Woodard JL: Development of a computer-based battery designed to screen adults for neuropsychological impairment. Neurotoxicol Teratol 1996; 18(4):365–370Crossref, Medline, Google Scholar
39 Ganguli M, Dodge HH, Chen P, et al: Ten-year incidence of dementia in a rural elderly US community population: the MoVIES Project. Neurol 2000; 54:1109–1116Crossref, Medline, Google Scholar
40 Albert M, Smith LA, Scherr PA, et al: Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci 1991; 57:167–178Crossref, Medline, Google Scholar



