Behavioral Problems in Dementia Patients and Salivary Cortisol Patterns in Caregivers
Abstract
This study examines cortisol profiles in caregivers of dementia patients and their relationship to patients’ behavioral problems. Salivary cortisol profiles and cortisol response to awakening were measured in 57 caregivers and 55 noncaregiver comparison subjects. Caregivers showed significantly higher levels of cortisol at the time of morning awakening than did comparison subjects, with a smaller increase after awakening. A higher cortisol awakening response was found in caregivers of patients with high versus low levels of behavioral and psychological symptoms of dementia (BPSD). Elevated morning cortisol levels could predispose caregivers to negative health consequences, with caregivers of patients with BPSD at greater risk.
The behavioral and psychological symptoms of dementia (BPSD) exert a major influence on the quality of life of patients and their caregivers. These symptoms are reported by caregivers as difficult to manage and a major source of stress.1–3 Several studies demonstrated that patient behavioral problems are stronger predictors of caregiver distress than patient cognitive or functional impairment.1,4–8 However, little data about the impact of BPSD on physiological stress responses in caregivers are available. Assessment of physiological reactions to stress adds objective evidence to subjective stress reports in caregivers. Furthermore, physiological responses to long-term stress generated by patient problem behavior may contribute to an increased vulnerability to disease in caregivers. Several studies report that caregivers of dementia patients have poorer subjective health,9 more frequently visit a general practitioner, and take more medications, compared to noncaregivers.10,11 The primary goal of this study was to explore the relationship between behavioral and psychological symptoms in dementia patients and stress responses in their primary caregivers.
One of the most extensively studied physiological responses to stress is change in the activity of the hypothalamic-pituitary-adrenal (HPA) axis, as reflected in cortisol secretion.12 Chronic stress can lead to either increases or decreases in overall cortisol levels.13,14 Because cortisol has a pronounced circadian rhythm, with high levels in the morning and a decline in levels during the day, diurnal cortisol profiles provide a better picture of HPA activity than measurements at a single point in time. Another measure of HPA activity that may be a useful tool in stress assessment is the cortisol response to morning awakening.15–18 Cortisol increases rapidly after awakening, with peak levels observed after 30–40 minutes. Chronic stress appears to influence the magnitude of this response, although it is not yet clear in which direction. Increased morning cortisol responses were found in individuals experiencing chronic work overload.16 In contrast, blunted cortisol responses to awakening have been reported in subjects with burnout.13 Possible confounding factors include awakening time, health status, age, and sleep quality.19,20
A number of studies have investigated the effects of chronic stress on HPA activity in caregivers of dementia patients. Among these studies, most reported elevated daytime cortisol levels in caregivers, compared with healthy comparison subjects,21–24 and two found no difference in cortisol levels, when compared with comparison subjects.25,26 Another study found that cortisol levels in caregivers were associated with self-reported distress.27 Of these studies mentioned above, however, each assessed daytime cortisol profiles only and not the morning response in caregivers. To date, there has also been no attempt to relate changes in cortisol levels to patient functioning as a potential source of chronic stress in caregivers.
This study tests the hypothesis that diurnal levels of salivary cortisol and the cortisol awakening response differ between caregivers and noncaregivers. Second, we examined the influence of behavioral and psychological syndromes in dementia patients on cortisol measures in their caregivers.
METHOD
Subjects
Participants were 57 primary caregivers of dementia patients and 55 noncaregiver, volunteer comparison subjects. The caregivers were participants in the Maastricht Study of Behavior in Dementia (MAASBED),28 a 2-year follow up study of the course and risk factors for BPSD. Patients were in treatment at the Memory Clinic of the Academic Hospital Maastricht or the geriatric division of the Regional Institute for Ambulatory Mental Health Care (RIAGG), Maastricht, the Netherlands. Caregivers were included if they were the primary caregiver and had contact with the patient at least once a week. Noncaregiver comparison subjects, matched with caregivers on sex, age, and education, were recruited by telephone from a pool of volunteers for previous studies. Caregivers and comparison subjects were excluded if they were taking medication that could have influenced cortisol levels. The study was approved by the Hospital Medical Ethics Committee, and informed consent was obtained from all participants.
Of the caregivers, 28 were spouses, 26 were children, and 3 were other family members or close friends. Mean length of caregiving was 31.1 months (range=3–120), with a mean contact time per week of 85.6 hours (range=2–168). Patients were diagnosed as having Alzheimer disease (N=46), vascular dementia (N=7), frontal lobe dementia (N=1), Parkinson’s disease (N=1), or mixed dementia (N=2). There were 21 male and 36 female patients, with a mean age of 76.2 (range=54–96) and a mean Mini-Mental State Examination score of 18.0 (range=5–27).
Saliva Collection
Salivary cortisol correlates well with free plasma cortisol,12 and sample collection is noninvasive. Subjects were given both oral and written instructions for collecting samples at home. Saliva samples were obtained directly after awakening, 30 minutes after awakening, at 4:00 p.m., and at 9:00 p.m. Subjects were free to use an alarm clock or to wake spontaneously. They were instructed not to eat, drink, or smoke in the 30 minutes before a saliva collection and to record the exact collection time. Samples were collected with a cotton dental roll and stored in a capped plastic vial (Salivette; Sarstedt, Etten-Leur, the Netherlands).
Additional Measures.
Patients.
Behavioral and psychological symptoms of dementia were measured with the Neuropsychiatric Inventory (NPI),29 a structured interview with the caregiver that evaluates 12 behavioral and psychological symptoms. The total score on each item can range from 1 to 12, obtained by multiplying severity (1 = “mild” to 3 = “severe”) by frequency (1 = “sometimes” to 5 = “very often”).
Caregivers.
For each BPSD on the NPI, caregivers rated the level of distress they experienced on a scale from 0 (none) to 5 (extreme). Neuropsychiatric Inventory-D Score is the sum of these 12 ratings.
Physical health complaints were measured with the physical functioning subscale of the RAND-36 Questionnaire.30 Ten items were rated on 3-point scales ranging from severely impaired to not at all impaired, with higher scores representing less impairment.
Caregivers and Comparison Subjects.
Overall subjective stress was measured with the Perceived Stress Scale, 10-item version (PSS).31 This questionnaire is a global measure of the degree to which situations in one’s life in the past month are appraised as stressful. Items were rated on a 5-point scale ranging from 0 (never) to 4 (very often).
The Symptom Checklist-90 (SCL-90)32 total score was used as a measure of general psychological distress.
We used the Montgomery-Åsberg Depression Rating Scale (MADRS),33 a structured interview, to measure depressive symptoms. Ratings (from 0 to 6) on 10 items were summed.
Caregivers and comparison subjects were asked to report any physical health problems and habitual mean hours of sleep per night.
Biochemical Analysis
Saliva samples were stored at −20°C until analysis. Salivary-free cortisol levels were determined in duplicate by direct radioimmunoassay (using a high-performance-liquid-chromatography-purified preparation of cortisol-3-CMO-histamine and antiserum made against the 3-CMO-BSA conjugate by Dr. J. Sulon, University of Liege, Belgium).
Statistical Analysis
Cortisol values were logarithmically transformed to normalize distributions. One comparison subject was excluded because cortisol levels were far outside the normal range (>55.2 nmol/liter). Six subjects (1 comparison subject, 5 caregivers) were excluded from analyses of awakening response due to deviant (<15 minute, >45 minute) or unknown time intervals between samples 1 and 2.
The awakening response was defined as the change in cortisol level from the first to the second sample (directly after awakening and 30 minutes later). Cortisol measures directly after waking up, at 4:00 p.m., and at 9:00 p.m. were standardized within time of day and averaged to compute daily average cortisol (DAC).34
Univariate comparisons were performed with Student’s t test, Chi Square test, and Mann-Whitney U test. Differences in diurnal cortisol profiles between caregivers and comparison subjects were analyzed using repeated measures multiple analysis of covariance (MANCOVA) with group (caregivers versus comparison subjects) and sex as between-subject factors and time (time of saliva samples 1, 3, and 4) as within-subject factors. Age and time of the first sample were covariates. To examine the awakening response, this analysis was repeated for the cortisol measures at awakening and 30 minutes later.
In a separate analysis, caregivers were assigned to a low BPSD and a high BPSD group, according to a median-split on BPSD total scores. Differences in cortisol levels between these two groups were analyzed, as above, with repeated measures MANCOVA, with BPSD (high versus low) as between-subject factors this time. Greenhouse-Geisser corrections were applied when appropriate. Finally, a forced entry regression analysis was performed to predict the cortisol awakening response by caregiver distress (NPI-D, RAND-36, MADRS and hours of sleep). Significance was tested with two-tailed tests, with alpha=0.05.
RESULTS
Group Characteristics
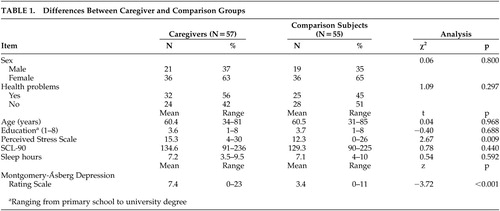
Caregiver and control characteristics are shown in Table 1. There were no significant differences between the two groups in age, education, or sex distribution. However, caregivers reported significantly more subjective stress (PSS) and more depressive symptoms (MADRS) than comparison subjects. No differences were found in general psychological distress (SCL-90) and sleep hours. Awakening time on the sampling day ranged from 04:10 a.m. to 10:20 a.m. and did not differ between comparison subjects and caregivers (t=0.04, df=108, p=0.967).
Differences Between Caregivers and Comparison Subjects in Cortisol Patterns
Multivariate analysis of covariance (MANCOVA) results showed a significant main effect of time (F=6.0, df=1.8, 184.5, p=0.004) and a time by group interaction effect (F=6.9, df=1.8, 184.5, p=0.002). Men had higher cortisol levels than women (F=4.7, df=1,100, p=0.033). There were no significant main effects of group, age, or time of awakening. As shown in Figure 1, cortisol levels were high in the morning and declined over the day in both groups. Post hoc comparisons revealed significantly higher cortisol levels for caregivers directly after waking (t=3.51, df=102, p=0.001).
For the cortisol awakening response, MANCOVA results showed no main effect of time but rather a significant time by group interaction effect (F=7.3, df=1, 96, p=0.008) as well as a significant main effect of group (F=4.5, df=1, 96, p=0.036), indicating that the cortisol increase after awakening differed between groups, with caregivers having higher overall cortisol levels in the morning and a smaller cortisol awakening response. There were no significant effects of sex, age, or time of awakening. Higher cortisol levels at awakening were related to an attenuated subsequent response (r=−0.58, p<0.001).
Association Between BPSD and Cortisol Patterns in Caregivers
Caregivers who reported many behavioral problems in the patient (high BPSD) were compared with those who reported fewer behavioral problems (low BPSD). The groups did not differ in the nature of the relationship between caregiver and patient (spouse versus offspringχ2=2.11, df=1, p=0.189), length of caregiving (t=−0.05, df=55, p=0.964), or contact hours per week (t=1.14, df=55, p=0.259). High BPSD caregivers reported significantly more distress related to patients’ symptoms (Mann-Whitney U Test; z=−5.47, p<0.001), more physical health complaints (Mann-Whitney U Test; Z=−2.62, p=0.009), and greater perceived stress (t=−2.11, df=53, p=0.040). The two groups did not differ in depressive symptoms (Mann-Whitney U; z=−0.57, p=0.570) or sleep hours (t=1.48, df=54, p=0.145).
To examine whether differences in cortisol levels in caregivers were related to behavioral problems in the patient, diurnal cortisol levels at awakening, 4:00 p.m., and 9:00 p.m. were compared in the high versus low BPSD groups (Figure 2). Multivariate analysis of covariance results showed a main effect of sampling time only (F=3.3, df=2,96, p=0.040).
With respect to the cortisol awakening response, results showed a significant time by group interaction effect (F=4.7, df=1, 46, p=0.035), with a larger response in caregivers in the high BPSD group. There was no main effect for sampling time, group, sex, age, or time of awakening. Post hoc comparisons at the two sampling times revealed significantly higher cortisol levels for caregivers in the high BPSD group 30 minutes after waking (t=−2.5, df=50, p=0.017).
To explore the association between the cortisol response to awakening and caregiver distress, a forced entry regression analysis was performed, with cortisol awakening responses as dependent variables. Independent variables were distress related to behavioral problems in the patient (NPI-D), physical health complaints (RAND-36 subscale), depressive symptoms (MADRS), and hours of sleep. Of these, only distress related to behavioral problems was a significant predictor (t=2.15, p=0.037), with greater distress associated with larger responses.
DISCUSSION
This study supports the hypothesis that salivary cortisol patterns change in relation to the stress of caregiving. This was particularly true for morning measures. Caregivers showed significantly higher levels of cortisol at the time of morning awakening than comparison subjects, with a smaller increase after awakening. In the caregiver group, a higher cortisol awakening response was found in caregivers of patients with high levels of BPSD. In addition to subjective measures, these findings provide evidence for physiological changes as indicators of chronic stress in caregivers.
Elevated morning cortisol levels in caregivers indicate an increased HPA activity, which corresponds with several previous investigations.22–24 To our knowledge, this is the first study to examine the cortisol awakening response in dementia patients' caregivers. We found elevated cortisol levels in caregivers directly after waking, which was associated with blunted subsequent response when matched against comparison subjects. This pattern is consistent with previous findings of blunted cortisol responses to awakening in teachers reporting high levels of burnout, and it may reflect an inability to cope with prolonged stress.13 It is important to note, however, that these diminished cortisol responses to awakening were associated with higher initial hormone levels, which has also been observed in other studies.17,19
We investigated changes in cortisol levels in caregivers in relation to BPSD, a potential source of stress. In this case, caregivers who reported high levels of patient BPSD displayed a higher cortisol increase after awakening than caregivers who reported low levels of BPSD, and they showed higher cortisol levels 30 minutes after awakening. A greater cortisol response to awakening has also been found in individuals with chronic work overload18 or highly perceived stress.35 The question as to why caregivers showed no higher cortisol increase after awakening, when evaluated against comparison subjects, arises. A possible explanation could be that the higher cortisol increase after awakening in high BPSD caregivers appeared to reflect specific distress due to behavioral problems in the patient. This may represent a more specific type of stress than the general stress that differentiates caregivers from comparison subjects. However, it remains unclear whether higher cortisol at awakening or the cortisol response over the next 30 minutes is a better index of chronic stress in caregivers.
Some limitations should be considered, however. Although there were no differences between caregivers and comparison subjects in habitual sleep duration, we have no way of assessing whether differences in sleep problems in the past night might have influenced cortisol levels the next morning. In addition, sampling cortisol over 2 days instead of 1 day, with more frequent samples per day, would have increased the reliability of individual diurnal cortisol profiles.
The caregiver sample was heterogeneous, which may have obscured subtle patterns. Included in the sample were spouses and children, over a wide age range, with varying intensity and duration of caregiving activity. However, caregivers in the high and low BPSD groups did not differ in the nature of the relationship between caregiver and patient, number of contact hours per week, or months of caregiving.
In conclusion, this study provides evidence for increased HPA activity in the morning in caregivers, as reflected by elevated salivary cortisol levels. With respect to the hypothesis that severity of patients’ behavioral symptoms influences cortisol levels in caregivers, increased awakening responses were found in caregivers reporting high levels of BPSD. The absence of significant associations between caregiver status and cortisol levels later in the day suggests that morning cortisol measures may be especially sensitive markers of this type of chronic stress, which should be taken into account in future research.
Previous reports have stated that increased HPA activity can lead to poorer immune function and subsequent increased disease vulnerability.23 The elevation of morning cortisol levels could predispose caregivers to negative health consequences, with caregivers of patients with high levels of BPSD at greater risk. Longitudinal data from the MAASBED study may provide more information on both the causal relationship between chronic stress and cortisol secretory patterns and the long-term consequences of stress-related HPA abnormalities in dementia patients' caregivers.
ACKNOWLEDGMENTS
This study was funded by the Dutch Research Council (NWO: 940–33-039) and the Adriana van Rinsum Ponssen Foundation.
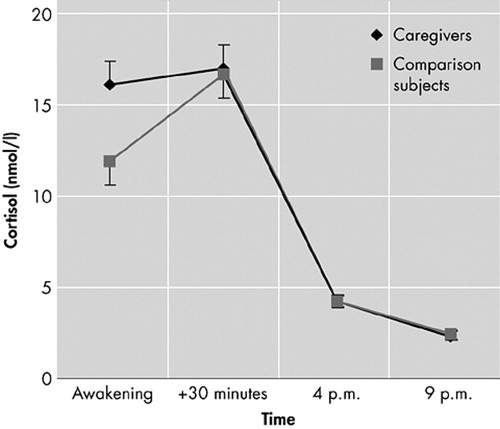
FIGURE 1. Diurnal Cortisol Levels (mean ± SEM) in Caregivers (N=49) and Comparison Subjects (N=51)
SEM=standard error of the mean
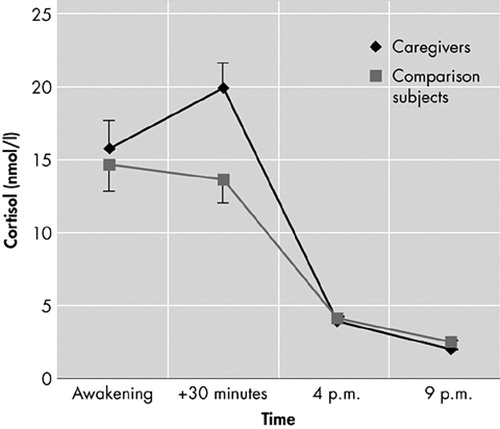
FIGURE 2. Diurnal Cortisol Levels (mean ± SEM) in Caregivers Reporting High BPSD (N=26) and Caregivers Reporting Low BPSD (N=23)
BPSD=Behavioral and psychological symptoms of dementia
SEM=standard error of the mean
 |
1 Donaldson C, Tarrier N, Burns A: Determinants of carer stress in Alzheimer’s disease. Int J Geriatr Psychiatry 1998; 13:248–256Crossref, Medline, Google Scholar
2 Gonzalez-Salvador MT, Arango C, Lyketsos CG, et al: The stress and psychological morbidity of the Alzheimer patient caregiver. Int J Geriatr Psychiatry 1999; 14:701–710Crossref, Medline, Google Scholar
3 Draper BM, Poulos RG, Poulos CJ, et al: Risk factors for stress in elderly caregivers. Int J Geriatr Psychiatry 1995; 11:227–231Crossref, Google Scholar
4 Vugt de ME, Stevens F, Aalten P, et al: Behavioural disturbances in dementia patients and quality of the marital relationship. Int J Geriatr Psychiatry 2003; 18:149–154Crossref, Medline, Google Scholar
5 Deimling GT, Bass DM: Symptoms of mental impairment among elderly adults and their effects on family caregivers. J Gerontol 1986; 41:778–784Crossref, Medline, Google Scholar
6 Gaugler JE, Davey A, Pearlin LI, et al: Modeling caregiver adaptation over time: the longitudinal impact of behavior problems. Psychol Aging 2000; 15:437–450Crossref, Medline, Google Scholar
7 Kaufer DI, Cummings JL, Christine D, et al: Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc 1998; 46:2109–2215Google Scholar
8 Pruchno RA, Resch NL: Aberrant behaviors and Alzheimer’s disease: mental health effects on spouse caregivers. J Gerontol 1989; 44:S177-S182Google Scholar
9 Baumgarten M, Battista RN, Infante-Rivard C, et al: The psychological and physical health of family members caring for an elderly person with dementia. J Clin Epidemiol 1992; 45:61–70Crossref, Medline, Google Scholar
10 Kiecolt-Glaser JK, Dura JR, Speicher CE, et al: Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom Med 1991; 53:345–362Crossref, Medline, Google Scholar
11 Katon W, Kleinman A, Rosen G: Depression and somatization: a review. Am J Med 1982; 72:127–135Crossref, Medline, Google Scholar
12 Kirschbaum C, Hellhamer DH: Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 1994; 19:313–333Crossref, Medline, Google Scholar
13 Pruessner JC, Hellhamer DH: Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med 1999; 61:197–204Crossref, Medline, Google Scholar
14 Melamed S, Ugarten U, Shirom A, et al: Chronic burnout, somatic arousal and elevated salivary cortisol levels. J Psychosom Res 1999; 61:197–204Google Scholar
15 Pruessner JC, Wolf OT, Hellhammer DH, et al: Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 1997; 61:2539–2549Crossref, Medline, Google Scholar
16 Schulz P, Kirschbaum C, Preussner J, et al: Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med 1998; 14:91–97Crossref, Google Scholar
17 Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, et al: The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci 1999; 64:1653–1660Crossref, Medline, Google Scholar
18 Wust S, Wolf J, Hellhammer DH, et al: The cortisol awakening response: normal values and confounds. Noise and Health 2000; 7:77–85Google Scholar
19 Kudielka BM, Kirschbaum C: Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology 2003; 28:35–47Crossref, Medline, Google Scholar
20 Nicolson NA, van Diest R: Salivary cortisol patterns in vital exhaustion. J Psychosom Res 2000; 49:335–342Crossref, Medline, Google Scholar
21 Cacioppo JT, Burleson MH, Poehlmann KM, et al: Autonomic and neuroendocrine responses to mild psychological stressors: effects of chronic stress on older women. Ann Behav Med 2000; 22:140–148Crossref, Medline, Google Scholar
22 Vedhara K, Cox NKM, Wilcock GK, et al: Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet 1999; 353:627–631Crossref, Medline, Google Scholar
23 Bauer ME, Vedhara K, Perks P, et al: Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. J Neuroimmunol 2000; 103:84–92Crossref, Medline, Google Scholar
24 Da Roza Davis JM, Cowen PJ: Biochemical stress of caring. Psychol Med 2001; 31:1475–1478Crossref, Medline, Google Scholar
25 Irwin M, Hauger R, Patterson TL, et al: Alzheimer caregiver stress: basal natural killer cell activity, pituitary-adrenal cortical function, and sympathetic tone. Ann Behav Med 1997; 19:83–90Crossref, Medline, Google Scholar
26 Mills PJ, Ziegler MG, Patterson T, et al: Plasma catecholamine and lymphocyte beta-2-adrenergic receptor alterations in elderly Alzheimer caregivers under stress. Psychosom Med 1997; 59:251–256Crossref, Medline, Google Scholar
27 Tarrier N, Barrowclough C, Ward J, et al: Expressed emotion and attributions in the carers of patients with Alzheimer’s disease: the effect on carer burden. J Abnorm Psychol 2002; 111:340–349Crossref, Medline, Google Scholar
28 Aalten P, Vugt ME, Lousberg R, et al: Behavioral problems in dementia: a factor analysis of the Neuropsychiatric Inventory (NPI). Dement Geriatr Cogn Disord (in press.)Google Scholar
29 Cummings JL, Mega M, Gray K, et al: The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
30 Van der Zee KI, Sanderman R: Het meten van de algemene gezondheidstoestand met de RAND-36: een handleiding, Measurement of General Health With the RAND-36: A Manual. Groningen, Netherlands, Noordelijk Centrum voor Gezondheidsvraagstukken NCG, 1993Google Scholar
31 Cohen S, Williamson GM: Perceived stress in a probability sample of the United States, in The Social Psychology of Health. Edited by Spacepan S, Oskamp S. Newbury Park, Calif, Sage Publications, 1988, pp 31–67Google Scholar
32 Arrindell WA, Ettema JHM: SCL-90: Handleiding bij een multidimensionele psychopathologie indicator. Lisse, Netherlands, Swets & Zeitlinger, 1986Google Scholar
33 Montgomery S, Åsberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
34 Gunnar MR, Morison SJ, Chisholm K, et al: Salivary cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol 2001; 13:611–628Crossref, Medline, Google Scholar
35 Wust S, Federenko I, Hellhammer DH, et al: Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology 2000; 25:707–720Crossref, Medline, Google Scholar



