Mild Traumatic Brain Injury: Neuroimaging of Sports-Related Concussion
Public awareness of sports-related head injuries came to the forefront in the early twentieth century. Between 1869 and 1905, there were 18 deaths and 159 documented serious injuries attributed to the game of football. In response to these alarming numbers, President Theodore Roosevelt convened representatives from the academic institutions governing football to discuss reforming the game. The American Football Rules Committee arose from these conferences.5–8
In 1962, the American Medical Association’s Committee on Medical Aspects of Sports organized a conference addressing head protection in athletes and associated matters in sports medicine.5 A greater understanding of the need to determine patterns of injury in order to reduce morbidity in the professional and recreational athletic arenas resulted. Although football was the main focus on the American front, emphasis was placed on head protection in other sports arenas elsewhere. In Sweden, for example, the use of hockey helmets became mandatory in 1963. This was subsequent to an insurance survey that found over 100 closed head injuries, including 1 death, 22 mild traumatic brain injuries (MTBIs) and 3 facial fractures as a result of hockey participation.9
Today, multiple sports are associated with concussive events. In excess of 1.5 million people participate in football (i.e. recreational, high school, collegiate, and professional) annually. The estimated annual incidence of MTBI’s in football is 4–20%.5 A systematic review of the literature from 1985 to 2000 found ice hockey and rugby to have the highest incidence of concussion for high school, college, and amateur athletes, while soccer had the lowest.10 At the recreational level, female taekwondo participants and male boxers had the highest frequency of concussion.10 Of the injuries, 6.2% were concussive in a three-year prospective study among intercollegiate athletes.11 According to a survey of 1,659 children participating in contact sports, 3% suffered concussions.12 In addition, an epidemiologic study of collegiate and high school football players found that players who sustain one concussion are three times more likely to sustain a second one in the same season.13
Concussion and Postconcussive Syndrome
The term concussion is derived from the Latin word concutere (to strike together).14 Another synonymous term, commotion cerebri, was introduced in the 16th century by the French military surgeon Pare.15 Although the symptoms associated with concussion have been recognized for centuries, the term “postconcussion syndrome” was first used in 1934 to describe the “subjective posttraumatic syndrome…due directly to the blow on the head.”16,17 Symptoms associated with postconcussion syndrome may include persistent headache, irritability, inability to concentrate, memory impairment, generalized fatigue, dizziness, or a generalized loss of well-being. Commonly, the course is self-limited, resolving usually within 6-8 weeks of the incident.5
Most recently, it has been recognized that MTBI may occur as a result of concussion. The Centers for Disease Control and Prevention (CDC) Mild Traumatic Injury Workgroup conceptually defined MTBI as “an injury to the head as a result of blunt trauma or acceleration or deceleration forces that result in one or more of the following conditions: (1) Any period of observed or self-reported transient confusion, disorientation, impaired consciousness, dysfunction of memory around the time of injury, or loss of consciousness lasting less than 30 minutes. (2) Observed signs of neurological or neuropsychological dysfunction, headache, dizziness, irritability, fatigue or poor concentration.”18 According to the CDC (based on the 1991 National Health Interview Survey), the incidence of athletic TBIs was approximately 300,000, with only 12% of those injuries requiring hospitalization.19 The remaining 88% most likely include many MTBIs based on currently accepted definitions.19 It is important to note that this survey included weighted data collected from 46,761 households where the main outcome measure was the occurrence of one or more incidences of head injury with loss of consciousness. As many concussions in athletics do not result in loss of consciousness, actual occurrence of MTBIs in the athletic arena is likely much higher. A survey of high school football players found that only 47.3% of the athletes who sustained a concussion had reported it at the time of injury.20 The most common reason for not reporting was belief that the injury was not serious enough to need medical attention (66.4%). Other cited reasons include not wanting to leave the game (41%), not recognizing that a concussion had occurred (36.3%), and not wanting to disappoint their teammates (22.1%).
The large numbers of athletic-related MTBIs raise concerns about possible short and long term sequelae of repetitive injury.9 Dementia Pugilistica in boxers results from repetitive head injury and leads to chronic disability.5,21 Second Impact Syndrome (SIS)is rare and potentially fatal. SIS occurs when “…an athlete who has sustained an initial head injury, most often a concussion, sustains a second head injury before symptoms associated with the first have fully cleared.”22,23 SIS may result from increased vascular congestion creating cerebral swelling resulting in transtentorial herniation and subsequent death. There were 35 probable cases of SIS in football between 1980-1993.5 There are reports of possible SIS in other sports including field hockey and skiing. The existence of SIS is controversial as not all cases fully satisfy diagnostic criteria.22
Post concussive symptoms can also contribute to performance limitations and overall decreased functionality. Athletes who do not exhibit on field mental status changes or report any post concussive symptoms may still be impaired on neuropsychological testing.24 A National Collegiate Athletic Association (NCAA) Concussion Study found that college football players with a history of concussion are likely to have future concussive injuries with a seven to ten day window of increased susceptibility. Repetitive concussions may be associated with slower recovery of neurological function.9 Another study found a prolonged course of recovery in high school as compared to college athletes. Significant memory impairment was present up to 7 days after injury in high school athletes but for only 1 day in college athletes.25,26 Thus, the age of the athlete must be considered when addressing MTBI management.
Concussion is characterized by a disturbance in neural function that can be profound, causing brief loss of consciousness. Early studies utilizing animal models of concussion indicated that loss of consciousness occurred when the head was unrestrained and hit by an angular acceleration/deceleration process.27 Recently, finite element (FE) modeling based on professional football head-to-head field collisions has found that rotational acceleration produced maximum shear stress (Cover and Figure 1).28 No significant relationship was found between translational acceleration and sheer stress. This theoretical analysis is supported by multiple sports studies. A six year investigation of National Football League players demonstrated that the most susceptible to concussion were quarterbacks, wide receivers and defensive secondaries who received a facemask impact at an oblique angle.29 Concussion in hockey is commonly associated with an eccentric blow to the head, a strike to the face or jaw, or a hit directed to the chin.30 In taekwondo, concussion is primarily due to a round-house kick (i.e. angular kick) to the temporal region of the head.31 Additional proposed biomechanical mechanisms of injury include: sudden movement of the head about the axis of the neck severely stressing the craniospinal junction; hemisphere movement causing stretch of brainstem neurons; rotational loading causing violent impact between the skull and brain; propagation of intracranial pressure waves deforming brain tissue and depressing skull bone.32
Several theories of concussion have been developed to explain the immediate loss of consciousness.27 Reticular theory is based on the premise that a concussive blow temporarily paralyzes the brainstem reticular formation. Centripetal hypothesis attributes concussion to mechanically induced strains disrupting brain function. Activation of cholinergic neurons resulting in suppression of behavioral responses is central to pontine cholinergic system theory. Convulsive hypothesis attributes concussion to generalized neuronal firing. This theory is supported by the sequence of cerebral hyperexcitability followed by a longer period of depression acutely after head injury.32–34
A neurochemical cascade develops immediately following biomechanical insult to the brain, bringing a multitude of cellular changes.32 Disruption of neuronal membranes and axonal stretching leads to an increase in extracellular potassium and subsequent depolarization and release of excitatory neurotransmitters. This has been termed “neurotransmitter storm.” Thereafter, neuronal suppression occurs diffusely throughout the brain, activating membrane pumps and increasing glucose utilization. Increased lactate production follows. The resultant accumulation may leave neurons more susceptible to further injury.32 Cerebral blood flow also decreases. N-methyl-D-asparate (NMDA) receptors are activated. There is an influx of calcium into the cell with accumulation in the mitochondria. This impairs oxidative metabolism, leading to energy failure and the possibility of microtubule breakdown. Other neurochemical changes may include: a decrease in gamma-aminobutyric acid (GABA) and other inhibitory neurotransmitters, possibly lowering the seizure threshold; decreased magnesium leading to impaired energy production and neurologic deficits; and loss of forebrain cholinergic neurons resulting in impaired neurotransmission with possible learning and memory difficulties.32
Neuropsychological Testing
Neuropsychological testing is increasingly used in initial assessment of concussion, both on and off the field. In the late 1980’s, The University of Virginia completed the first large scale research study on MTBI in athletes.35 Neuropsychological testing was utilized to assess cognitive function prior to and following concussion. The Pittsburg Steelers began using neuropsychological testing in the 1990’s to determine return to play decisions. It is now used in other areas of professional athletics, including ice hockey and auto racing.36 Neuropsychological batteries are also utilized for assessment of short and long term post concussive symptoms. Several return to play guidelines have been developed, based on the degree of symptomatology experienced by the athlete. These guidelines have slight differences in their definitions of grades of concussion and recommendations. At present, all are based on expert opinion rather than prospective studies.15,37–39 Ideally, baseline testing is obtained at the beginning of the season. At the time of injury, testing is repeated. The choice of specific neuropsychological tests varies, but a battery is chosen to assess cognitive skills including immediate and delayed recall, orientation, verbal memory, attention span, word fluency, visual scanning and coordination.36 Methods of testing range from sideline assessment to computer based inventories (e.g. Immediate Post Concussion Assessment and Cognitive Testing (ImPACT)).40
Imaging
Imaging of diffuse axonal injury was recently reviewed.41 In brief, computed tomography (CT) is the initial method of choice to evaluate and exclude hemorrhage. T2 weighted magnetic resonance imaging (MRI), particularly fluid attenuated inversion recovery (FLAIR) MRI, is more sensitive to traumatic lesions.42,43 Gradient echo MRI is better at detecting hemorrhagic change. However, studies have not been able to correlate abnormal findings on MRI with either post concussive symptoms or long term outcome.42 Diffusion weighted imaging (DWI) has been shown to identify shearing injuries not evident on T2/FLAIR or gradient echo sequences, thus making it valuable in evaluating closed head injuries.44 Diffusion tensor imaging (DTI) examines the integrity of the white matter tracts by measuring the degree and direction of water diffusion, providing a potential marker for white matter injury.45,46
Studies utilizing positron emission tomography (PET) and single-photon emission computed tomography (SPECT) have demonstrated frontal and/or temporal hypometabolism following MTBI at rest and during working memory tasks.47,48 This has been hypothesized to correlate with decreased memory function. Several recent studies have assessed the potential of functional MRI (fMRI) to provide more thorough assessment following concussion. fMRI provides information regarding neural function during task performance and is noninvasive.1–3 Tasks can be tailored to obtain information regarding specific neurological functions. A small prospective study of college football players compared individual brain activation patterns prior to and following concussive injury.1 Brain activation was more widespread following concussion compared to both pre-injury levels and uninjured subjects during the performance of various memory and sensorimotor tasks (Figure 2). Performance was unchanged compared to baseline measures. The motor sequencing tasks were the most sensitive to concussion. The authors note that these results are consistent with cognitive-load induced recruitment of neural resources. Another fMRI study evaluated working memory in adult athletes who had sustained a concussion (1-14 months prior to study) and were experiencing post concussive symptoms.2 Athletes with concussions had less task-related activation in the mid-dorsolateral prefrontal cortex (important for working memory) than comparison subjects (Figure 2). There was an inverse correlation between right dorsolateral prefrontal cortex activation and severity of symptoms. None of the symptomatic athletes had evidence of axonal injury on structural MRI. Thus, functional impairment may be present in the absence of abnormalities on clinical imaging. In addition, concussed athletes had widespread activations in areas not activated in comparison subjects. In one subject, a follow-up study showed that resolution of symptoms was accompanied by normalization of the activation pattern. Finally, less than normal activation was found in dorsolateral prefrontal cortex in a task requiring response inhibition (Figure 2).3 These studies provide the framework for the possible clinical utility of fMRI in concussive injury in athletes.
Standard electroencephalographic (EEG) techniques have had limited value in the assessment of MTBI.49,50 One study utilized EEG patterns in isolation and during postural tasks in comparison subjects versus asymptomatic athletes post-injury (mean of 89.4 days) to evaluate any residual effects of concussion on global cortical function.51 The authors reported an overall decrease in EEG power in all bandwidths studied, an effect most prominent during standing postures. This suggests a possible explanation for the reduced functional capabilities, such as postural instability, observed in athletes subsequent to concussive injury.52,53
Studies utilizing Evoked Potentials (EP) and Event-Related Potentials (ERP) in the evaluation of MTBI have shown more promising results. Both EP and ERP represent the averaged EEG signal in response to a given stimulus. EPs are thought to represent processing in the primary sensory pathways, whereas ERPs are associated with cognitive processes. EP studies have consistently found the cortical waveform to be briefly extinguished immediately after concussion in animal models.54 The brainstem auditory evoked potential (BAEP) has been primarily utilized as it is a marker of brainstem function. There have been reports of change in BAEP latency subsequent to concussion, as well as studies that report no change.50,54 Thus, to date, results are mixed.
ERP’s may be more useful. One study examined ERPs evoked following MTBI in college athletes.4 Concussed athletes demonstrated a significant decrease in the waveform around 300msec (P300) which was related to the severity of post-concussive symptoms (Figure 3). Another study compared the effects of concussion on attention and ERPs in athletes based on their symptomatology.55 Longer reaction times were exhibited by symptomatic athletes compared to asymptomatic ones. In addition, there was an inverse relationship between severity of postconcussion symptoms and P300 amplitude. This effect was not influenced by the length of time since injury. This suggests that concussive symptomatology affects attentional capacities. As ERPs are resistant to practice effects, this approach is promising as a possible diagnostic tool in MTBI.
A relatively new approach to evaluation of MTBI is Magnetic Source Imaging (MSI). MSI integrates anatomic data from MRI with electrophysiology data from Magnetoencephalography (MEG).56 MEG measures the neuromagnetic field of the dendrites organized parallel to the skull surface (as compared to EEG that measures the potential gradients of dendrites perpendicular to the skull surface). It allows tracking of real-time brain activity without distortions by differences in conductivity between the brain, skull and scalp.57 One study compared MRI and resting EEG with resting MSI in post concussive subjects versus comparison subjects.56 Results indicated that MSI detected more patients with post concussive symptoms than either EEG or MRI alone. All patients with abnormal EEG or MRI also had abnormal MSI. To date, there are no published studies evaluating MTBI with MSI in athletes.
CONCLUSION
Recognition of the high incidence of sports-related concussions has increased interest in MTBI assessment and prognosis. Neuropsychological testing is beginning to play a more integral role in the evaluation of the concussed athlete. Functional imaging studies allow comparison pre- and postinjury. In this context, fMRI, ERP, and MSI are promising tools in the evaluative process. It is critical to find a means of detecting possible neurological consequences of MTBI, however subtle, and identifying the neuropsychological and neurophysiological impairments caused by a sports-related concussion. Specifically, assessing recovery for several brain functions and their underlying neuronal mechanisms will permit development of tools for rapid and efficient diagnosis. Further, a better understanding of recovery will guide return to play decisions as well as special measures and accommodations that need to be taken to carry out daily activities.
Cover
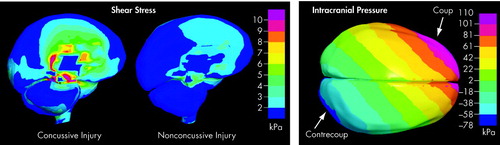
FIGURE 1. Computer Modeling of Forces (expressed as pressure, kPa) Within the Brain Following a Strong Blow to the Head. The Predicted Sheer Stresses (left panel) Differentiate Concussive From Nonconcussive Injury. Note the High Sheer Stress (pink) in the Central Core of the Brain. The Predicted Intracranial Pressure Wave Following a Concussive Blow is Shown in the Right Panel. Note the Drastic Change From Positive Pressure (pink, coup) to Negative Pressure (blue, contrecoup) Across the Brain.
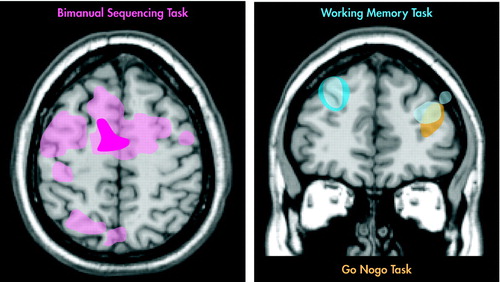
FIGURE 2.
Task-Related Brain Activation on fMRI May be Changed Post Concussion. 1–3 Athletes With Concussions (shown in light shades) Have Many More Regions of Activation Than Those Without (shown in dark shades) During a Bimanual Sequencing Task (pink, left) or Working Memory Task (blue, right).1,2 In Addition, Concussion May Be Associated With Decreased Activation in Areas Critical for Task Performance. Athletes With Concussion Had Reduced Activation in Right Dorsolateral Prefrontal Cortex During a Working Memory Task (light blue, right).2 In Another Study, Comparison Subjects Showed More Activation in Left Dorsolateral Prefrontal Cortex (gold, right) Than Athletes With Concussion During a Go-NoGo Task.3
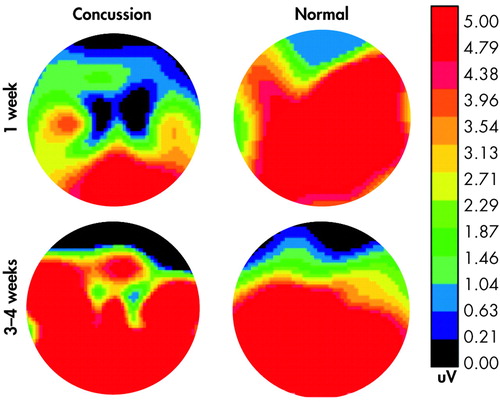
FIGURE 3. Bidimensional View of the Scalp Distribution of the P300 Component of the Event-Related Potential Associated With the Oddball Effect (obtained by subtracting the P300 component elicited by the frequent stimulus from that elicited by the rare stimulus) in an Athlete Who Suffered a Mild (grade 1) Concussion. At 1 Week, When the Subject Was Clearly Displaying Post Concussive Symptoms, the Oddball Effect Was Quite Small. By 4 Weeks the Patient’s Symptoms Had Resolved and a Strong Oddball Effect Was Seen, Very Similar to That Exhibited by the Comparison Athlete (matched for age, sex, education) Tested at the Same Time Intervals. The Color Bar Presents Level of Activation From Greatest (orange) to Least (black) (used with permission)
1 Jantzen KJ, Anderson B, Steinberg FL, et al: A prospective functional MR imaging study of mild traumatic brain injury in college football players. AJNR 2004; 25:738–745Medline, Google Scholar
2 Chen JK, Johnston KM, Frey S, et al: Functional abnormalities n symptomatic concussed athletes: an fMRI study. Neuroimage 2004; 22:68–82Crossref, Medline, Google Scholar
3 Easdon C, Levine B, O'Connor C, et al: Neural activity associated with response inhibition following traumatic brain injury: An event-related fMRI investigation. Brain Cogn 2004; 54:136–138Medline, Google Scholar
4 Dupuis F, Johnston KM, Lavoie M, et al: Concussions in athletes produce brain dysfunction as revealed by event-related potentials. Neuroreport 2000; 11:4087–4092Crossref, Medline, Google Scholar
5 Bailes JE, Cantu RC: Head injury in athletes. Neurosurgery 2001; 48:26–46Medline, Google Scholar
6 The NCAA Century Series Part 1 : 1900–1939. The NCAA News,Google Scholar
7 The Roosevelt Rough Writer: The Newsletter for Volunteers in Part at Sagamore Hill. 2005; 1Google Scholar
8 Levy ML, Ozgur BM, Berry C, et al: Birth and evolution of the football helmet. Neurosurgery 2004; 55:656–661Crossref, Medline, Google Scholar
9 Guskiewicz KM, McCrea M, Marshall SW, et al: Cumulative effects associated with recurrent concussion in collegiate football players. JAMA 2003; 290:2549–2555Crossref, Medline, Google Scholar
10 Koh JO, Cassidy JD, Watkinson EJ: the incidence of concussion in contact sports: a systemic review of the evidence. Brain Inj 2005; 17:901–917Crossref, Google Scholar
11 Covassin T, Swanik CB, Sachs ML: Epidemiological considerations of concussions among intercollegiate athletes. Appl Neuropsychol 2003; 10:12–22Crossref, Medline, Google Scholar
12 Radelet MA, Lephart SM, Rubinstein EN, et al: Survey of the injury rate for children in community sports. Pediatrics 2002; 110:e28-Google Scholar
13 Guskiewicz KM, Weaver NL, Padua DA, et al: Epidemiology of concussion in collegiate and high school football players. The American Journal of Sports Medicine 2000; 28:643–650Crossref, Medline, Google Scholar
14 The American Heritage Dictionary of the English Language, fourth edition. 2000Google Scholar
15 Maroon JC, Lovell MR, Norwig J, et al: Cerebral concussion in athletes: evaluation and neuropscyhological testing. Neurosurgery 2000; 47:659–669Medline, Google Scholar
16 Pellman EJ, Viano DC, Casson IR, et al: Concussion in professional football: injuries involving 7 or more days out—part 5. Neurosurgery 2004; 55:1100–1119Crossref, Medline, Google Scholar
17 Strauss I, Savitsky N: Head injury: neurologic and psychiatric aspects. Arch Neurol Psychiatry 1934; 31:955Crossref, Google Scholar
18 Gerberding JL, Binder S: Report to congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. 2003Google Scholar
19 Sosin DM, Sniezek JE, Thurman DJ: Incidence of mild and moderate brain injury in the United States 1991. Brain Inj 1996; 10:47–54Crossref, Medline, Google Scholar
20 McCrea M, Hammeke T, Olsen G, et al: Unreported concussion in high school football players: implications for prevention. Clin J Sports Med 2004; 14:13–17Crossref, Medline, Google Scholar
21 Rabadi MH, Jordan BD: The cumulative effect of repetitive concussion in sports. Clin J Sports Med 2001; 11:194–198Crossref, Medline, Google Scholar
22 McCrory P: Does second impact syndrome exist? Clin J Sports Med 2001; 11:144–149Crossref, Medline, Google Scholar
23 Cantu RC, Voy R: Second impact syndrome: a risk in any contact sport. Phys Sportsmed 1995; 23:27–34Crossref, Google Scholar
24 Lovell MR, Collins MW, Iverson GL, et al: Grade 1 or “ding” concussions in high school athletes. Am J Sports Med 2004; 32:47–54Crossref, Medline, Google Scholar
25 Lovell MR, Collins MW, Iverson GL, et al: Recovery from mild concussion in high school athletes. J Neurosurg 2003; 98:296–301Crossref, Medline, Google Scholar
26 Field M, Collins MW, Lovell MR, et al: Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr 2003; 142:546–553Crossref, Medline, Google Scholar
27 Shaw N: The neurophyiology of concussion. Prog Neurobiol 2002; 67:281–344Crossref, Medline, Google Scholar
28 Zhang L, Yang KH, King AI: A proposed injury threshold for mild traumatic brain injury. J Biomech Eng 2004; 126:226–236Crossref, Medline, Google Scholar
29 Pellman EJ, Viano DC, Tucker AM, et al: Concussion in professional football: location and direction of helmet impacts—part 2. Neurosurgery 2003; 53:1328–1340Crossref, Medline, Google Scholar
30 Biasca N, Wirth S, Tegner Y: The avoidability of head and neck injuries in ice hockey: an historical review. Br J Sports Med 2002; 36:410–427Crossref, Medline, Google Scholar
31 Roh JO, Watkinson EJ: Video analysis of blows to the head and face at the World Taekwondo Championships. J Sports Med Phys Fitness 2002; 42:348–353Medline, Google Scholar
32 Giza CC, Hovda DA: The neurometabolic cascade of concussion. J Athl Train 2001; 36:228–235Medline, Google Scholar
33 Hayes RL, Dixon CE: Neurochemical changes in mild head injury. Semin Neurol 2005; 14:25–31Crossref, Google Scholar
34 Regner A, Alves LB, Chemale I, et al: Neurochemical characterization of traumatic brain injury in humans. J Neurotrauma 2005; 18:783–792Crossref, Google Scholar
35 Alves WM, Rimel RW, Nelson WE: University of Virginia prospective study of football-induced minor head injury: status report. Clin Sports Med 1987; 6:211–218Medline, Google Scholar
36 Pellman EJ, Lovell MR, Viano DC, et al: Concussion in professional football: neuropsychological testing—part 6. Neurosurgery 2004; 55:1290–1305Crossref, Medline, Google Scholar
37 Cantu RC: Return to play guidelines after a head injury. Clin Sports Med 1998; 17:45–60Crossref, Medline, Google Scholar
38 Fick DS: Management of concussion in collision sports. Guidelines for the sidelines. Postgrad Med 1995; 97:53–60Crossref, Medline, Google Scholar
39 Kelly JP, Rosenberg JH: The development of guidelines for the management of concussion in sports. J Head Trauma Rehabil 1998; 13:53–65Crossref, Medline, Google Scholar
40 Iverson GL, Lovell MR, Collins M.W: Interpreting changes on ImPACT following sports concussion. Clin Neuropsychol 2005; 17:460–467Crossref, Google Scholar
41 Hurley RA, McGowan JC, Arfanakis K, et al: Traumatic axonal injury: Novel insights into evolution and identification. J Neuropsychiatry Clin Neurosci 2004; 16:1–7Link, Google Scholar
42 Hughes DG, Jackson A, Mason DL, et al: Abnormalities on magnetic resonance imaging seen acutely following mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology 2004; 46:550–558Medline, Google Scholar
43 Kelly AB, Zimmerman RD, Snow RB, et al: Head trauma: comparison of MR and CT—experience in 100 patients. AJNR 1988; 9:699–708Medline, Google Scholar
44 Huisman TAGM, Sorensen AG, Hergan K, et al: Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head injury. J Comput Assist Tomogr 2003; 27:5–11Crossref, Medline, Google Scholar
45 Huisman TA, Schwamm LH, Schaefer PW, et al: Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR 2004; 25:370–376Medline, Google Scholar
46 Arfanakis K, Haughton VM, Carew JD, et al: Diffusion tensor MR imaging in diffuse axonal injury. AJNR 2002; 23:794–802Medline, Google Scholar
47 Chen SHA, Kareken DA, Fastenau PS, et al: A study of persistent post-concussion symptoms in mild head trauma using positron emission tomography. J Neurol Neurosurg Psychiatry 2003; 74:326–332Crossref, Medline, Google Scholar
48 Umile EM, Sandel ME, Alavi A, et al: Dynamic imaging in mild traumatic brain injury: Support for the theory of medial temporal vulnerability. Arch Phys Med Rehabil 2002; 83:1506–1513Crossref, Medline, Google Scholar
49 Pointinger H, Sarahrudi K, Poeschl G, et al: Electroencephalography in primary diagnosis of mild head trauma. Brain Inj 2002; 16:799–805Crossref, Medline, Google Scholar
50 Gaetz M, Bernstein DM: The current status of electrophysiologic procedures for the assessment of mild traumatic brain injury. J Head Trauma Rehabil 2001; 16:386–405Crossref, Medline, Google Scholar
51 Thompson J, Sebastianelli W, Slobounov S: EEG and postural correlates of mild traumatic brain injury in athletes. Neurosci Lett 2005; 377:158–163Crossref, Medline, Google Scholar
52 Bleiberg J, Cernich AN, Cameron K, et al: Duration of cognitive impairment after sports concussion. Neurosurgery 2005; 54:1073–1078Crossref, Google Scholar
53 Echemendia RJ, Putukian M, Mackin RS, et al: Neuropsychological test performance prior to and following sports-related mild traumatic brain injury. Clin J Sports Med 2001; 11:23–31Crossref, Medline, Google Scholar
54 Noseworthy JH, Miller J, Murray TJ, et al: Auditory brainstem reponses in postconcussive syndrome. Arch Neurol 1981; 38:275–278Crossref, Medline, Google Scholar
55 Lavoie ME, Dupuis F, Johnston KM, et al: Visual p300 effects beyond symptoms in concussed college athletes. J Clin Exp Neuropsychol 2004; 26:55–73Crossref, Medline, Google Scholar
56 Lewine JD, Davis JT, Sloan JH, et al: Neuromagnetic assessment of pathophysiologic brain activity induced by minor head trauma. AJNR 1999; 20:857–866Medline, Google Scholar
57 Wheless JW, Castillo E, Maggio V, et al: Magnetoencephalography (MEG) and magnetic source imaging (MSI). Neurologist 2004; 10:138–153Crossref, Medline, Google Scholar



