Impact of Frontal Systems Behavioral Functioning in Dementia on Caregiver Burden
One of the most significant challenges faced by dementia caregivers is behavioral problems in the patient. Between 70% and 90% of patients with dementia experience problems with depression, irritability, restlessness, agitation, and verbal and physical aggression at some point during their illness. 10 , 11 These problems typically occur on a daily basis and can precipitate institutionalization. 12 , 13 Unlike cognition, however, behavioral problems do not show a clear linear pattern of decline. Behavioral and personality changes are often more unpredictable and variable at different points of the illness, thereby causing significant distress to the caregiver. In fact, the frequency of behavioral problems has been identified as one of the strongest predictors of caregiver distress. 14 – 16 Not surprisingly, other characteristics of the patient with dementia, such as length and severity of illness and the patient’s awareness of his or her cognitive impairment, are also predictive of emotional distress in caregivers. 17 – 19
Although increases in problem behaviors among patients with dementia predicted caregivers’ mental and physical health in a longitudinal study, these relationships were mediated through stress appraisal, 20 and caregivers’ reactions to memory and behavior problems were more strongly related to distress than the frequency of problems. 21 Taken together, it appears that caregiver burden may reflect a combination of objective stressors and the caregiver’s perception and appraisal of the caregiving context. Caregivers may perceive behavioral problems as particularly distressing because of their unpredictable nature and limited benefits of treatment.
This study investigated the contribution of frontal systems behavioral functioning (i.e., apathy, executive dysfunction, and disinhibition) on caregiver burden. A better understanding of the types of behaviors that affect caregivers’ perceived burden and distress may inform psychosocial interventions designed to reduce caregiver distress.
METHOD
Participants
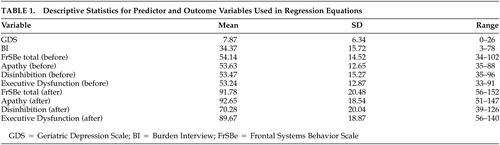
Participants comprised 72 caregivers of patients with mild (N=47) or moderate (N=25) dementia. Caregivers were recruited from the community and memory disorder clinics. All caregivers resided with the care recipient and were providing a minimum of 4 hours of daily care for at least 6 months. Caregivers reported an average of 18.24 hours of daily contact with the care recipient (SD=7.19). Caregivers residing in a nursing home or assisted living facility were excluded from the study. The majority of caregivers were women (N=56), spouses of the patients (N=44), and Caucasian (N=69). Caregivers had been providing care for an average of 39.68 months (SD=35.57), and the average length of dementia diagnosis was 39.71 months (SD=35.90). Caregivers’ average age was 64.36 (SD=11.66). Care recipients’ average age was 77.18 (SD=9.47).
The diagnosis of dementia was confirmed by the treating physician. The Clinical Dementia Rating scale (CDR) was administered to the caregiver over the telephone by a neuropsychologist (JDD) and was used to measure dementia severity. 22 Only care recipients with a CDR rating of 1 or 2 (reflecting mild or moderate dementia) were included in the study. Clinical diagnoses by the treating physician were used to identify dementia subtypes. Based on these clinical classifications, the sample comprised the following dementia subtypes: probable Alzheimer’s disease (N=42), vascular dementia (N=4), mixed dementia (N=4), frontotemporal dementia (N=5), diffuse lewy-body disease (N=4), Parkinson’s dementia (N=3), hydrocephalus (N=2), progressive supranuclear palsy (N=1), dementia not otherwise specified (N=2), and unknown (N=5).
Instruments and Procedures
Caregivers completed self-report measures of their mood, perceived burden, and patient ratings of frontal systems behavioral problems as part of a baseline assessment of a large-scale, psychosocial intervention study. Assessments were completed at the caregivers’ homes and administered by a trained research assistant. Informed consent was obtained from all participants prior to participating in the study.
Frontal Systems Behavior Scale
The Frontal Systems Behavior Scale (FrSBe) 23 is a 46-item behavior rating scale completed by the caregiver. It is designed to measure behavior associated with damage to the frontal lobes and frontal systems of the brain. The FrSBe targets three behavioral subtypes thought to be subserved by the frontal systems, including executive dysfunction, disinhibition, and apathy. Each item is rated on a 5-point Likert scale. Caregivers rated behavior in these three domains prior to the onset of dementia (“before” scores) and currently (“after” scores). The FrSBe yields a total score as well as scores for the three subscales. Raw scores were converted to age- and education-corrected t scores, which were used in data analyses.
Activities of Daily Living Questionnaire
The caregiver completed the Activities of Daily Living Questionnaire (ADL), 24 a 14-item self-report questionnaire. This instrument measures degree of independence in basic (e.g., dressing and grooming) and instrumental (e.g., medication and financial management) daily activities. Each item is scored on a 3-point scale, reflecting independence, the need for assistance, or dependence. Total scores range from 0 to 28, with higher scores reflecting greater functioning independence.
Burden Interview
The Burden Interview 25 (BI) is a 22-item, self-report measure of perceived burden. The instrument measures caregivers’ psychological health, emotional well-being, social and family life, finances, and degree of control over one’s life. Each question is scored on a 5-point Likert scale. Total scores range from 0 (low burden) to 88 (high burden).
Geriatric Depression Scale
The Geriatric Depression Scale (GDS) 26 is a self-report instrument comprising 30 yes/no questions. Higher scores reflect greater depressive symptoms.
Statistical Analyses
To investigate the relationship between frontal systems behavioral problems in the patient and perceived burden, hierarchical regression equations with four steps of predictor variables were fitted. Total score on the Lawton-Brody Activities of Daily Living Questionnaire was added in the first step to control for care recipients’ severity of activity of daily living impairment. GDS total score was entered next to control for caregiver depression. To control for caregivers’ response style on self-report measures and the patients’ baseline personality and behavior, t scores for the overall FrSBe “before” ratings were added in the third step. Finally, t scores for the overall FrSBe “after” rating were added in the fourth step. Total scores on the Burden Interview served as the outcome variable. Descriptive statistics for predictor and outcome variables are presented in Table 1 .
 |
RESULTS
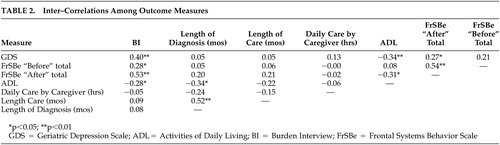
Table 2 summarizes the intercorrelations among measures. As predicted, perceived burden was moderately correlated with caregiver depression and frontal systems behavioral problems in the care recipient. Length of diagnosis, length of caregiving, and hours of daily care provided were unrelated to burden. There was a modest relationship between total activities of daily living and burden.
 |
To determine relative contributions of dementia severity, caregiver depression, and frontal systems behavioral problems on burden, we used hierarchical regression analysis, with total score on the BI as the dependent variable. We entered variables in the following order: ADL, GDS, total FrSBe “before” scores, and total FrSBe “after” scores.
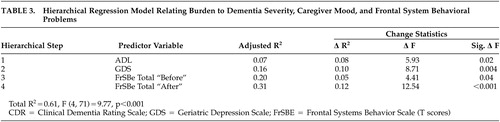
Results of the hierarchical regression analyses are presented in Table 3 . As expected, ADL scores significantly accounted for variance in BI scores (8%, two-tailed p<0.05). GDS scores added in the second step accounted for a significant increase in the variance of BI scores, R 2 =0.10, F (1, 69)=8.7, two-tailed p<0.01. FrSBe “before” scores were significant, R 2 =0.05, F (1, 68)=4.11, two-tailed p<0.05. FrSBe “after” scores, added in the fourth step, accounted for a significant increase in the variance in BI scores, R 2 =0.12, F (1, 67)=12.54, two-tailed p<0.001 ( Table 3 ).
 |
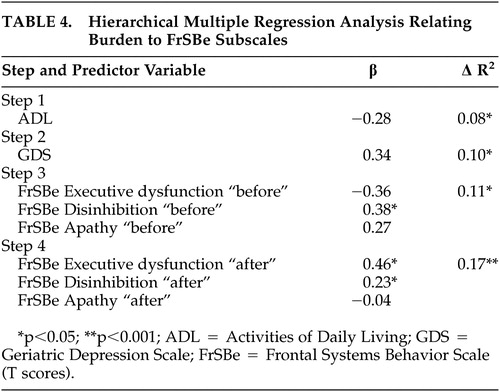
We conducted a second hierarchical regression analysis to further investigate the specific problem behaviors contributing to burden. ADL scores were entered into the first step, followed by GDS, FrSBe “before” subscale scores (apathy, disinhibition, and executive dysfunction), and finally FrSBe “after” subscale scores. Again, FrSBe “after” scores accounted for a significant proportion of the variance in BI scores, R 2 =0.17, F (3, 63)=6.51, two-tailed p<0.001, above and beyond the contribution of GDS, ADL, and FrSBe “before” scores. Examination of individual predictors in these models indicated that FrSBe executive dysfunction (t=2.09, p<0.01, β=0.36) and disinhibition subscales were significantly predictive of BI scores. Results are summarized in Table 4 .
 |
To investigate the impact of frontal systems behaviors on depression as a primary outcome, we ran an additional regression with GDS as the outcome variable and ADL and FrSBe as predictors. Total score on the Lawton-Brody, followed by FrSBe “before” scores and FrSBe “after” scores were entered. Results indicated that ADL scores significantly accounted for variance in GDS scores (12%, two-tailed p<0.01). In the second step, FrSBe “before” scores accounted for a significant increase in variance in GDS scores, R 2 =0.05, F (1, 69)=4.84, two-tailed p=0.03. However, FrSBe “after” scores, added in the third step, failed to account for a significant increase in the variance in GDS, R 2 =0.001, F (1, 68)=0.12, two-tailed p=0.73.
Patients with a variety of dementia subtypes were enrolled in the study, with the majority being Alzheimer’s disease patients. To evaluate any group differences in both caregiver mood/burden and ratings of patient behaviors between these distinct dementia subtypes, participants were divided into groups of Alzheimer’s disease versus “other” dementia subtypes. Independent sample t tests showed no differences in caregiver burden, caregiver depression, overall or subscale ratings of behavioral problems in the patient, disease severity (i.e., CDR sum of boxes), activities of daily living, length of caregiving, or duration of dementia.
Finally, spousal and adult child caregivers were compared on outcome measures to explore possible important distinctions related to caregiver relationship. Dementia severity, as measured by the CDR sum of boxes, was greater in the care recipients of the adult child, t (70)=−3.0, p<0.05. This was also reflected in adult child caregivers reporting that the care recipient was functioning at a lower level for both basic and instrumental daily activities compared to the care recipients of spousal caregivers, t (69)=3.63, p<0.05, and t (69)=2.23, p<0.05, respectively. There were no other differences between spousal and adult child caregivers in ratings of caregiver depression, caregiver burden, or behavior problems in the patient.
DISCUSSION
Results from this study suggest that frontal systems behavioral problems in the care recipient predict caregiver burden after controlling for dementia severity and caregiver depression. Closer analysis of the subscales revealed that behaviors associated with executive dysfunction and disinhibition were predictive of burden, whereas apathy in the patient was not predictive of caregiver burden. Interestingly, frontal systems behavioral problems failed to predict caregiver depression after controlling for dementia severity. There were no differences on outcome measures when comparing dementia subtypes. Spousal and adult child caregivers reported similar levels of perceived burden, depressive symptoms, and severity of behavioral problems, despite the fact that spousal caregivers reported more intact activities of daily living in the care recipient than adult child caregivers.
The results from this study are consistent with previous findings demonstrating that behavioral disturbance in the patient is one of the strongest contributors to caregiver burden 6 , 15 , 19 , 27 – 29 and partially replicates the study of Rymer et al., 19 which found that frontal systems behavioral problems uniquely contribute to caregiver burden in Alzheimer’s disease above and beyond the contribution of disease severity and patient awareness of memory and behavioral problems. The current findings are an important extension of Rymer et al.’s findings: we demonstrated that this relationship between caregiver burden and frontal systems dysfunction remains after accounting for the variability associated with caregiver depression. Furthermore, we demonstrated that this relationship persists in a sample reflecting multiple dementia subtypes and in a larger sample of caregivers with strict inclusion criteria in terms of quantity of daily care, place of residence, and relationship to the care recipient.
When considering the specific problem types, our findings suggest that behaviors associated with executive dysfunction and disinhibition were predictive of burden, whereas apathy was less burdensome to caregivers. Executive functioning refers to a constellation of behaviors, such as repeating actions, difficulty sequencing multistep behaviors, failure to learn from previous mistakes, disorganization, lack of insight, poor problem-solving skills, rigidity, and poor planning. Although previous research suggests that objective measures of cognition are less reliably associated with burden, the current study demonstrated that executive dysfunction is a strong predictor of caregiver burden.
There is a strong relationship between executive dysfunction and functional status in both community elderly 30 , 31 and dementia patients. 32 Therefore, one explanation for the current findings is that executive dysfunction affects daily functioning to a greater extent than do memory changes, thereby necessitating more care. Similarly, our findings suggest that disinhibition in the patient is quite distressing to caregivers. Taken together, these findings suggest that these active behaviors may be more difficult to manage and more burdensome to caregivers than the more passive behaviors associated with apathy. Behavioral interventions for patients aimed at increasing daily structure, creating routines, assisting with sequencing and behavioral strategies to reduce unwanted, inappropriate behaviors may be particularly useful in dementia caregiver interventions. Caregiver interventions for managing patient behavior may be further tailored by examining objective executive functioning in the patient with neuropsychological measures. Our group is currently investigating the impact on caregiver burden of a family-based intervention that relies heavily on problem-solving techniques.
It is important to emphasize that caregiver burden was related to caregiver depression in the current study. This is consistent with many other reports demonstrating significant overlap between depressive symptoms and feelings of burden and strain. 4 , 16 , 33 , 34 We controlled for depression in the initial regression analyses to focus on the unique relationship between behavioral problems and burden after accounting for caregiver depression. In our follow-up analyses, we examined caregiver depression as a primary outcome to determine whether the same factors that predict caregiver burden also predict caregiver depression. Results indicated that the severity of frontal systems behavioral problems in the patient do not predict caregiver depression. This discrepancy between the predictors of caregiver burden and depression is somewhat surprising and highlights the complexities of understanding mental health outcomes in dementia caregivers. Our data suggest that burden may encompass depression, as well as additional constructs, such as perception and attributions about the caregiving situation. These attributions may be critical in predicting mental health outcomes in caregivers of patients with dementia.
Certainly, the relationship between depression and burden requires more investigation and is beyond the scope of our data. Recent data from Clyburn et al. 27 suggest that burden may mediate caregiver mood. As such, future studies should employ longitudinal, structural equation models to address whether behavioral problems directly affect burden, thereby affecting caregiver depression. Our data do suggest, however, that burden and depression are not interchangeable constructs and may have distinct predictors.
We found an unexpected correlation between caregivers’ reports of patients’ premorbid behavior and current burden. The meaning of this relationship in our study is somewhat unclear. It may reflect method variance on the FrSBe or possibly a relationship between premorbid personality characteristics and burden (i.e., individuals with difficult premorbid personalities may be more likely to be difficult following onset of dementia). Our group recently demonstrated that poor premorbid relationship satisfaction is associated with greater caregiver burden in both spouse and adult child dementia caregivers. These findings were independent of relationship type (e.g., spousal versus parental), length of caregiving, disease severity, and care recipient daily functioning. 35
The current study may have been limited by the use of a self-report questionnaire of depressive symptoms rather than a clinician-rated interview, and by lack of objective neuropsychological measures of executive dysfunction. In addition, several authors have demonstrated important differences between male and female caregivers, 36 , 37 though this relationship remains unclear. We were unable to address these differences due to the overrepresentation of women in our sample. However, future research may benefit from incorporating objective neuropsychological testing and utilizing more comprehensive measures of depressive symptomatology that would allow for more detailed analyses of the impact of important caregiver demographic factors and dementia subtype differences on caregiver depression and burden. Finally, the study population may not be representative of all caregivers of patients with mild to moderate dementia, as they were recruited into the study to participate in a psychosocial intervention study. As such, it is difficult to determine whether the level of burden and behavior problems reported in our sample is similar to a community-based sample of dementia caregivers.
Overall, findings from the present study demonstrate the unique contribution of caregivers’ perceptions of frontal systems behavior dysfunction, particularly executive dysfunction and disinhibition, on caregiver burden. Results support caregiver interventions that address both caregiver mood and specific types of behavioral disturbance in the patient. For example, problem-solving strategies to compensate for executive dysfunction and behavioral interventions to address inappropriate behaviors in the patient may be beneficial.
1. Beard CM, Kokmen E, O’Brien PC, et al: The prevalence of dementia is changing over time in Rochester, Minn. Neurology 1995; 45:75–79Google Scholar
2. Evans DA, Funkenstein HH, Albert MS, et al: Prevalence of Alzheimer’s disease in a community population of older persons: higher than previously reported. JAMA 1989; 262:2551–2556Google Scholar
3. Aneshensel CS, Pearlin LI, Schuler RH: Stress, role captivity, and the cessation of caregiving. J Health Soc Behav 1993; 34:54–70Google Scholar
4. Stommel M, Collins CE, Given BA: The costs of family contributions to care of persons with dementia. Gerontologist 1994; 34:199–205Google Scholar
5. Schulz R, O’Brien AT, Bookwala J, et al: Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist 1995; 35:771–791Google Scholar
6. Baumgarten M, Battista RN, Infante-Rivard C, et al: The psychological and physical health of family members caring for an elderly person with dementia. J Clin Epidem 1992; 45:61–70Google Scholar
7. Draper BM, Poulos CJ, Cole AM, et al: A comparison of caregivers for elderly stroke and dementia victims. J Am Geriatr Soc 1992; 49:896–901Google Scholar
8. Kiecolt-Glaser JK, Dura JR, Speicher CE, et al: Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom Med 1991; 53:345–362Google Scholar
9. Schulz R, Beach SR: Caregiving as a risk factor for mortality: the caregiver health effects study. JAMA 1999; 282:2259–2260Google Scholar
10. Teri L: Behavior and caregiver burden: behavioral problems in patients with Alzheimer’s disease and its association with caregiver distress. Alzheimer Dis Assoc Disord 1997; 11:S35–S38Google Scholar
11. Teri L, Larson E, Reifler BV: Behavioral disturbance in dementia of the Alzheimer’s type. J Am Geriatr Soc 1988; 36:1–6Google Scholar
12. Teri L, Traux P, Logsdon R, et al: Assessment of behavioral problems in dementia: the Revised Memory and Behavior Problems Checklist. Psychol Aging 1992; 7:622–631Google Scholar
13. Zarit SH, Reever KE, Bach-Peterson J: Relatives of impaired elderly: correlates of feelings of burden. Gerontologist 1980; 20:640–655Google Scholar
14. Coen RF, O’Boyle CA, Coakley D, et al: Dementia care education and patient behaviour disturbance. Int J Geriatr Psychiatry 1999; 14:302–306Google Scholar
15. Pinquart M, Sorensen S: Associations of stressors and uplifts of caregiver with caregiver burden and depressive mood: a meta-analysis. J Gerontol B Psychol Sci Soc Sci 2003; 58:112–128Google Scholar
16. Song L-Y, Biegel DE, Milligan SE: Predictors of depressive symptomatology among lower social class caregivers of persons with chronic mental illness. Community Ment Health J 1997; 33:269–286Google Scholar
17. Haley WE, Brown SL, Levine EG: Experimental evaluation of the effectiveness of group intervention for dementia caregivers. Gerontologist 1987; 27:376–382Google Scholar
18. Pruchno RA, Michaels JE, Potashnik SL: Predictors of institutionalization among Alzheimer’s disease victims with caregiving spouses. J Gerontol Soc Sci 1990; 45:S259–S266Google Scholar
19. Rymer S, Salloway S, Norton L, et al: Impaired awareness, behavior disturbance, and caregiver burden in Alzheimer’s disease. Alzheimer Dis Assoc Disord 2002; 16:248–253Google Scholar
20. Hooker K, Bowman SR, Coehlo DP, et al: Behavioral change in persons with dementia: relationship with mental and physical health of caregivers. J Gerontol B Psychol Sci Soc Sci 2002; 57:453–460Google Scholar
21. Robinson KM, Adkisson P, Weinrich S: Problem behaviour, caregiver reactions, and impact among caregivers of persons with Alzheimer’s disease. J Adv Nurs 2001; 36:573–582Google Scholar
22. Hughes CP, Berg L, Danziger WL, et al: A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140:566–572Google Scholar
23. Grace J, Malloy P: Frontal Systems Behavior Scale (FrSBe): Professional Manual. Lutz, Fla, Psychological Assessment Resources, 2001Google Scholar
24. Lawton MP, Brody EM: Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9:179–188Google Scholar
25. Zarit SH, Reever KE, Bach-Peterson J: Relatives of impaired elderly: correlates of feelings of burden. Gerontologist 1980; 20:649–655Google Scholar
26. Yesavage JA, Brink TL, Rose TL, et al: Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1983; 17:37–49Google Scholar
27. Clyburn LD, Stones MJ, Hadjistavropoulos T, et al: Predicting caregiver burden and depression in Alzheimer’s disease. J Gerontol Soc Sci 2000; 1:S2–S13Google Scholar
28. Donaldson C, Tarrier N, Burns A: The impact of the symptoms of dementia on caregivers. Br J Psychiatry 1997; 170:62–68Google Scholar
29. Nagaratnum N, Lewis-Jones M, Scott D, et al: Behavioral and psychiatric manifestations in dementia patients in a community: caregiver burden and outcome. Alzheimer Dis Assoc Disord 1998; 12:330–334Google Scholar
30. Cahn-Weiner DA, Boyle PA, Malloy PF: Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol 2002; 9:197–191Google Scholar
31. Cahn-Weiner DA, Malloy PF, Boyle PA, et al: Prediction of functional status from neuropsychological test in community-dwelling elderly individuals. Clin Neuropsychol 2000; 14:187–195Google Scholar
32. Norton LE, Malloy PF, Salloway S: The impact of behavioral symptoms on activities of daily living in patients with dementia. Am J Geriatr Psychiatry 2001; 9:41–48Google Scholar
33. Gallant MP, Connell CM: The stress process among dementia spouse caregivers: are caregivers at risk for negative health behavior change? Res Aging 1998; 20:267–297Google Scholar
34. Pearson JL, Teri L, Wagner A, et al: The relationship of problem behaviors in dementia patients to the depression and burden of caregiving spouses. Am J Alz Dis Related Disord Res 1991; 8:15–22Google Scholar
35. Steadman PL, Tremont G, Davis JD: Premorbid relationship satisfaction and caregiver burden in dementia caregivers. J Geriatr Psychiatry Neurol (in press)Google Scholar
36. Bookwala J, Schulz R: A comparison of primary stressors, secondary stressors, and depressive symptoms between elderly caregiving husbands and wives: the caregiver health effects study. Psychol Aging 2000; 15:607–616Google Scholar
37. Ingersoll-Dayton B, Raschick M: The relationship between care-recipient behaviors and spousal caregiving stress. Gerontologist 2004; 44:318–327Google Scholar



