An Exploratory Study of Diagnostic Criteria for Delirium in Older Medical Inpatients
In the older medical inpatients, however, DSM criteria identify a disorder with low rates of recovery, 3 persistence of symptoms for up to 1 year 3 and significant increases in length of hospital stay, 4 , 5 rates of institutionalization, 6 , 7 functional disability, 6 – 8 and rates of death, 9 – 11 independent of many sociodemographic and clinical variables. Thus, the prognosis of delirium in older medical inpatients appears to be poor, perhaps related to the frequent presence of dementia or multiple chronic medical conditions.
Frequent dementia and multiple chronic medical conditions notwithstanding, it is possible that the presenting symptoms of delirium change with age and that current diagnostic criteria do not identify delirium (that recovers) in this population. 12 – 14 This possibility would have to be considered seriously if the presenting symptoms of delirium among patients who recover can be shown to differ from those of patients who do not recover. Therefore, the goal of the present study was to explore the presenting symptoms of delirium among older medical inpatients who did or did not recover from delirium.
METHOD
Subjects
The study was a secondary analysis of data collected in a study of the prognosis of delirium. 6 The original study was conducted at St. Mary’s Hospital, a 400-bed primary acute care university-affiliated hospital in Montreal, Quebec. A study nurse with master’s level training in geriatrics was responsible for patient screening and enrollment in the two studies. Patients ages 65 or older who were admitted from the emergency department to the general medical or geriatric services were included in the study. Patients excluded were those with primary diagnosis of stroke, those admitted to the oncology unit, those admitted to the intensive care unit (ICU) or cardiac monitoring unit (CMU), unless they were transferred to a medical ward within 48 hours of admission, and those who did not speak English or French.
The study nurse administered the Confusion Assessment Method (CAM) 15 to those whose initial Short Portable Mental Status Questionnaire (SPMSQ) 16 score was 3 or more or whose nursing notes indicated symptoms of delirium. The study nurse used various data sources to complete the CAM (chart, family, nursing staff) and assessed the patient at several points in time, if necessary. Those with an initial SPMSQ score of less than 3 and those with an initial SPMSQ of 3 or more but who did not have delirium were rescreened with the SPMSQ daily for the following week. The CAM was readministered if the SPMSQ score increased or there was evidence from the nursing notes of symptoms of delirium. Patients with delirium were asked to assent to participate in the study, and a family member was asked for informed consent. The study was approved by the hospital Research Ethics Committee.
Procedures
Patients with delirium were assessed at enrollment by the study nurse and a research assistant, then several times during the first week, and weekly for 4 weeks by the research assistant, who also interviewed a family member. At enrollment, the study nurse collected demographic data (e.g., age, gender) and completed a Clinical Severity of Illness measure. 17 The research assistant, independent of the study nurse’s information and diagnosis, completed the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) 18 and the Delirium Index (DI). 19 At follow-up, the research assistant completed the DI. The Charlson Comorbidity Index (CCI) 20 and the Acute Physiology Score (APS) 21 were determined by chart review at the end of the study. Note that an operational implementation of DSM-IV criteria for delirium was based on symptoms recorded by the CAM.
Measures
The SPMSQ 16 is a widely used, observer-rated 10-item questionnaire that evaluates orientation, memory, and concentration; scale scores range from 0 (no impairment) to 10 (severe impairment). The CAM 15 is a structured instrument that operationalizes the 10 symptoms of delirium specified in DSM-III-R: 22 acute onset, fluctuating course, inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation or retardation, and sleep/wake disturbance. The DI 19 is an instrument developed for the measurement of the severity of seven symptoms of delirium (inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation or retardation); subscale scores are 0 (absent), 1 (mild), 2 (moderate) and 3 (severe); total scores range from 0 (no symptoms) to 21 (maximum severity); the scale has demonstrated reliability and validity. 19 , 23 The presence of dementia was assessed from the IQCODE, an instrument with high internal consistency and test-retest reliability. 18 We used a cutoff of more than 3.5 to define dementia. Three measures of illness severity were used: the Charlson Comorbidity Index (CCI) (higher scores indicating greater comorbidity), 20 the Acute Physiology Score (APS), derived from the APACHE II scale (score ranges from 0 [no impairment] to 44 [severe impairment]) 21 and Clinical Severity of Illness (score ranges from 1 [minimal] to 9 [most severe]). 17
The interrater agreement for delirium versus no delirium using the CAM was excellent (kappa value 1.0) (N=14); the study nurse diagnosis of delirium had a sensitivity of 0.89 (N=87) and a specificity of 1.0 compared to a consensus diagnosis. 24 The interrater concordance correlation coefficients (CCC) for the other measures ranged from 0.90 to 0.99 (N=28).
Statistical Analysis
Through some data exploration, we arrived at the following definition of recovery using the DI. We decided to use a DI measure of recovery because this measure indicates the presence or absence of core features of delirium; moreover, the DI was highly correlated with other measures of recovery (i.e., cognition and basic activities of daily living) in our population. The definition of recovery was as follows: there were at least two DI measures over 4 weeks; from the first to the last measure the score decreased by 3 points or more; and the last DI measure was 4 or less in patients with no dementia and 5 or less in patients with dementia. 25 Thus, the outcome variable “recovery” was a binary variable taking value 1 if the patient satisfied all of the above conditions and 0 otherwise.
Predictors were individually compared across delirium groups by calculating odds ratios (OR) and confidence intervals by means of univariate logistic regression. To obtain a prediction model for recovery among patients diagnosed with DSM-IV delirium, we used both multivariate logistic regression with backward variable selection and tree analysis. For tree construction, we used the RECPAM (Recursive Partitioning and Amalgamation) approach, 26 which has the desirable feature of allowing a direct comparison with logistic regression, since both methods are based on maximizing a likelihood function. Comparisons of the tree model and the logistic regression model (both models built from the data) were based on the cross-validated deviances (twice the negative of the log-likelihood) and generalized R 2 . Cross-validation was performed as in Ciampi et al. 27 All calculations were performed in SAS and S-PLUS.
RESULTS
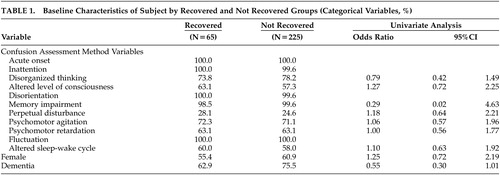
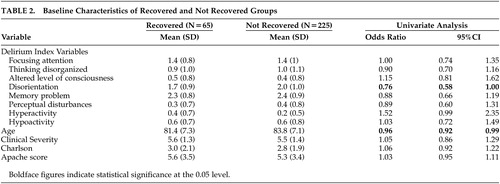
Two hundred ninety patients met DSM-IV criteria for delirium; 65 patients with delirium met the criteria for recovery and 225 did not. Characteristics of the 290 patients in recovered and not recovered groups are presented in Tables 1 and 2 . In univariate analyses, there were statistically significant differences between recovered and not recovered groups for only age (OR=0.96, 95% CI: 0.92, 0.99) and DI disorientation (OR=0.76, 95% CI: 0.58, 1.0) and marginally for dementia status (OR=0.55, 95% CI: 0.30, 1.01). In a multivariate logistic model (with backward elimination) to predict recovery based on dementia status, CAM, and DI variables (including interactions of these with dementia), only DI hyperactivity and the interaction between dementia and DI disorientation were statistically significant at the 0.05 level ( Table 3 ); dementia as a main effect was retained in the model, though it was not significant. The interpretation of the model, taking the interaction into account, is as follows: in patients with dementia, increasing disorientation decreased the probability of recovery (OR=0.64, p value=0.0131); in patients with or without dementia, increasing hyperactivity increased the probability of recovery. Notably, in the dementia group 42 patients recovered (19%), while in the nondementia group 23 patients (32%) recovered (Pearson’s chi-square=5.0029, p value=0.0253); thus, absence of dementia can also be considered an important predictor of recovery. We also developed a prediction model for patients with and without dementia separately (details not shown); the results were similar. It should be noted that CAM1 (acute onset) and CAM10 (fluctuation) have variation 0 on this sample and therefore do not appear in the analysis.
 |
 |
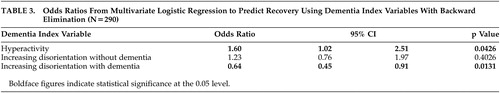 |
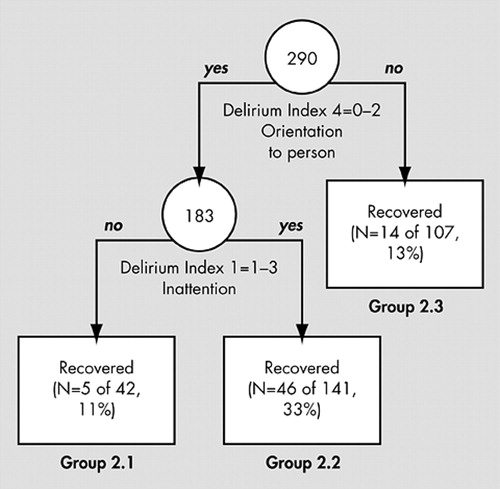
Figure 1 presents the results of the RECPAM regression tree analysis of the 290 patients with delirium, using dementia, CAM and DI variables as predictors. According to the tree diagram, patients most likely to recover (33% recovery) were those with DI orientation to person (DI4=0 to 2) and any DI inattention (DI1=1 to 3, group 2.2). Patients in the other two groups were less likely to recover: those with orientation to person and without inattention (Group 2.1) had a recovery rate of 11%, and those without orientation to person (Group 2.3) had a recovery rate of 13%. Cross-validation showed that the RECPAM tree was a better predictor than the logistic regression model. Indeed the cross-validated deviance was 100.10 for the tree model and 101.80 for the regression model, both with standard errors of 0.40. Also, the generalized R 2 was 0.06 for the tree model and 0.03 for the regression model, both with standard errors of 0.010. Although dementia does not appear in the tree, direct cross-tabulations show that dementia is highly prevalent (92 of 107 patients or 86%) in Group 2.3 and that absence of dementia significantly predicts recovery only in this group (Fisher’s exact test p value=0.026).
DISCUSSION
This study proposed to explore the presenting symptoms of delirium among older medical inpatients who did or did not recover from delirium. In the multivariate logistic regression model, among patients without dementia, those who were hyperactive were more likely to recover; among patients with dementia, those who were less disoriented and hyperactive were more likely to recover. The RECPAM tree analysis emphasized the presence of orientation to person and any inattention in predicting recovery. It is possible that one of the symptoms, orientation to person, identified a severity threshold below which recovery was unlikely. Of note, all predictive variables were DI items (not CAM items), possibly because the DI items were entered in the analysis as continuous variables whereas the CAM variables were entered as categorical variables.
This study has three potential limitations. First, our definition of recovery may have been too conservative (only 22% of patients with delirium recovered); however, for purposes of this study, it was important to avoid misclassifying patients who did not recover as “recovered.” Second, the 4-week follow-up period may have been too short to allow maximum rates of recovery. Third, our measure of motor disturbances was based on a single observation at baseline and may have resulted in misclassification of patients with mixed hyper- and hypoactivity.
To conclude, three presenting symptoms of delirium, orientation to person, hyperactivity, and inattention, distinguished older medical inpatients who recovered from those who did not recover. If delirium is conceptualized as a transient condition (as in DSM), these results suggest it may be necessary to place increased emphasis on these presenting symptoms when diagnosing delirium in this population.
1. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Association, 1994Google Scholar
2. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Washington, DC, American Psychiatric Association, 1980Google Scholar
3. McCusker J, Cole MG, Dendukuri N, et al: The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med 2003; 18:696–704Google Scholar
4. McCusker J, Cole MG, Dendukuri N, et al: Does delirium increase hospital stay? J Am Geriatr Soc 2003; 51:1539–1546Google Scholar
5. O’Keeffe S, Lavan J: The prognostic significance of delirium in older hospital patients. J Am Geriatr Soc 1997; 45:174–178Google Scholar
6. McCusker J, Cole MG, Dendukuri N, et al: Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. Can Med Assoc J 2001; 165:575–583Google Scholar
7. Inouye SK, Rushing JT, Foreman MD, et al: Does delirium contribute to poor hospital outcomes? a three-site epidemiologic study. J Gen Intern Med 1998; 13:234–242Google Scholar
8. Murray AM, Levkoff SE, Wetle TT, et al: Acute delirium and functional decline in the hospitalized elderly patient. J Gerontol 1993; 48:M181–M186Google Scholar
9. Francis J, Martin D, Kapoor WN: A prospective study of delirium in hospitalized elderly. JAMA 1990; 263:1097–1101Google Scholar
10. McCusker J, Cole MG, Abrahamowicz M, et al: Delirium predicts 12-month mortality. Arch Intern Med 2002; 162:457–463Google Scholar
11. Rockwood K, Cosway S, Carver D, et al: The risk of dementia and death after delirium. Age Ageing 1999; 28:551–556Google Scholar
12. Treloar AJ, Macdonald A: Outcome of delirium: outcome of delirium diagnosed by DSM-III-R, ICD-10 and CAMDEX and derivation of the reversible cognitive dysfunction scale among acute geriatric inpatients (part 1). Int J Geriatr Psychiatry 1997; 12:609–613Google Scholar
13. Treloar AJ, Macdonald AJD: Outcome of delirium: clinical features of reversible cognitive dysfunction—are they the same as accepted definitions of delirium? (part 2). Int J Geriatr Psychiatry 1997; 12:614–618Google Scholar
14. Cole MG: Delirium in elderly patients. Am J Geriatr Psychiatry 2004; 12:7–21Google Scholar
15. Inouye SK, VanDyck CH, Alessi CA, et al: Clarifying confusion: the Confusion Assessment Method, a new method for detection of delirium. Ann Intern Med 1990; 113:941–948Google Scholar
16. Pfeiffer E: A Short Portable Mental Status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975; 23:433–441Google Scholar
17. Charlson ME, Sax FL, MacKenzie R, et al: Assessing illness severity: does clinical judgment work? J Chronic Dis 1986; 39:439–452Google Scholar
18. Jorm AF: A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994; 24:145–153Google Scholar
19. McCusker J, Cole MG, Bellavance F, et al: Reliability and validity of a new measure of severity of delirium. Int Psychogeriatr 1998; 10:421–433Google Scholar
20. Charlson ME, Pompei P, Ales KL, et al: A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383Google Scholar
21. Knaus W, Draper E, Wagner D, et al: Apache II: a severity of disease classification system. Crit Care Med 1985; 13:818–829Google Scholar
22. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Washington, DC, American Psychiatric Association, 1987Google Scholar
23. McCusker J, Cole M, Dendukuri N, et al: The Delirium Index, a measure of the severity of delirium: new findings on reliability, validity and responsiveness. J Am Geriatr Soc 2004; 52:1744–1749Google Scholar
24. Zou Y, Cole MG, Primeau FJ, et al: Detection and diagnosis of delirium in the elderly: psychiatrist diagnosis, Confusion Assessment Method or consensus diagnosis? Int Psychogeriatr 1998; 10:303–308Google Scholar
25. Cole MG, McCusker J, Dendukuri N, et al: Symptoms of delirium among elderly medical inpatients with or without dementia. J Neuropsychiatry Clin Neurosci 2002; 14:167–175Google Scholar
26. Ciampi A: Generalized regression trees. Comput Stat Data Anal 1991; 12:57–78Google Scholar
27. Ciampi A, Couturier A, Li S: Prediction trees with soft nodes for binary outcomes. Stat Med 2002; 21:1145–1165Google Scholar



