Predictors of Abnormal Involuntary Movement in an African Schizophrenia Population
Studies clearly indicate that there are several possible risk factors associated with involuntary movements (including tardive dyskinesia). Increasing age and the use of antipsychotic medication are the factors most consistently associated with the development of tardive dyskinesia. 7 – 10 Nevertheless, Waddington et al. 11 , 12 could not distinguish between patients with or without involuntary movements based on conventional indices of neuroleptic or anticholinergic treatment. Furthermore, involuntary movements can also occur in treatment-naive patients. 13 Waddington et al. 11 , 12 could not distinguish tardive dyskinesia and non-tardive dyskinesia by age and gender in this Irish sample. Oosthuizen et al. 7 did not find any correlation with gender, race, or duration of untreated psychosis in a study of tardive dyskinesia in subjects with first-episode psychosis. A number of studies, however, do suggest a role for gender in tardive dyskinesia, with females seemingly at higher risk for developing tardive dyskinesia, although Gervin et al. 1 and van Os et al. 14 found either no influence or a higher risk in males. Age of first neuroleptic exposure, type of neuroleptic, diagnosis, and use of other psychiatric medication has been shown to have little or no effect. The abuse of alcohol or cannabis has been associated with a higher incidence of involuntary movements. 15 , 16
Several studies have investigated the correlation between tardive dyskinesia and negative symptoms. The most consistent, but not uniform, finding is that the severity of tardive dyskinesia is positively associated with negative symptoms. 17 Brown and White 18 divided patients into groups scoring high and low on the Scale for the Assessment of Negative Symptoms (SANS), formed by segregating around the median value. The group with the high SANS scores was found to have significantly more severe tardive dyskinesia. This is in keeping with findings from Caligiuri et al. 10 and Gervin et al. 1 The latter study demonstrated an increase in overall negative symptoms in patients with dyskinesia compared to those without. Liddle 19 found that both orofacial and limb dyskinesia were associated with negative symptoms. On the other hand, Bartzokis et al. 20 found a small correlation between more severe tardive dyskinesia and fewer negative symptoms in 25 patients, while Chiu et al. 21 found no association between negative symptoms and tardive dyskinesia.
It would be of interest to investigate whether the above-mentioned risk factors, especially negative symptoms, are associated with involuntary movements in an African Xhosa population, as ethnic differences have been suggested before. 22 The Xhosa is an indigenous Nguni-speaking population mainly living in the Western and Eastern Cape provinces of South Africa. Previous explanatory and confirmatory factor analysis in this population group showed that the core symptom domains (negative symptom, positive symptom, positive thought disorder, and bizarre behavior domains) of schizophrenia in the Xhosa population are similar to those reported in Caucasian samples. 23 However, preferential response to antipsychotic treatment, ethno-specific loci, and specific comorbidity patterns within this African study population suggest heterogeneity in risk factors for schizophrenia. The question now arises whether unique risk factors for involuntary movements exist in this African population group.
METHOD
One hundred seventy Xhosa patients with a diagnosis of schizophrenia were recruited, as part of a larger genetic study, from primary, secondary, and tertiary health services in the Western Cape based on inclusion and exclusion criteria. The inclusion criteria included a diagnosis of schizophrenia or schizoaffective disorder according the DSM-IV criteria, Xhosa ethnic origin (4/4 grandparents reported to be of Xhosa origin), and written consent from subjects and/or their legal caregivers. Subjects with known medical disorders that may cause psychosis were excluded.
Recruitment Process
Mental health workers were asked to identify possible participants, who were then screened for suitability and diagnosed according to DSM-IV criteria. Patients were included in the study after providing written, informed consent as stipulated in the protocol approved by the local Institutional Review Board of the University of Stellenbosch, South Africa. The father, mother, and/or unaffected sibling of the proband were contracted to the study in order to provide phenotypical and genealogical information.
Assessment
Primary Assessment Measure
The Diagnostic Interview for Genetic Studies, version 2.0, 24 and the Abnormal Involuntary Movement Scale (AIMS) 25 were the primary assessment tools and provided the data for statistical analysis of clinical measurements. The Diagnostic Interview for Genetic Studies is a clinical assessment tool designed for diagnosing major mood and psychotic spectrum disorders and includes the Scale for the Assessment of Negative Symptoms (SANS) and the Scale for the Assessment of Positive Symptoms (SAPS), validated assessment scales for positive and negative symptom complexes. The AIMS measures seven domains of involuntary movements on a scale of 0 to 4 (0=absence of involuntary movement, 2=presence of involuntary movement, and 4=severe involuntary movement). The interviewers also used hospital chart records (when available) and information gathered from family members to supplement these interviews. Relevant demographic data, medical history, treatment history, and pedigree information were collected from the proband and family. Each subject underwent a urinary drug screen test for drugs of abuse (including cannabis).
A trained psychiatrist and/or Xhosa psychiatric nurse with extensive clinical experience interviewed each participant, using an English (oral translation into Xhosa) version of the standardized instrument (the Diagnostic Interview for Genetic Studies). When necessary, the help of an interpreter was utilized. In order to maintain optimal rating consistency over the 2-year period of recruitment, regular calibration meetings were held and all the participants were recruited at a single study site, using the same interviewers.
Statistical Methods
Subjects were divided into two groups, those with involuntary movements (AIMS>0) and those without involuntary movements, which defined the dependent variable in the statistical analysis. The decision to consider any positive score on the AIMS on a cross-sectional assessment as indicative of abnormal movements is significantly less stringent than the Schooler-Kane criteria 5 and may be overinclusive of movements that would not necessarily lead to functional impairment. Nevertheless, we do report on the number of participants from this group who met criteria for tardive dyskinesia and a post hoc classification was done according to the Morgenstern-Glazer criteria 26 for the presence of tardive dyskinesia to allow for a direct comparison with Patterson et al. 3 This criterion specifies a score of “mild” on at least two items or “moderate” on any one item of the seven measured domains. However, the potential outcome of this study had to consider the structure and availability of health resources within South Africa. As the majority of mental health treatment takes place in a community setting under the care of nursing personnel, the use of our criteria (presence of any positive score on AIMS) was within the abilities of the available health resources and assisted in the screening of patients with possible tardive dyskinesia. Various potentially explanatory factors of involuntary movement were considered, including age at interview, gender, subsections of the SANS, duration of illness, drug use history, and smoking. The binary variable, presence/absence of involuntary movement, was modeled using a multivariate logistical (logit) model. The results were confirmed by substituting a probit model for the logit model.
RESULTS
Description of the Data
The sample consisted of 167 individuals with consistent data (three individuals excluded based on missing variables) across the various categories of interest, of which 33 had involuntary movements. A large majority (N=137; 82%) of subjects were male. Twelve of the 33 participants with involuntary movements (8 men) fulfilled Morgenstern-Glazer criteria for tardive dyskinesia. No significant differences for age (p=0.167), duration of illness (p=0.439), or duration of treatment (p=0.900) were detected between those who met tardive dyskinesia criteria (TD+ group) and those with abnormal involuntary movement who did not meet the tardive dyskinesia criteria (TD− group). The tardive dyskinesia group had an average AIMS score of 14.90 (SD=2.37) compared to 6.4 (SD=3.17) in the group with abnormal involuntary movement who did not fulfill the tardive dyskinesia criteria (p<0.001). Seven of the 12 tardive dyskinesia cases and 17 of the 21 non-tardive dyskinesia cases (but who exhibited abnormal involuntary movements) had a cannabis use or abuse history.
The primary aim of this study, however, was to investigate the association between involuntary movements, as defined by an AIMS score of more than 1 on any of the domains, and various potentially relevant characteristics. The association between characteristics and abnormal involuntary movements are reported in a bivariate manner in the next section, followed by a multivariate logit model that combines the explanatory power of the various characteristics that appeared to be individually relevant.
Bivariate Analysis of Potential Explanatory Factors
The potential explanatory factors were divided into continuous variables (e.g., age at interview) and categorical variables (e.g., handedness).
Continuous Explanatory Factors
The following potential explanatory factors were measured on a continuous scale: age at interview, duration of illness, and duration of medication use. The measurements of these factors were not, in fact, continuous, but on a categorical scale with a large number of categories relative to the size of the data set. Treating these as categorical variables would have resulted in a number of thin categories.
Age at Interview
The variable “age at interview” measures the age at last birthday and showed a range of 17 to 59 years of age (mean=32.7; SD=9.96). Two hypothesis tests, first assuming similar variance for both groups and the second allowing for differences in variance, both rejected the hypothesis of equal mean for age at interview (p<0.0001). This result held for one-sided and two-sided alternative hypotheses. Further evidence of a likely positive relationship between involuntary movements and age at interview is found by estimating a bivariate logistic model in which age at interview is the only predictor of the (log) odds ratio for involuntary movements. Adding the squared value of age at interview to the logistic regression improved the explanatory power of the regression significantly: coefficient for age at interview =−0.2629 (p=0.078); age at interview squared =0.005 (p=0.018); Pearson goodness of fit statistic =37.5 (0.4003).
The nonlinearity in the relationship emerged clearly as the probability of involuntary movements rose only from 0.097 to 0.109 between the 25th percentile for age at interview and the median but almost doubled between the median and the 75th percentile (0.211). The hypothesis tests suggest that involuntary movements are more prevalent in older individuals in this sample and the logistic regressions suggest that this relationship might be nonlinear.
Duration of Illness and Duration of Treatment
The variable “duration of illness” measures each individual’s duration of illness in years. The duration of illness ranged between 0 and 39 years (mean=9.6; SD=8.6). Both versions of the test reject the null hypothesis (single and two-tailed) of equal means for the involuntary movements and no involuntary movements groups over duration of illness. The bivariate logistic model in which duration of illness is the only predictor of the (log) odds ratio for involuntary movements provides further evidence of a possible positive relationship between duration of illness and involuntary movements. An examination of a potential nonlinear effect (similar to that for age at interview) did not reveal evidence of a nonlinear relationship between involuntary movements and duration of illness (implied probabilities were 0.122, 25%; 0.161, 50%; 0.241, 75%).
The measurement of duration of treatment in weeks was problematic in that it was truncated at long durations with all durations in excess of 999 weeks grouped in one category. The data set was divided into the full data set and a reduced data set obtained by deleting all individuals with duration of treatment in the highest category. The reduced data set contained 134 individuals of which 24 had involuntary movements with a mean duration of 187.9 weeks (SD=217.5).
An apparent positive association was seen between duration of treatment and involuntary movements, but seemed to be driven by the large interquartile range of the full data set. The latter was, in turn, the result of the truncated manner in which this variable was recorded at the longest durations. When the truncated upper category was removed in the reduced data set, the two distributions seemed a lot more similar, even if the involuntary movements groups still showed a large interquartile range and slightly higher median.
The box plots of both the full and the reduced data sets suggest that the involuntary movement groups have a larger dispersion of treatment duration. This places the emphasis on the test-statistics using unequal variances. Assuming a one-tailed alternative hypothesis the two versions of the data yield different conclusions at conventional levels of significance. The hypothesis of equal means for involuntary movements and noninvoluntary movement groups is rejected at a 10% level of significance for the full data set, but not for the reduced data set. However, even the reduced data set shows evidence of dissimilar means between the two groups, and this difference is significant at 12%. A bivariate logistic regression was used to investigate this potential relationship further. The possibility of a nonlinear relationship was investigated through the inclusion of a squared duration of treatment term, but this term did not contribute significantly to an explanation of involuntary movements. Further, the logistic regression confirmed the message from the hypotheses tests that the positive relationship between involuntary movements and duration of treatment is stronger in the full data set (including the truncated duration of treatment data) than in the reduced data set. The relatively small difference between the probabilities at the 25th percentile (full data set 0.156 and reduced data set 0.147), median (0.175 and 0.16), and 75th percentile (0.233 and 0.196) demonstrates that variation in duration of treatment offers a less powerful explanation for the presence of involuntary movements.
Categorical Explanatory Factors
A number of potentially relevant explanatory factors were analyzed as categorical variables, including: gender, handedness, substance abuse (alcohol, cannabis), and smoking. In addition, a number of negative symptoms potentially associated with involuntary movements were also considered.
Gender
Of the 167 individuals, 137 were men (of which 25 had involuntary movements) and 30 female (of which 8 had involuntary movements). Neither the chi-square test nor Fisher’s exact test suggest that the gender difference is statistically significant in this sample. A bivariate logistic regression was done with gender as explanatory factor for involuntary movements, but it was not significant.
Handedness
The data set contained information on the handedness of the individuals, with 153 right-handed individuals, 12 left-handed and 2 ambidextrous. Test statistics for the formal hypothesis test offer no evidence to reject the null hypothesis of independence at conventional levels of significance. Handedness was also an insignificant explanatory factor in a bivariate logistic regression.
Substance Abuse
Substance abuse could be documented for 109 of the individuals in the sample. This consisted of only two substances: cannabis use or abuse (N=100) and alcohol abuse (N=53). Though alcohol abuse was slightly more prevalent among those individuals without involuntary movements than would have been expected under a hypothesis of independence (45 cases versus 43) and involuntary movements were relatively more prevalent among those with no record of alcohol abuse (25 versus 23), these deviations from the expected frequencies did not suggest evidence against the hypothesis of independence at conventional levels of significance, nor was the alcohol a significant explanatory factor in a bivariate logistic regression function.
In the involuntary movement group, cannabis use or abuse was less prevalent than expected (15 cases versus 20), while in the group without involuntary movements, cannabis use or abuse was more prevalent (85 versus 80) than would have been expected if involuntary movements and cannabis use or abuse were independent. The reported test statistics reject the null hypothesis of independence at a 10% level of significance.
The implied probability (0.269) of involuntary movements in subjects with no history of cannabis use or abuse is considerably larger than the probability of involuntary movement in a subject with a history of cannabis use or abuse (0.15). This implies that an individual with schizophrenia and a history of cannabis use or abuse was less likely to show abnormal involuntary movements at assessment.
Further continuous data were available on the duration of cannabis use or abuse and the age of first use (of cannabis). These variables entered the data set as interaction effects with cannabis use or abuse (i.e., they showed zero values for respondents without a history of cannabis use or abuse and positive values for subjects with a history of cannabis use or abuse). The “age of first use” data were combined with the “age at interview” data to create a new variable “maximum duration of cannabis use or abuse” which is the difference between “age at interview” and “age of first use.” This variable was introduced due to the fact that the variable “duration of cannabis use or abuse” relies on memory recall (supplemented with collateral information) and thus “maximum duration of cannabis use or abuse” will introduce a worst-case scenario where we assume that a participant has abused cannabis continuously. These data were not available for all the subjects with cannabis use or abuse in the full data set. Accordingly, the data set had to be reduced to 102 subjects who satisfied the criteria of either no history of cannabis use or abuse or known duration of cannabis use or abuse and known first date of cannabis use.
Cross tabulation between involuntary movements and cannabis use or abuse for this reduced data set showed that, in the involuntary movement group, cannabis use or abuse was less prevalent than expected (4 cases versus 9), while in the group without involuntary movements, cannabis use or abuse was more prevalent (40 versus 35) than would have been expected if involuntary movements and cannabis use or abuse were independent. The odds ratio for involuntary movements in subjects with no history of cannabis use or abuse (0.415) was four times larger than the odds ratio for subjects with a history of cannabis use or abuse (0.100). The reported test statistics reject the null hypothesis of independence at a 5% level of significance (p=0.012), i.e., a stronger result than reported for the full data set.
The “duration of cannabis use” (8–1508 weeks; mean=346 weeks; SD=335.47 weeks), “age at first cannabis use” (10–32 years old; mean=18 years; SD=18.682) and “max duration of use” (34 years; mean=8 years; SD=8.361) were used as interaction variables. To determine whether these three interaction variables (combining binary data for subjects with no history of cannabis use or abuse and continuous information for subjects with a history of cannabis use or abuse) add additional information to the binary indicator of cannabis use or abuse for this reduced data set, four bivariate logistic regressions were estimated. An additional metric, the area under the receiver operating characteristic curve, is reported for each regression. Following Peng and So 27 the preferred model is the model with the greatest area under the receiver operating characteristic curve (which indicates the greater ability to discriminate between cases with and without involuntary movements across the full range of cutoff probabilities). This analysis confirms (in this reduced data set) the significance of cannabis use or abuse as an explanatory factor for involuntary movements, with cannabis use or abuse reducing the odds of involuntary movements relative to noninvoluntary movements. However, the continuous information added by the two variables “duration of cannabis use or abuse” and “maximum duration of cannabis use or abuse” yields a more complicated picture. These variables suggest that longer maximum duration of cannabis use or abuse and longer duration of cannabis use or abuse raise the odds of involuntary movements.
In addition to the extra continuous data examined above, further binary data were available from a urine test conducted on the subjects. These data provide a binary indicator of the presence or otherwise of cannabis in the subject’s urine at the time of the interview. Data were available for 131 of the subjects and a cross tabulation between involuntary movements and cannabis use or abuse for this reduced data set was done (data not shown). This analysis did not report evidence against the hypothesis that involuntary movements and urine test results are independent and suggests that a relationship between cannabis use or abuse and involuntary movements is not driven by cannabis present in the subject at the time of the interview. However, the small number of data points in the group “involuntary movements with cannabis use or abuse” should be a note of caution.
Smoking
Smoking is a further potential substance related explanatory factor. Smoking (136 users) had no significant explanatory power for the prevalence of involuntary movements in this sample. Furthermore, smoking was not a significant explanatory variable in a bivariate logistic regression with involuntary movements as a dependent variable.
Negative Symptoms
Avolition, alogia, anhedonia, and affective changes were investigated as potential risk factors. The SANS for measuring the severity of the negative symptoms is a categorical scale ranging from 0 (absent) to 5 (most severe). To avoid the problem of thin cells in the cross tabulation, this scale was aggregated to a three-point scale where 0 corresponded to 0, 1 on the original scale; 1 corresponded to 2, 3 on the original scale; and 2 corresponded to 4, 5. No support for a hypothesis of a relationship between involuntary movements and affect, avolition, or alogia was found.
Anhedonia
Both the chi-square test and Fisher’s exact test reject the hypothesis of independence of anhedonia and involuntary movements at conventional levels of significance in this sample. More specifically, this would mean that there were more subjects with involuntary movements in the group without symptoms of anhedonia than would have been expected if involuntary movements and anhedonia were independent. Further, there were fewer cases with involuntary movements in the group with severe symptoms of anhedonia than would have been expected if involuntary movements and anhedonia were independent. The inverse association between anhedonia and involuntary movements was confirmed by the bivariate logistic regression (data not shown). The implied probabilities of involuntary movements at three positions in the distribution of anhedonia for the regression function show a considerable change in the probability of involuntary movements between the 25th percentile and the median of the distribution.
Multivariate Analysis
The potential explanatory factors for involuntary movements were then investigated factor by factor with a series of bivariate tests. This section proposes a multivariate model of the relevant explanatory factors using a multivariate logistical (logit) model. The multivariate extension is important to determine which of the identified bivariate relationships contribute independent information.
A number of logit models were estimated for the full (data not shown) and reduced data set ( Table 1 ). The reduced data set was obtained by eliminating the problematic data points related to duration of treatment and includes the extra binary and continuous information about cannabis use or abuse. Handedness had to be dropped as a potential explanatory factor from the reduced data set as there was insufficient variation in the series (all the involuntary movement cases in the reduced data set were right handed). This is not a serious loss of information given the lack of significance reported in the full data set and univariate analysis.
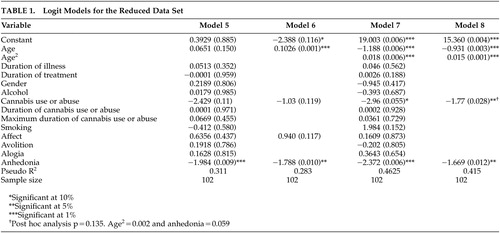 |
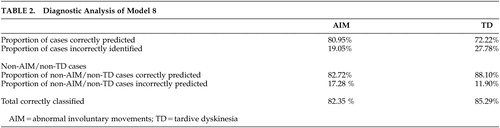
In the reduced data set, cannabis use or abuse enters the preferred model significantly and with a negative coefficient. Age at interview is nonlinear in the same way as in the full data set. A model ( Table 2 ) which combines age at interview, age-squared, cannabis use or abuse, and anhedonia successfully identifies 81% of the involuntary movement cases in the reduced data set while 83% of the cases without involuntary movement cases were correctly identified. The results of the post-hoc analysis based on the stricter Morgenstern-Glazer criteria 26 did not substantially change the discriminative value of our model ( Table 2 ) despite a lower p value for the variable cannabis use or abuse.
 |
DISCUSSION
This study found involuntary movements, as defined by a positive AIMS score, in 19.4% of this group of Xhosa schizophrenia patients. This is within the lower range of previously reported data in Caucasian and African samples. It is, however, important to note that previous studies in African population groups used samples with mixed diagnoses 3 , 4 , 6 and limited their reports to predefined tardive dyskinesia criteria.
Our results support the notion (in keeping with previous findings by Gatere et al. 6 ) that involuntary movements increases with increasing age, but in a nonlinear manner. Age had a nonlinear effect in the logit model, with opposing signs for age (p=0.003) and age-squared (p=0.001). The implication is that age has little impact on the probability of involuntary movements at an earlier age, but an increasing impact as age progresses. In contrast to Patterson et al., 3 neither the duration of illness nor the duration of treatment in our sample had any significant effect on the probability of involuntary movements. However, Patterson’s findings only held true for “documented” treatment duration; when patient collateral was taken into account, the significance disappeared. This issue is partly reflected by our data, where a large category exists with undetermined length of treatment (category 999). This could be due to the difficulties associated with recall bias in reporting treatment duration and the problems associated with the availability of treatment records at other hospitals. Since both Patterson’s study and our study were done in the same country, it clearly illustrates the challenge of establishing reliable treatment records over the period of treatment.
Waddington et al. 11 , 12 found that those patients with and without involuntary movements could not be distinguished by type of neuroleptic treatment. Since more than 95% of our sample used typical antipsychotic treatment, it was not possible for us to evaluate atypical versus typical antipsychotics as a risk factor for involuntary movements. Although it can be argued that the current trend toward the use of atypical antipsychotics makes tardive dyskinesia or involuntary movements less of an issue, the direct cost of atypical treatment remains a stumbling block for its use in many developing countries. Involuntary movements due to the use of typical antipsychotics will, for the foreseeable future, remain a reality in these countries.
Modeling of the data set shows that a model which combines age at interview, age-squared, cannabis use or abuse, and anhedonia successfully identifies 82.35% (85.29% of tardive dyskinesia) of abnormal involuntary movements cases overall. The value of this model is that is provides a set of clinical risk factors to identify individuals at risk for the development of involuntary movements in an area with limited community health resources. The three factors (age at interview, cannabis use or abuse, and anhedonia) are relatively easy to monitor and will not be affected significantly by cross-sectional assessments or recall bias. Previously published data by our group 23 on an independent Xhosa schizophrenia sample suggested that negative symptoms may remain relatively stable and should thus be a reliable cross-sectional marker to use in future predictor studies.
Cannabis remains one of the most common drugs of abuse in South Africa. Our results seem to suggest that a history of cannabis use or abuse may be a protective factor against the development of involuntary movements. This protection seems not to significantly depend on current (at time of assessment) urine drug screen status or duration of use or abuse. We regard this as a fascinating, albeit not an altogether unexpected, finding as several lines of evidence suggest that cannabinoid receptors play an active role in dopamine homeostasis. Animal studies indicate that tetrahydrocannabinol (THC) increases dopamine synthesis and release in the CNS while inhibiting dopamine clearance via the dopamine transporter. Furthermore, antagonists to cannabinoid 1 (CB1) receptors reduce the activity of dopaminergic neurons in the mesolimbic area in animal models, and interaction between THC and the cannabanoid receptor leads to dopamine release in the mesolimbic pathway, which mediates the reinforcing properties of cannabis. 28 , 29
Postmortem studies in human subjects have suggested that CB1 receptors are increased in the frontal cortex of individuals with schizophrenia who used cannabis shortly before death, 30 while elevated endogenous cannabinoid levels have been detected in the cerebro-spinal fluid of subjects with schizophrenia. 31 Also, THC seems to stimulate the nigrostriatal dopaminergic pathway, which projects from dopaminergic cell bodies in the substantia nigra of the brainstem via axons terminating in the basal ganglia or striatum. This pathway is part of the extrapyramidal nervous system and controls motor movements. Deficiencies in this pathway may cause movement disorders and THC mediated stimulation of this pathway could, in part, explain a protective role for cannabis in the development of movement disorders. 32
Anandamide, an endogenous ligand for cannabinoid receptors, increases the release of dopamine in the striatum and the mesolimbic system by activating the CB1 receptors. 33 Also, stimulation of cannabanoid receptors can increase gamma-aminobutyric acid (GABA) in the lateral segment of the globus pallidus and reduce glutamate release in the striatum. This, in addition to Dean et al.’s 30 finding of reduced dopamine transporter in the caudate of THC negative schizophrenia patients, suggests that cannabis use may reverse dopamine transporter associated changes found in schizophrenia.
Furthermore, CB1 receptors are encoded by the CNRI gene, which was cloned by Matsuda et al. 34 Ujike and Morita 29 demonstrated that certain alleles or genotypes of the CNRI gene may confer a susceptibility for schizophrenia, especially the subtype with predominantly negative symptoms. Brown and White 35 found that patients with high SANS scores had significantly more severe tardive dyskinesia. Put together, these findings could indicate that the common denominator linking involuntary movements and negative symptoms in schizophrenia may have a genetic underpinning.
The amotivational syndrome linked to long-term use of cannabis could theoretically influence the findings on the negative symptom scale of the SANS. However, this syndrome more closely resembles asociality, avolition, and apathy, which did not have a significant effect in our study. It is therefore possible that a relationship between involuntary movements and the latter group of negative symptoms may be obscured by the amotivational syndrome of long-term cannabis use.
According to Ujike and Morita 29 an increase in CB1 receptors can be demonstrated in the caudate and putamen in schizophrenia. It is thought that this increase in receptors may be associated with negative symptoms and with some of the cognitive disturbances found in schizophrenia. However, in our study, except for anhedonia, no significant effect for specific subcategories of the SANS (affective changes, alogia or avolition/apathy) were found.
What is interesting, however, is that, in our study, anhedonia was associated with the group that displayed fewer involuntary movements. Anhedonia can be defined as the loss of interest in, and withdrawal from, all regular and pleasurable activities. Anhedonia and other predominantly negative symptoms may be caused by a dysfunction in the ventral striatum, including the nucleus accumbens—a core region of the brain reward system. Ventral striatal activity has been associated with pleasant emotions of anticipation and ventral striatal dysfunction has long been associated with reduced motivation or anhedonia. 36 As the striatum also forms a central part of the basic motor loop that controls movement, it is possible that abnormal involuntary movement and anhedonia share a common underlying pathological mechanism.
One of the strengths of this study was the use of structured assessment tools in a relatively homogenous (schizophrenia) clinical population. Nevertheless, our study criteria for involuntary movement are specifically not limited to tardive dyskinesia. However, the post-hoc analysis to a large extent supports the validity of the results for tardive dyskinesia as well.
This study demonstrates that involuntary movements occur frequently in Xhosa patients with schizophrenia and that increasing age, as well as a negative cannabis use history and the absence of anhedonia, is a significant predictor of abnormal involuntary movements. The use of treatments that can effectively address negative symptoms may therefore have the possibility of reducing involuntary movements in patients with schizophrenia. Cannabinoid receptors may also serve as potential drug targets for the treatment or prevention of involuntary movements. In fact, Fox et al. 37 tested the hypothesis that the cannabanoid receptor agonist nabilone would alleviate L -dopa-induced dyskinesia. They demonstrated that coadministration of nabilone with L -dopa was associated with significantly less dyskinesia. It will also be important to investigate possible underlying genetic factors that could contribute to these findings, including Cytochrome P450 and cannabinoid receptor polymorphisms.
1 . Gervin M, Browne S, Lane A, et al: Spontaneous abnormal involuntary movements in first-episode schizophrenia and schizophreniform disorder: baseline rate in a group of patients from an Irish catchment area. Am J Psychiatry 1998; 155:1202–1206Google Scholar
2 . Altamura AC, Cavallaro R, Regazzetti MG: Prevalence and risk factors for tardive dyskinesia: a study in an Italian population of chronic schizophrenics. Eur Arch Psychiatry Clin Neurosci 1990; 240:9–12Google Scholar
3 . Patterson BD, Swingler D, Willows S: Prevalence of and risk factors for tardive dyskinesia in a Xhosa population in the Eastern Cape of South Africa. Schizophr Res 2005; 76:89–97Google Scholar
4 . Holden TJ: Tardive dyskinesia in long-term hospitalised Zulu psychiatric patients: a prevalence study. S Afr Med J 1987; 71:88–90Google Scholar
5 . Schooler NR, Kane JM: Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry 1982; 39:486–487Google Scholar
6 . Gatere N, Othieno CJ, Kathuku DM: Prevalence of tardive dyskinesia among psychiatric in-patients at Mathari Hospital, Nairobi. East Afr Med J 2002; 79:547–549Google Scholar
7 . Oosthuizen PP, Emsley RA, Maritz JS, Turner JA, Keyter N: Incidence of tardive dyskinesia in first-episode psychosis patients treated with low-dose haloperidol. J Clin Psychiatry 2003; 64:1075–1080Google Scholar
8 . Guy W, Ban TA, Wilson WH: The prevalence of abnormal involuntary movements among chronic schizophrenics. Int Clin Psychopharmacol 1986; 1:134–144Google Scholar
9 . Rittmannsberger H, Schony W: [Prevalence of tardive dyskinesia in long-term hospitalized schizophrenic patients]. Nervenarzt 1986; 57:116–118 (German)Google Scholar
10 . Caligiuri MP, Lacro JP, Rockwell E, et al: Incidence and risk factors for severe tardive dyskinesia in older patients. Br J Psychiatry 1997; 171:148–153Google Scholar
11 . Waddington JL, Youssef HA: An unusual cluster of tardive dyskinesia in schizophrenia: association with cognitive dysfunction and negative symptoms. Am J Psychiatry 1986; 143:1162–1165Google Scholar
12 . Waddington JL: Schizophrenia, affective psychoses, and other disorders treated with neuroleptic drugs: the enigma of tardive dyskinesia, its neurobiological determinants, and the conflict of paradigms. Int Rev Neurobiol 1989; 31:297–353Google Scholar
13 . Fenton WS: Prevalence of spontaneous dyskinesia in schizophrenia. J Clin Psychiatry 2000; 61:S10–14Google Scholar
14 . van Os J, Walsh E, van Horn E, Tattan T, Bale R, Thompson SG: Tardive dyskinesia in psychosis: are women really more at risk? UK700. Group Acta Psychiatr Scand 1999; 99:288–293Google Scholar
15 . Dixon L, Weiden PJ, Haas G, et al: Increased tardive dyskinesia in alcohol-abusing schizophrenic patients. Compr Psychiatry 1992; 33:121–122Google Scholar
16 . Zaretsky A, Rector NA, Seeman MV, et al: Current cannabis use and tardive dyskinesia. Schizophr Res 1993; 11:3–8Google Scholar
17 . Chen EY, Lam LC, Chen RY, et al: Negative symptoms, neurological signs and neuropsychological impairments in 204 Hong Kong Chinese patients with schizophrenia. Br J Psychiatry 1996; 168:227–233Google Scholar
18 . Brown KW, White T: The association among negative symptoms, movement disorders, and frontal lobe psychological deficits in schizophrenic patients. Biol Psychiatry 1991; 30:1182–1190Google Scholar
19 . Liddle PF, Barnes TR, Speller J, et al: Negative symptoms as a risk factor for tardive dyskinesia in schizophrenia. Br J Psychiatry 1993; 163:776–780Google Scholar
20 . Bartzokis G, Hill MA, Altshuler L, et al: Tardive dyskinesia in schizophrenic patients: correlation with negative symptoms. Psychiatry Res 1989; 28:145–151Google Scholar
21 . Chiu H, Lau J, Lam L, et al: Association of negative symptoms with tardive dyskinesia in schizophrenic patients. Aust N Z J Psychiatry 1993; 27:228–232Google Scholar
22 . Glazer WM, Morgenstern H, Doucette J: Race and tardive dyskinesia among outpatients at a CMHC. Hosp Community Psychiatry 1994; 45:38–42Google Scholar
23 . Niehaus DJ, Koen L, Laurent C, et al: Positive and negative symptoms in affected sib pairs with schizophrenia: implications for genetic studies in an African Xhosa sample. Schizophr Res 2005; 79:239–249Google Scholar
24 . Nurnberger JI Jr, Blehar MC, Kaufmann CA, et al: Diagnostic interview for genetic studies: rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry 1994; 51:849–859Google Scholar
25 . Guy W: Abnormal Involuntary Movement Scale (AIMS). Washington, DC, Department of Health and Human Services, 1976, pp 534–537Google Scholar
26 . Morgenstern H, Glazer WM: Identifying risk factors for tardive dyskinesia among long-term outpatients maintained with neuroleptic medications: results of the Yale tardive dyskinesia study. Arch Gen Psychiatry 1993; 50:723–733Google Scholar
27 . Peng C-YJ, So TH: Logistic regression analysis and reporting: a primer. Understanding Statistics 2002; 1:31–70Google Scholar
28 . Nocerino E, Amato M, Izzo AA: Cannabis and cannabinoid receptors. Fitoterapia 2000; 71S:S6–12Google Scholar
29 . Ujike H, Morita Y: New perspectives in the studies on endocannabinoid and cannabis: cannabinoid receptors and schizophrenia. J Pharmacol Sci 2004; 96:376–381Google Scholar
30 . Dean B, Bradbury R, Copolov DL: Cannabis-sensitive dopaminergic markers in postmortem central nervous system: changes in schizophrenia. Biol Psychiatry 2003; 53:585–592Google Scholar
31 . Leweke FM, Giuffrida A, Wurster U, et al: Elevated endogenous cannabinoids in schizophrenia. Neuroreport 1999; 10:1665–1669Google Scholar
32 . Muller-Vahl KR: Cannabinoids reduce symptoms of Tourette’s syndrome. Expert Opin Pharmacother 2003; 4:1717–1725Google Scholar
33 . Schneider U, Leweke FM, Mueller-Vahl KR, et al: Cannabinoid/anandamide system and schizophrenia: is there evidence for association? Pharmacopsychiatry 1998; 31:S110–113Google Scholar
34 . Matsuda LA, Bonner TI, Lolait SJ: Cannabinoid receptors: which cells, where, how, and why? NIDA Res Monogr 1992; 126:48–56Google Scholar
35 . Brown KW, White T: Sub-syndromes of tardive dyskinesia and some clinical correlates. Psychol Med 1992; 22:923–927Google Scholar
36 . Goldstein RZ, Volkow ND: Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 2002; 159:1642–1652Google Scholar
37 . Fox SH, Kellett M, Moore AP, et al: Randomised, double-blind, placebo-controlled trial to assess the potential of cannabinoid receptor stimulation in the treatment of dystonia. Mov Disord 2002; 17:145–149Google Scholar