Anosognosia Is a Significant Predictor of Apathy in Alzheimer’s Disease
Cross-sectional studies reported a significant association between anosognosia and apathy, 4 but to our knowledge, whether anosognosia may predict apathy or vice versa has never been examined. Furthermore, longitudinal studies of apathy and anosognosia in Alzheimer’s disease are few. 5 , 6 In recent longitudinal studies we found that apathy in Alzheimer’s disease is a significant predictor of depression, faster functional and cognitive decline, and more severe parkinsonism. 5 , 7
The present study is, to our knowledge, the first to examine the longitudinal association between anosognosia and apathy in Alzheimer’s disease. We expected both anosognosia and apathy to increase in parallel with increasing cognitive decline, which may be related to progression of pathology in brain regions common to apathy and anosognosia. Alternatively, we expected anosognosia to predict apathy based on the hypothesis that patients with anosognosia may have more limitations in adapting to their functional deficits due to their poor insight and more severe disinhibition and irritability. 4 , 8
METHODS
Participants
The Alzheimer’s disease group included a consecutive series of 354 outpatients attending the Dementia Clinic at a tertiary neurology center in Buenos Aires, Argentina, between January 1996 and October 2001 for evaluation and treatment of progressive cognitive decline (more information on this sample was reported elsewhere). 5 , 7 The main aim of the original study was to examine the longitudinal progression of apathy in Alzheimer’s disease, and assessments of anosognosia were started after the study was commenced. Therefore, 213 of the 354 patients were assessed with scales of anosognosia, and this is the sample included for our present study.
All patients met the following inclusion criteria: National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable Alzheimer’s disease 9 ; no history of closed head injuries with loss of consciousness, strokes, or other neurological disorder with CNS involvement; normal results on laboratory tests (to rule out other causes of dementia); no focal lesions on MRI scan; and a Hachinski Ischemic Scale score less than 4. 10 The institutional human subjects committee approved the study.
Psychiatric Examination
After written informed consent was obtained from patients and their respective caregivers, a psychiatrist blind to the neurological findings assessed patients with the following instruments.
The Structured Clinical Interview for DSM-IV (SCID) 11 is a semistructured diagnostic interview for making the major axis I DSM-IV diagnoses. Based on the SCID responses, the DSM-IV axis I diagnosis of major depressive episode and the DSM-IV research diagnosis of minor depression were made. 12
The Mini-Mental State Exam (MMSE) 13 is an 11-item examination found to be valid and reliable in assessing a limited range of cognitive functions in a global way.
The Apathy Scale 14 includes 14 items which are scored by the patient’s relative or caregiver. We have demonstrated the reliability and validity of the Apathy Scale in Alzheimer’s disease. 14 Diagnoses of apathy were generated based on caregivers’ ratings on the Apathy Scale using the procedure and the diagnostic criteria for apathy previously validated. 15
The Anosognosia Questionnaire for Dementia (AQ-D) 4 is a 30-item questionnaire divided into two sections. The first section assesses performance of basic and instrumental activities of daily living, whereas the second section examines changes in mood and behavior. There are two forms for this questionnaire: Form A is answered by the patient alone and Form B is answered by a next of kin or caregiver. Forms A and B are rated blind to each other, and the final score is obtained by subtracting the scores on Form B from those on Form A. Thus, positive scores indicated that the caregiver rated the patient as more impaired than the patient’s own self-evaluation. Patients were interviewed first. Simultaneously, caregivers, who were blind to the results of these interviews, rated the AQ-D. Finally, the psychiatrist administered the SCID to each patient, with both the patient and the caregiver present. We demonstrated the reliability and validity of the above instruments in Alzheimer’s disease. 4 , 8 , 14 , 16 , 17 Diagnoses of anosognosia were generated based on AQ-D discrepancy scores using the procedure validated in a recent publication. 1
The Hamilton Depression Rating Scale (HAM-D) is a 17-item interviewer-rated scale that measures psychological and autonomic symptoms of depression. 18
Follow-Up Examination
A follow-up evaluation was carried out on 154 of the 213 patients (72%) between 1 and 4 years after the initial evaluation using the same instruments assessed at baseline. Lack of follow-up was due to death during the follow-up period (n=12, 6%), severe dementia that precluded assessment (n=27, 13%), relocation to another city or inability to be traced (n=9, 4%), or refusal to sit for another evaluation (n=11, 5%). There were no significant demographic or clinical differences between patients in the follow-up group and those not in the follow-up group on the following factors: age (mean years=71.7 [SD=7.1] compared with mean years=69.6 [SD=7.6], respectively; t=1.87, df=211, p=0.06); education (mean years=12.9 [SD=7.9] compared with mean years=13.0 [SD=6.0], respectively; t=0.14, df=211, p=0.88); and duration of illness (mean years=4.45 [SD=12.3] compared with mean years=6.58 [SD=19.0], respectively; t=0.89, df=211, p=0.37).
Statistical Analysis
Statistical analysis was carried out using means and standard deviations, one-way and repeated measures analysis of variance (ANOVA) and covariance (ANCOVA) followed by Tukey’s Honestly Significant Difference. Frequency distributions were calculated using chi-square and Fisher’s exact tests. All p values are two-tailed, and the alpha value was set at 0.05.
RESULTS
Frequencies of Anosognosia and Apathy
Fifty-five patients (36%) had no anosognosia at baseline or follow-up, 32 patients (21%) with no anosognosia at baseline developed anosognosia during the follow-up period, 17 patients (11%) with anosognosia at baseline had no anosognosia at follow-up, and 50 patients (32%) had anosognosia at both baseline and follow-up.
Seventy-nine patients (51%) had no apathy at baseline and follow-up, 28 patients (18%) with no apathy at baseline developed apathy during the follow-up period, six patients (4%) with apathy at baseline had no apathy at follow-up, and 41 patients (27%) had apathy at both baseline and follow-up.
Anosognosia as a Predictor of Apathy
This analysis included patients with (n=29) or without (n=48) anosognosia at baseline and follow-up, and no apathy at baseline (i.e., patients with apathy at baseline were excluded from the comparison). There were no significant between-group differences on age, education, gender, duration of illness, and follow-up interval between patients with no anosognosia at baseline and follow-up and patients with anosognosia at both time points ( Table 1 ). Patients with anosognosia had the expected higher scores on the AQ-D compared with patients without anosognosia (t=11.0, df=75, p<0.0001), as well as lower MMSE scores (t=2.60, df=75, p<0.05) and higher Apathy Scale scores (t=2.57, df=75, p<0.05) ( Table 1 ).
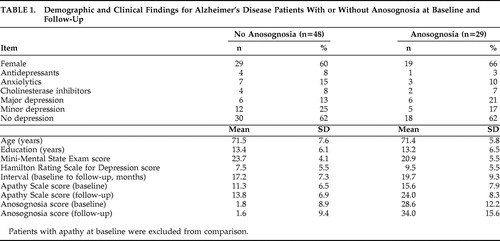 |
A two-way ANCOVA was calculated with presence of anosognosia as the grouping variable, Apathy Scale scores as the repeated measure, and baseline MMSE as the covariate. There was a significant group effect (F=19.1, df=1, 74, p<0.0001): patients with anosognosia had significantly higher Apathy Scale scores than patients without anosognosia. The time effect was significant (F=36.2, df=1, 75, p<0.0001): there was an increase on apathy scores over time. Finally, there was a significant group × time interaction (F=10.6, df=1, 75, p=0.001): patients with anosognosia showed a significantly higher increase on Apathy Scale scores over time than patients without anosognosia ( Table 1 ). When HAM-D scores were entered as an additional covariate, the group × time interaction remained statistically significant (F=6.52, df=1, 75, p=0.01).
Apathy as a Predictor of Anosognosia
This analysis included patients with (n=16) or without (n=59) apathy at baseline and follow-up, and no anosognosia at baseline. There were no significant between-group differences on age, education, gender, duration of illness, MMSE scores, AQ-D scores at baseline and follow-up, or interval between patients with no apathy at baseline and follow-up or apathy at both time points ( Table 2 ). Patients with apathy at baseline had the expected higher scores on the Apathy Scale relative to patients without apathy (t=8.52, df=73, p<0.0001) ( Table 2 ). While there was no significant group × time interaction for apathy scores (F=2.37, df=1, 72, p=0.12), lack of significance may be related to a ceiling effect for apathy scores for the apathy group.
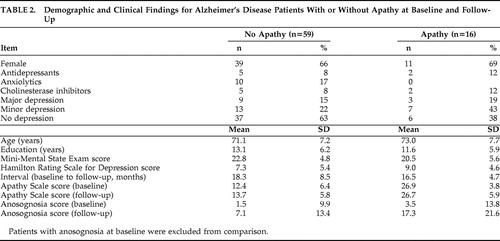 |
A two-way ANCOVA was calculated with presence of apathy as the grouping variable, AQ-D scores as the repeated measure, and baseline MMSE scores as the covariate. There was no significant group effect (F=3.36, df=1, 72, p=0.070): patients with or without apathy had overall similar AQ-D scores. The time effect was significant (F=15.5, df=1, 73, p=0.0001): there was an increase on AQ-D scores over time. Finally, there was no significant group × time interaction (F=2.78, df=1, 73, p=0.10): patients with or without apathy showed a similar increase on AQ-D scores over time ( Table 2 ).
DISCUSSION
To our knowledge, this is the first study to examine the association between apathy and anosognosia among patients with Alzheimer’s disease in the context of a longitudinal study. The main finding was that anosognosia at baseline was a significant predictor of more severe apathy at follow-up. Additional relevant findings were that the severity of both anosognosia and apathy significantly increased over time, suggesting that these phenomena are robust psychological and behavioral constructs in Alzheimer’s disease, and that remission is rare.
Before further comments, several limitations of our study should be pointed out. First, 24% of our baseline sample did not have a follow-up. However, there were no significant differences between patients with or without a follow-up on the main demographic variables. Second, the follow-up assessment ranged from 1 to 4 years after baseline, but there were no between-group differences on the mean duration of follow-up. Third, a small group of patients (11% of the sample) had anosognosia at baseline but no anosognosia at follow-up, and this interesting phenomenon of improved awareness in dementia will require further studies in larger samples. This finding could be related to patients being repeatedly confronted with their functional limitations in the context of preserved self-knowledge learning abilities. Finally, an important question is whether patients with anosognosia throughout follow-up had more severe apathy at follow-up than patients who developed anosognosia during the follow-up. We only had eight patients who developed anosognosia during the follow-up period and had no apathy at baseline, and future studies with larger samples should examine this interesting issue.
Anosognosia is a clinically relevant phenomenon in Alzheimer’s disease. In a recent study, we found that anosognosia is already present in about one-third of patients with mild dementia and is associated with memory and language deficits. 1 Apathy is among the most frequent relevant behavioral changes in Alzheimer’s disease, and we have recently demonstrated that it predicts more severe depression, a faster cognitive and functional decline, and more severe parkinsonism. 5 , 7
Cross-sectional studies reported a significant association between anosognosia and apathy in Alzheimer’s disease. 14 Our present study examined the direction of this association, and the main finding was that patients with anosognosia had a significantly greater increment on apathy scores over time relative to patients without anosognosia. The question now arises as to the mechanism of this association. One possibility is that patients with depression may have an increased rating for developing apathy, but we demonstrated recently that depression at baseline does not predict apathy at follow-up. 5
Anosognosia and apathy are both related to frontal lobe dysfunction. Recent studies showed a significant association between apathy in Alzheimer’s disease and metabolic and pathological changes in specific regions of the frontal lobes. We found a significant association between apathy and the volume of frontal white matter hyperintensities in Alzheimer’s disease patients assessed with MRI volumetry. 19 Marshall et al. 20 found that apathy scores (as measured with the Neuropsychiatric Inventory) were significantly correlated with neurofibrillary tangle counts in the anterior cingulate. Using structural MRI, the same group reported a significant positive correlation between apathy severity and gray matter atrophy in the bilateral anterior cingulate and the left medial frontal cortex. 21 Using fluorodeoxyglucose positron emission tomography (FDG-PET), Marshall et al. 22 reported that Alzheimer’s disease patients with apathy had significantly more severe hypometabolism in the bilateral anterior cingulate region than subjects without apathy. Finally, an MRI volumetric study 23 confirmed the association between apathy and more severe bilateral gray matter atrophy in the anterior cingulate, orbitofrontal cortex, and frontal dorsolateral cortex. The involvement of the anterior cingulate in most of the above studies is of interest, since this structure has been consistently related to the initiation of motivated goal-orientated behaviors. 24
Similarly, anosognosia in Alzheimer’s disease has also been related to frontal lobe dysfunction. In an early study using single photon emission CT that included 12 Alzheimer’s disease patients with anosognosia and 12 patients without anosognosia matched for age, duration of illness, and cognitive impairment, we found that patients with anosognosia had significant perfusion deficits in the right frontal lobe relative to the comparison group. 25 More recent FDG-PET studies showed significant correlations between increased anosognosia scores and lower metabolism in bilateral dorsolateral frontal temporo-parietal, left inferior frontal, and orbitofrontal regions. 26
To summarize, there is strong evidence that both anosognosia and apathy are related to dysfunction in specific frontal regions. The present finding that anosognosia predicts more severe apathy suggests an asynchrony in frontal lobe involvement in Alzheimer’s disease: whereas anosognosia may arise as an early response to frontal lobe damage, apathy may develop with further frontal involvement.
An alternative explanation for the present findings is that patients with anosognosia may have a poorer adaptation response to their functional limitations than patients without anosognosia. More specifically, when Alzheimer’s disease patients with good awareness are faced with severe limitations performing some of their usual interests and chores due to the increasing cognitive impairment, they may look for and engage in activities that are compatible with their current functional capacities. On the other hand, patients with anosognosia may fail to search for alternative activities due to their inability to recognize their increasing functional limitations. Patients with anosognosia may become frustrated (which may account for the increased irritability often reported in this group 27 ) and may eventually lose motivation for most activities.
In conclusion, our study demonstrated that both anosognosia and apathy in Alzheimer’s disease increased significantly in severity after a mean period of 18 months. We also demonstrated that anosognosia is a significant predictor of apathy in Alzheimer’s disease. Future studies should examine whether this clinically relevant association is related to regional variances in the progression of Alzheimer’s disease neuropathology and/or to behavioral changes and adjustment difficulties produced by loss of awareness.
1. Starkstein SE, Jorge R, Mizrahi R, et al: A diagnostic formulation for anosognosia in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2006; 77:719–725Google Scholar
2. Levy ML, Cummings JL, Fairbanks LA, et al: Apathy is not depression. J Neuropsychiatry Clin Neurosci 1998; 10:314–319Google Scholar
3. Landes AM, Sperry SD, Strauss ME: Prevalence of apathy, dysphoria, and depression in relation to dementia severity in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 2005; 17:342–349Google Scholar
4. Migliorelli R, Teson A, Sabe L, et al: Anosognosia in Alzheimer’s disease: a study of associated factors. J Neuropsychiatry Clin Neurosci 1995; 7:338–344Google Scholar
5. Starkstein SE, Jorge R, Mizrahi R, et al: A prospective longitudinal study of apathy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2006; 77:8–11Google Scholar
6. Starkstein SE, Chemerinski E, Sabe L, et al: Prospective longitudinal study of depression and anosognosia in Alzheimer’s disease. Br J Psychiatry 1997; 171:47–52Google Scholar
7. Starkstein SE, Merello M, Brockman S, et al: Apathy predicts more severe parkinsonism in Alzheimer’s disease. Am J Geriatr Psychiatry 2009; 17:291–298Google Scholar
8. Starkstein SE, Garau ML, Cao A: Prevalence and clinical correlates of disinhibition in dementia. Cogn Behav Neurol 2004; 17:139–147Google Scholar
9. McKhann G, Drachman D, Folstein M, et al: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Google Scholar
10. Hachinski VC, Lassen NA, Marshall J: Multi-infarct dementia: a cause of mental deterioration in the elderly. Lancet 1974; 2:207–210Google Scholar
11. Spitzer RL, Williams JB, Gibbon M, et al: The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Google Scholar
12. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Association, 1994Google Scholar
13. Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
14. Starkstein SE, Migliorelli R, Manes F, et al: The prevalence and clinical correlates of apathy and irritability in Alzheimer’s disease. Eur J Neurol 1995; 2:540–546Google Scholar
15. Starkstein SE, Petracca G, Chemerinski E, et al: Syndromic validity of apathy in Alzheimer’s disease. Am J Psychiatry 2001; 158:872–877Google Scholar
16. Migliorelli R, Petracca G, Teson A, et al: Neuropsychiatric and neuropsychological correlates of delusions in Alzheimer’s disease. Psychol Med 1995; 25:505–513Google Scholar
17. Migliorelli R, Teson A, Sabe L, et al: Prevalence and correlates of dysthymia and major depression among patients with Alzheimer’s disease. Am J Psychiatry 1995; 152:37–44Google Scholar
18. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Google Scholar
19. Starkstein SE, Mizrahi R, Capizzano AA, et al: Neuroimaging correlates of apathy and depression in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 2009; 21:259–265Google Scholar
20. Marshall GA, Fairbanks LA, Tekin S, et al: Neuropathologic correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord 2006; 21:144–147Google Scholar
21. Apostolova LG, Akopyan GG, Partiali N, et al: Structural correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord 2007; 24:91–97Google Scholar
22. Marshall GA, Monserratt L, Harwood D, et al: Positron emission tomography metabolic correlates of apathy in Alzheimer’s disease. Arch Neurol 2007; 64:1015–1020Google Scholar
23. Bruen PD, McGeown WJ, Shanks MF, et al: Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain 2008; 131(part 9):2455–2463Google Scholar
24. Devinsky O, Morrell MJ, Vogt BA: Contributions of anterior cingulate cortex to behavior. Brain 1995; 118:279–306Google Scholar
25. Starkstein SE, Vazquez S, Migliorelli R, et al: A single-photon emission computed tomographic study of anosognosia in Alzheimer’s disease. Arch Neurol 1995; 52:415–420Google Scholar
26. Harwood DG, Sultzer DL, Feil D, et al: Frontal lobe hypometabolism and impaired insight in Alzheimer’s disease [comment]. Am J Geriatr Psychiatry 2005; 13:934–941Google Scholar
27. Starkstein SE, Jorge R, Mizrahi R, et al: Insight and danger in Alzheimer’s disease. Eur J Neurol 2007; 14:455–460Google Scholar



