Anxiety Disorders in Children and Adolescents in the First Six Months After Traumatic Brain Injury
Abstract
The study's objective was to assess the nature, rate, predictive factors, and neuroimaging correlates of novel (new-onset) definite anxiety disorders and novel definite/subclinical anxiety disorders (in a broader group of children with at least subclinical anxiety disorders) after traumatic brain injury (TBI). Children with TBI from consecutive admissions to five trauma centers were enrolled and studied with psychiatric interviews soon after injury (baseline) and again 6 months post-injury. Novel definite anxiety disorder and novel definite/subclinical anxiety disorders were heterogeneous and occurred in 8.5% (N=12) and 17% (N=24) of participants, respectively, in the first 6 months after injury. Novel definite anxiety disorder was significantly associated with younger age at injury and tended to be associated with novel depressive disorder, as well as lesions of the superior frontal gyrus. Novel definite/subclinical anxiety disorder was significantly associated with concurrent psychiatric problems of personality change due to TBI and novel definite/subclinical depressive disorder, as well as with lesions of the superior frontal gyrus and a trend-association with frontal lobe white-matter lesions. These findings suggest that anxiety after childhood TBI may be part of a broader problem of affective dysregulation related to damaged dorsal frontal lobe and frontal white-matter systems, with younger children being at greatest risk for developing novel anxiety disorder after TBI.
Traumatic brain injury (TBI) in children and adolescents is a major public health problem.1 Research indicates that TBI is associated with an increase in psychiatric disorders.2 Among these problems is novel (new-onset) anxiety beginning after the TBI, which is an important area that has received scant attention.3–9 This article focuses on novel anxiety problems and investigates their incidence, as well as their demographic, psychosocial, and neuroimaging correlates.
The incidence of anxiety problems appears to increase after TBI, although findings are mixed, depending on the measure of anxiety, whether disorders or symptom-counts.3,5 The extant literature suggests that when anxiety symptoms or disorders develop after TBI, they are varied and include posttraumatic stress disorder (PTSD),7–10 phobic disorders,3,11 obsessive-compulsive disorder (OCD),6 and generalized anxiety disorder (GAD).5 There are only four cohorts of pediatric TBI patients studied thus far from which correlates of anxiety have been examined.3,5,7,8 In one study, younger age at injury was a predictor of post-injury anxiety symptoms, although not of anxiety disorders, as such.3 Furthermore, pre-injury anxiety symptoms correlated significantly with post-injury anxiety symptoms.3 Another study showed that post-injury level of stress and greater severity of injury were significant predictors of “new-onset mood and/or anxiety disorder.”5 A neuroimaging analysis showed a negative correlation between new-onset anxiety and lesion volume and number of lesions in the orbital frontal cortex.4 Findings differ when analyses focus on specific subgroups of anxiety presentation, such as PTSD4,7–10 or obsessive-compulsive symptoms,6 rather than the inclusive category of anxiety. No study has addressed the influence of family history of anxiety disorder on post-injury anxiety despite the fact that family history of anxiety increases risk for anxiety in non-injured children.12
The neurobiology of perception and regulation of emotions, including anxiety, remains to be fully elucidated. Evidence suggests that a ventral system (including the amygdala, insula, ventral striatum, and ventral regions of the anterior cingulate gyrus and prefrontal cortex) is important for the identification of emotional significance of environmental stimuli and the generation of affective states.13,14 A dorsal-frontal neural system (dorsolateral prefrontal cortex and dorsomedial prefrontal cortex, including the superior frontal gyrus, dorsal anterior cingulate gyrus, hippocampus) is key for effortful regulation of affective states resulting from the activity of the ventral system. Studies of anxiety disorders unrelated to brain injury have suggested altered function within the ventral system, including increased reactivity to threat cues in the amygdala and an augmented interoceptive signal in the insula.15 Application of this model to a lesion model in children and adolescents with TBI would predict that anxiety is more likely to be present in the context of an intact ventral system that can generate an anxiety response (e.g., with fewer orbitofrontal lesions4) and/or a damaged and therefore poorly-regulated dorsal system. We have previously described problems with affective regulation in the form of irritability related to lesions of the dorsal system, specifically, superior frontal gyrus (SFG) lesions and frontal white-matter (FWM) lesions.2,16 For a comprehensive description of the prototypic disorder of affective regulation, personality change due to TBI, implying clinically significant affective lability, rage, disinhibition, apathy, or paranoia, the reader is referred to Max et al., 2001.17
On the basis of the above review, we hypothesized that novel anxiety in children and adolescents with TBI would be significantly associated with 1) demographic, 2) psychiatric, and 3) injury variables, as follows: 1) younger age at injury; 2) family history of anxiety disorder, pre-injury anxiety, other manifestations of affective dysregulation, for example, novel depression and personality change due to TBI; and 3) greater severity of injury, lesions of the dorsal-prefrontal region (specifically, the SFG and FWM lesions), and a lower incidence of orbitofrontal gyrus lesions.
METHOD
Participants
This study included 177 participants, age 5–14 years, who suffered TBI between 1998 and 2003. Participants were recruited from consecutive admissions to three academic medical centers in Texas; Rady Children's Hospital in San Diego, CA; and The Hospital for Sick Children in Toronto, Canada. Children with mild-to-severe TBI were recruited from all hospitals except San Diego, CA, where only mild TBI (as evidenced by abnormality on neuroimaging)-to-severe TBI patients were enrolled. Exclusion criteria included preexisting autistic disorder or schizophrenia, mental deficiency, and injury due to child abuse or penetrating-missile injury. In San Diego, CA, only, children were excluded if they had preexisting attention-deficit/hyperactivity disorder. Data concerning the number of children who were approached, the proportion who were eligible for recruitment, and participation rate among those eligible are missing, in part because of the fact that children were not required to answer eligibility questions before deciding whether to participate in the study. As required by the Institutional Review Boards, all children signed a consent form to participate in the study, and their legal guardians gave informed consent. Demographic, pre-injury psychosocial variables, and injury indices for participants are shown in Table 1.
 |
Measures
Psychosocial Assessments
DSM–IV psychiatric diagnoses were derived using the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version (K-SADS-PL)18 and Neuropsychiatric Rating Schedule (NPRS).19 The K-SADS-PL and NPRS were conducted at baseline (after resolution of posttraumatic amnesia) to record pre-injury diagnoses and were repeated 6 months after injury to record new-onset diagnoses. The K-SADS-PL is a semistructured, integrated parent/child interview designed to generate diagnoses in children and adolescents on the basis of DSM–IV criteria. The NPRS is structurally similar to the K-SADS-PL and assesses specifically for personality change due to TBI. Interviews were conducted by Master's- and Ph.D.-level clinicians, who were trained by the first author in a pre-study workshop and a mid-study workshop. A child psychiatrist (at four sites) or a child psychologist (at one site) supervised the assessments at each site. A second level of supervision involved the first author's reviewing written summaries composed by the interviewer and discussion of cases at monthly teleconferences between the first author and the interviewers. Diagnoses were based on a clinician's synthesizing data collected from the parent and child separately. The questions are concerned with present and lifetime symptoms and timing of the onset of these symptoms in relation to the TBI. The category of novel definite anxiety disorders consisted of children who met criteria for at least one of the following new post-injury onset diagnoses: OCD, PTSD, GAD, simple phobia, social phobia, panic disorder, and separation anxiety disorder, with onset within the first 6 months after injury. For example, novel anxiety disorder would be recorded in situations where a child with no pre-injury anxiety disorder or with pre-injury separation anxiety disorder developed panic disorder after the injury.
The category of novel definite/subclinical anxiety disorder consisted of children who met criteria for novel definite anxiety disorder described above and/or had a subclinical novel anxiety disorder. Therefore, all children with a novel definite anxiety disorder were also members of the novel definite/subclinical anxiety disorder group. A designation of subclinical anxiety disorder was made in situations where there was no clear functional impairment even though participants met or were one symptom short of meeting criteria for a specific anxiety disorder. We included children who had an adjustment disorder with anxious mood in the novel definite/subclinical anxiety disorder category because DSM–IV does not consider it under the anxiety disorder classification, and yet problematic anxiety is implied. The diagnoses definite and definite/subclinical were used for both pre-injury and post-injury anxiety.
Adaptive functioning was assessed using the Vineland Adaptive Behavior Scales.20 The Vineland is a nondirective interview conducted with the child's primary caretaker, surveying activities that the child habitually demonstrates in the environment, giving an overall adaptive-behavior composite score.
The Family Assessment Device General Functioning Scale21 was used to assess global family functioning. The scale consists a self-report questionnaire of 12 items with a 4-point scale. The scale is completed by the primary caretaker of each child. Each response is given a number from a range of 1–4. Lower scores represent healthier functioning.
The Family History Research Diagnostic Criteria22 interview was conducted by trained research assistants. At least one parent of each child was interviewed and answered questions regarding psychiatric disorders for every first-degree relative of the child with TBI. Criteria were modified to conform with DSM–IV. Family history of anxiety disorder was coded present/absent depending on whether a first-degree relative met diagnostic criteria.
Socioeconomic status (SES) was assessed with the Four-Factor Index.23 Scores are derived from a formula that takes into account both the maternal and paternal educational and occupational levels. The scores range from 8 to 66, with higher scores indicating higher educational and occupational levels, and therefore higher SES.
Neurological Assessments
MRI was conducted in most subjects 3 months after the injury, when lesions appear stable. The protocol included T1 volumetric spoiled gradient-recalled echo (1.5-mm slices) and fluid-attenuated-inversion recovery sequences (3-mm slices), acquired in coronal and sagittal planes according to a research protocol applied uniformly across sites. A total of 162 of the 177 enrolled children (92%) returned to complete their research MRI. A study neuroradiologist at each site coded each lesion from the multiple-slice, hard-copy films. Pathology (e.g., shearing, encephalomalacia, hemorrhage) was coded from a list. Similarly, anatomical location was coded from a list of multiple brain structures, including cortical gray matter (frontal, temporal, parietal, occipital), subcortical gray matter (thalamus, basal ganglia), and white matter.2 Specific coding of frontal-lobe gyri was made only when gray-matter lesions were present in these gyri. Since the coding of lesions was by judgment of expert neuroradiologists, and volumetric analyses were not conducted, there was no attempt to register images or to segment tissue types.
The Glasgow Coma Scale (GCS)24 was used to classify the severity of TBI, based on the lowest post-resuscitation score, which was recorded from clinical notes. The GCS is the standard measure of severity of acute brain injury associated with brain trauma. Score ranges for severe, moderate, and mild TBI are, respectively: 3–8, 9–12, and 13–15.
Statistical Analyses
The association of 6-month post-injury novel definite anxiety disorder or novel definite/subclinical anxiety disorder with the hypothesized associated variables was tested by two-tailed, independent-sample t-tests or χ2 analyses, as appropriate. The hypothesized associated variables analyzed with two-tailed, independent-sample t-tests were younger age at injury and severity of injury. The hypothesized associated variables analyzed with two-sided χ2 analyses were family history of anxiety disorder, novel depressive disorder (for novel definite anxiety disorder), novel definite/subclinical depressive disorder (for novel definite/subclinical anxiety disorder) testing a hypothesized relationship between subclinical forms of depression and anxiety, personality change due to TBI, pre-injury anxiety disorder (for novel definite anxiety disorder), pre-injury definite/subclinical anxiety disorder (for novel definite/subclinical anxiety disorder), and neuroimaging abnormalities, specifically, the presence of SFG lesions, FWM lesions, or the absence of orbitofrontal gyrus lesions. Other two-tailed, independent-sample t-test analyses were conducted to identify potential confounding relationships with the outcomes of interest; these included demographic (gender, SES, race) and pre-injury psychosocial variables (adaptive functioning, family functioning scores). Analyses of the children with 6-month postinjury novel definite anxiety disorder versus those who did not develop this disorder excluded children with 6-month post-injury novel subclinical anxiety.
In exploratory analyses, we repeated all of the above analyses after excluding children with a diagnosis of PTSD. This was done because PTSD may have a different mechanism than other anxiety disorders.
RESULTS
Of the original 177 children, 141 (80%) returned for the 6-month psychiatric assessment. Participants in the 6-month psychiatric assessment with severe, moderate, and mild TBI had MRI lesions visualized in 48/51 (94%), 12/17 (71%), and 34/63 (54%) of cases, respectively. The returning group was not significantly different from the children who did not return with respect to age, gender, race, GCS score, or SES. Lesion distribution was not significantly different in 21 of 22 areas, although children with basal ganglia lesions were more likely to be in the group not returning versus those returning (4/20 versus 6/131; Fisher's exact test: 0.028). The children who returned at 6 months also had significantly higher pre-injury adaptive functioning, as measured by the Vineland Adaptive Behavior Composite, than those who did not return (95.6 [SD: 14.6] versus 89.5 [SD: 18.0]; t = –2.02; df=163; p<0.05).
Occurrence
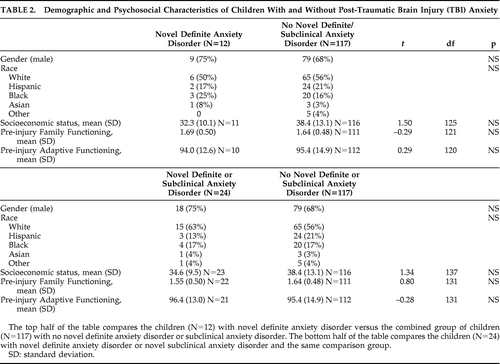
Novel definite anxiety disorder and novel definite/subclinical anxiety disorders occurred in 12 (8.5%) and 24 (17%) of participants, respectively, in the first 6 months after injury. Of those with mild TBI, 8/70 (11%) developed a definite anxiety disorder, and 14/70 (20%) developed a definite/subclinical anxiety disorder. For those with moderate TBI, 0/17 developed a definite anxiety disorder, and 4/17 (24%) a definite/subclinical anxiety disorder. Finally, for those with a severe TBI, 4/54 (7%) developed a definite anxiety disorder, and 6/54 (11%) developed a definite/subclinical anxiety disorder. Table 2 shows that children who developed versus those who did not develop anxiety problems were not significantly different regarding demographic variables (gender, race, SES) and pre-injury psychosocial variables (family functioning, adaptive functioning). The breakdown of novel anxiety problems: 9 children had PTSD (including 5 subclinical); 6 had separation anxiety (including 2 subclinical); 4 had simple phobia; 3 had GAD (including 1 subclinical); 3 children had adjustment disorder with anxious mood (all considered subclinical anxiety disorders); 3 had social phobia (including 2 subclinical); 1 child had panic disorder; and no child had OCD.
 |
Predictors and Correlates of Novel Definite Anxiety Disorders
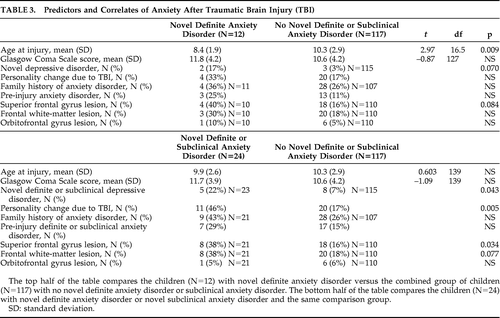
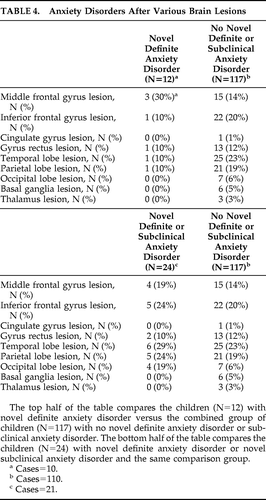
Table 3 shows the predictors and correlates of novel definite and novel definite/subclinical anxiety disorders. Younger age at injury was associated with novel definite anxiety disorder. The mean (SD) age of children with the disorder versus those without the disorder was 8.4 (1.9) years versus 10.3 (2.9) years, respectively (t=2.97; df=16.5; p=0.009). There was no significant relationship between novel definite anxiety disorder and family history of anxiety disorder. However, there was a trend for concurrent novel depressive disorder to be associated with novel definite anxiety disorder (2 of 12 children with novel definite anxiety disorder had novel depressive disorder, whereas 3 of 115 with no novel definite anxiety disorder had novel depressive disorder; Fisher's exact test: 0.070). There were no significant associations with other psychiatric disorders (personality change due to TBI, pre-injury anxiety disorder). There was a trend association for lesions of the SFG with novel definite anxiety disorder (4 of 10 children with novel definite anxiety disorder had an SFG lesion, whereas 18 of 110 with no novel definite anxiety disorder had an SFG lesion; Fisher's exact test: 0.084). There were no significant associations of novel definite anxiety disorder with severity of injury nor the other hypothesized lesions (FWM, orbitofrontal gyrus). For the interest of readers, Table 4 provides further data regarding the distribution of lesions according to the presence or absence of novel anxiety disorder and novel definite or subclinical anxiety disorder. Since these data are not related to our hypotheses, we have not subjected them to statistical analyses or corrected for multiple comparisons.
 |
 |
The analyses were repeated in an exploratory manner after excluding children with novel PTSD. The results were very similar, with three exceptions. The age-at-injury significant finding became a trend association. The mean (SD) age of children with the disorder versus those without the disorder was 8.5 (2.1) years versus 10.3 (2.9) years, respectively (t=2.24; df=8.8; p=0.052). The trend association with concurrent 6-month novel definite depressive disorder became significant (2 of 8 children with novel definite anxiety disorder had novel depressive disorder, whereas 3 of 115 with no novel definite anxiety disorder had novel depressive disorder; Fisher's exact test: 0.034). There emerged a significant association between novel definite anxiety disorder and concurrent personality change due to TBI (4 of 8 of children with novel definite anxiety disorder had personality change due to TBI, whereas 20 of 117 with no novel definite anxiety disorder had personality change due to TBI; Fisher's exact test: 0.044).
Predictors and Correlates of Novel Definite/Subclinical Anxiety Disorders
There was no significant association of novel definite/subclinical anxiety disorder with age or family history of anxiety disorder. Novel definite/subclinical anxiety disorder was significantly associated with concurrent personality change due to TBI (11 of 24 children with novel definite/subclinical anxiety disorder had personality change due to TBI, whereas 20 of 117 with no novel definite/subclinical anxiety disorder had personality change due to TBI; Fisher's exact test: 0.005), and novel definite/subclinical depressive disorder (5 of 23 children with novel definite/subclinical anxiety disorder had novel definite/subclinical depressive disorder; 8 of 115 with no novel definite/subclinical anxiety disorder had novel definite/subclinical depressive disorder; Fisher's exact test: 0.043). Pre-injury definite/subclinical anxiety disorder was not related to novel definite/subclinical anxiety disorder.
Novel definite/subclinical anxiety disorder was significantly associated with lesions of the SFG. Of children with novel definite/subclinical anxiety disorder, 8 of 21 (38%) had an SFG lesion, whereas 18 of 110 children (16%) with no novel definite/subclinical anxiety disorder had the lesion (Fisher's exact test: 0.034). There was a trend association between novel definite/subclinical anxiety disorder and FWM lesions. Specifically, 8 of 21 children (38%) with novel definite/subclinical anxiety disorder had an FWM lesion, whereas 20 of 110 (18%) with no novel definite/subclinical anxiety disorder had the lesion (Fisher's exact test: 0.077). There was no significant association with orbitofrontal gyrus lesions, nor with severity of injury. As noted above, Table 4 provides further data regarding lesion distribution and novel definite/subclinical anxiety disorder.
To evaluate the specificity of the significant relationship of novel definite/subclinical anxiety disorders to SFG lesions, we conducted two logistic-regression analyses, controlling for its significant psychiatric disorder correlates (personality change due to TBI and novel definite/subclinical depressive disorder). A logistic regression with novel definite/subclinical anxiety disorders as the dependent variable and personality change due to TBI and SFG lesions as the independent variables was significant (–2 log-likelihood χ2=9.67; df=2; p=0.008) and correctly classified 84% of children. Only personality change due to TBI was significant (Wald χ2=5.27; df=1; p=0.0218) in terms of unique contribution to this association. A second logistic regression, with novel definite/subclinical anxiety disorders as the dependent variable and novel definite/subclinical depressive disorder and SFG lesions as the independent variables, was significant (–2 log-likelihood χ2=9.03; df=2; p=0.011) and correctly classified 85% of children. Both novel definite/subclinical depressive disorder (Wald χ2=4.50; df=1; p=0.0339) and presence of an SFG lesion (Wald χ2=5.08; df=1; p=0.0242) significantly and independently accounted for novel definite/subclinical anxiety disorder. Taken together, these regressions suggest at least some specificity in the association between anxiety and SFG lesions.
The analyses were repeated in an exploratory manner after excluding children with novel PTSD. The results were very similar, with two exceptions. The significant association of novel definite/subclinical anxiety disorder and novel definite/subclinical depressive disorder was lost, as was the trend association with FWM lesions.
DISCUSSION
Our investigation into novel anxiety problems after pediatric TBI implicated several demographic (e.g., younger age at injury), concurrent psychopathology (novel depression; personality change due to TBI), and lesion (SFG; FWM) predictors and correlates. These findings could not be accounted for by differences in SES, gender, race, pre-injury psychiatric status, pre-injury family functioning, severity of injury, or pre-injury adaptive functioning.
Children who developed novel definite anxiety disorders, as compared with those who did not, were significantly younger at the time of injury. However, this finding did not apply to the prediction of novel definite/subclinical anxiety disorder. Our finding is not consistent with that from an earlier study,3 which reported that younger children had higher anxiety ratings but were not more likely to present with novel anxiety disorders. The differences across studies may be less important than the underlying theme that younger age poses a greater risk for anxiety problems after pediatric TBI. The issue of plasticity, that is, outcome in relation to age at the time of brain injury, has received continued attention.25 There is now robust evidence that younger age at the time of brain injury in children is an important risk factor for adverse immediate and long-term outcome.25 However, it is striking that, except in the case of novel anxiety, age at injury has not been found to influence outcome in terms of new psychiatric disorders after pediatric TBI. The underlying neurobiological and/or psychosocial mechanisms for this phenomenon have yet to be elucidated.
The phenomenology of novel anxiety was heterogeneous. In descending order of frequency were definite or subclinical PTSD, separation anxiety, simple phobia, adjustment disorder with anxious mood, generalized anxiety disorder, social phobia, and panic disorder. This pattern is compatible with previous findings.3 A notable absence was definite or even subclinical OCD. This reaffirms the rarity of the disorder but is not necessarily inconsistent with findings related to new obsessive-compulsive symptoms.6
Novel anxiety problems were associated with other new-onset disorders of affective regulation, specifically depression and personality change due to TBI, the dominant feature of which is irritability.2 It is unclear why this association was stronger when the anxiety-disorders group included subclinical anxiety. The finding of comorbidity among new-onset disorders of affective regulation mirrors the association in people who have not had a TBI. For example, up to 90% of people with anxiety disorders experience a clinical depression in their lifetime.26 Irritability is a common symptom in people with depressive and anxiety disorders. A close relationship between anxiety and depressive disorders is further suggested by response to similar medications and involvement of similar neural circuits.26 There was only a marginal effect of excluding PTSD from the anxiety disorder analyses except that it strengthened the association with personality change due to TBI and variably strengthened or weakened the association with depression, depending on the specific analysis.
Our investigation of neural circuits involved in novel anxiety problems relied on lesion–behavioral correlations. We demonstrated a trend relationship of novel definite anxiety disorder and SFG lesions, a significant relationship of novel definite/subclinical anxiety disorder and SFG, and a trend relationship with FWM lesions. This finding is similar to earlier analyses from this study, demonstrating a significant association of personality change due to TBI with SFG lesions during the first year after TBI and FWM lesions during the second year after TBI.2,16 Models of affective dysregulation13,27–29 propose that the ventral neural system (amygdala, insula, ventrolateral prefrontal cortex, orbitofrontal cortex, ventral anterior cingulate gyrus, thalamus, ventral striatum, and brainstem nuclei) is involved in the identification of emotional significance of environmental stimuli and the generation of an appropriate affective state. This model implicates the dorsal-frontal neural system, including the SFG, in the effortful regulation of inputs from the ventral neural system. Therefore, dysfunction in this area may result in poor regulation of the affective states generated by the ventral neural system and may play a role in the onset of new anxiety symptoms after injury. For example, studies found that Brodmann Area 9, which includes the SFG, was deactivated in uninjured PTSD patients and patients in memory-driven emotional states.29 The relationship of anxiety to FWM lesions underlines the importance of a connectivity problem resulting from diffuse axonal injury in the pathophysiology of TBI and its adverse influence on the efficiency of complex neural systems serving neurobehavioral functions including affective regulation.30–32
Family history of anxiety disorder was not significantly related to novel anxiety. This possibly suggests that anxiety related to brain damage is phenotypically rather than genotypically related to anxiety disorders prevalent in the community. However, replication in larger studies is required.
Our findings differed from earlier work finding that pre-injury anxiety symptoms correlated significantly with post-injury anxiety symptoms.3 This difference may be a function of statistical power related to our consideration of anxiety problems as a categorical variable (versus a nominal variable in the earlier study). In support of this difference is the finding in the current study that pre-injury anxiety was approximately twice as common in the groups that developed post-injury anxiety problems. Furthermore, the prevalence of novel anxiety disorders was similar to that in the earlier study (8.5% versus 9.5%).4 Differences may also be related to the wider range of severity of TBI (mild-to-severe) in the current study versus the predominantly severe TBI in other studies.3
The wide range of severity of TBI has exposed a testable pattern of differential occurrence of post-injury anxiety that is lowest in those who have severe TBI (7%–11%) versus those with mild (11%–20%) or moderate (0–24%) TBI. If this pattern is replicated and found to be statistically significant in a larger sample, it may suggest that perturbation of frontal networks increases the likelihood for the development of anxiety. However, if the perturbation is too great, the likelihood may decrease because the children may need the circuits to reexperience and be aware of their internal emotional response.4
There were several limitations in study methodology. First, we did not directly test interrater reliability for psychiatric diagnoses. However, the psychiatric assessments were supervised at each site by child psychiatrists or a child psychologist, and there were stringent procedures of quality control and training as described in the Methods section. Second, image-analyses did not include volumetric or tissue-segmentation measurements, nor were analyses of the correlation between number of lesions with anxiety-related outcomes conducted. These procedures might be used in future studies to delineate anxiety–lesion and anxiety–volumetric correlates that may involve smaller and more specific regions of interest than the large expanse of the SFG. Among these regions of interest are the hippocampus, amygdala, and basal ganglia, where we have previously documented significant volume loss in pediatric TBI.33 Other than using the same image-acquisition sequence, there were no procedures to enhance scanning compatibility across sites. Third, sample attrition was approximately 20%. The sample that participated at the 6-month follow-up was significantly different in that it had higher pre-injury adaptive functioning and fewer basal ganglia lesions. Neither of these variables was associated significantly with new anxiety problems. Fourth, the role of the environment, for example, parental anxiety, in the genesis of the child's anxiety was not evaluated and should be helpful to review in future studies.
There are several notable strengths of this study. This was the largest psychiatric interview study of consecutively-admitted, nonreferred children with TBI. The breadth and depth of assessments were extensive and included interview assessments of adaptive functioning, family psychiatric history, and psychopathology, as well as rating scales encompassing injury and other psychosocial risk factors for novel anxiety disorder. Also, lesion analysis was based on readings by skilled neuroradiologists.
The current findings have specific clinical and research implications. Clinicians should screen for anxiety disorders or symptoms after childhood TBI because they are not uncommon and may be clinically significant. Our findings suggest that younger children, those with other forms of affective dysregulation, and those with SFG and FWM lesions may be at higher risk for anxiety problems. Appropriate identification of anxiety problems may lead to effective treatment. It has been shown that 30% of non-injured anxious children developed new psychiatric disorders during a 3-year follow-up.34 If novel anxiety disorders after TBI similarly presage future psychopathology, these children should be monitored in follow-up. Research implications include further support via the lesion method for proposed neurological networks of affective regulation with relevance to understanding mechanisms of depression, anxiety, and anger problems.
1. : The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil 2005; 20:229–238Crossref, Medline, Google Scholar
2. : Predictors of personality change due to traumatic brain injury within six months after injury. J Am Acad Child Adolesc Psychiatry 2005; 44:435–442Google Scholar
3. : Anxiety after severe pediatric closed head injury. J Am Acad Child Adolesc Psychiatry 2002; 41:148–156Crossref, Medline, Google Scholar
4. : Neuroimaging correlates of anxiety after pediatric traumatic brain injury. Biol Psychiatry 2004; 55:208–216Crossref, Medline, Google Scholar
5. : Mood and anxiety disorders following pediatric traumatic brain injury: a prospective study. J Clin Exp Neuropsychol 2002; 24:270–279Crossref, Medline, Google Scholar
6. : New-onset obsessive-compulsive symptoms in children and adolescents with severe traumatic brain injury. Depress Anxiety 2008; 25:398–407Crossref, Medline, Google Scholar
7. : Posttraumatic stress symptomology after childhood traumatic brain injury. J Nerv Ment Disord 1998; 186:589–596Crossref, Medline, Google Scholar
8. : Posttraumatic stress symptoms in children following orthopedic or traumatic brain injury. J Clin Child Psychol 1999; 28:232–243Crossref, Medline, Google Scholar
9. : Is the spatial distribution of brain lesions associated with closed- head injury in children predictive of subsequent development of posttraumatic stress disorder? Radiology 2002; 224:345–351Crossref, Medline, Google Scholar
10. : Clinical predictors of posttraumatic stress disorder after closed head injury in children. J Am Acad Child Adolesc Psychiatry 2002; 41:157–165Crossref, Medline, Google Scholar
11. : Traumatic brain injury in children and adolescents: psychiatric disorders at two years. J Am Acad Child Adolesc Psychiatry 1997; 36:1278–1285Crossref, Medline, Google Scholar
12. : A developmental twin study of symptoms of anxiety and depression: evidence for genetic innovation and attenuation. Psychol Med 2008:1–9Google Scholar
13. : Neurobiology of emotion perception, I: the neural basis of normal emotion perception. Biol Psychiatry 2003; 54:504–514Crossref, Medline, Google Scholar
14. : Reciprocal limbic–cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156:675–682Medline, Google Scholar
15. : Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genet C Semin Med Genet 2008; 148:89–98Crossref, Google Scholar
16. : Predictors of personality change due to traumatic brain injury in children and adolescents 6 to 24 months after injury. J Neuropsychiatry Clin Neurosci 2006; 18:21–32Link, Google Scholar
17. : The phenomenology of personality change due to traumatic brain injury in children and adolescents. J Neuropsychiatry Clin Neurosci 2001; 13:161–170Link, Google Scholar
18. : Schedule for Affective Disorders and Schizophrenia for School-Age Children: Present and Lifetime Version (K-SADS–PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
19. : The Neuropsychiatric Rating Schedule: reliability and validity. J Am Acad Child Adolesc Psychiatry 1998; 37:297–304Crossref, Medline, Google Scholar
20. : The Vineland Adaptive Behavior Scales. Circle Pines, MN, American Guidance Services, 1984Google Scholar
21. : The Mcmaster Family Assessment Device: reliability and validity. J Marit Family Ther 1985; 11:345–356Crossref, Google Scholar
22. : The family history method using diagnostic criteria: reliability and validity. Arch Gen Psychiatry 1977; 34:1229–1235Crossref, Medline, Google Scholar
23. : Four-Factor Index of Social Status. New Haven, CT, Department of Sociology, Yale University, 1975Google Scholar
24. : Assessment of Coma and Impaired Consciousness: a practical scale. Lancet 1974; 2:81–84Crossref, Medline, Google Scholar
25. : Age-related differences in outcomes following childhood brain insults: an introduction and overview. J Int Neuropsychol Soc 1997; 3:555–567Crossref, Medline, Google Scholar
26. : Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 2007; 10:1116–1124Crossref, Medline, Google Scholar
27. : Limbic–cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 1997; 9:471–481Link, Google Scholar
28. : Neuroimaging studies of mood disorders. Biol Psychiatry 2000; 48:813–829Crossref, Medline, Google Scholar
29. : Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry 2000; 48:30–42Crossref, Medline, Google Scholar
30. : Diffuse damage in pediatric traumatic brain injury: a comparison of automated versus operator-controlled quantification methods. Neuroimage 2010; 50:1017–1026Crossref, Medline, Google Scholar
31. , Diffusion tensor imaging analysis of frontal lobes in pediatric traumatic brain injury. J Child Neurol 2010; 25:976–984Crossref, Medline, Google Scholar
32. : Early nonischemic oxidative metabolic dysfunction leads to chronic brain atrophy in traumatic brain injury. J Cereb Blood Flow Metab 2010; 30:883–894Crossref, Medline, Google Scholar
33. : Hippocampus, amygdala, and basal ganglia morphometrics in children after moderate-to-severe traumatic brain injury. Dev Med Child Neurol 2007; 49:294–299Crossref, Medline, Google Scholar
34. : A prospective study of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry 1996; 35:1502–1510Crossref, Medline, Google Scholar



