The Dorsal Hippocampal Commissure: When Functionality Matters
Case Report
“Mrs. A,” an ex-civil servant in her 50s, was referred to the tertiary center for consideration of a left temporal resection in view of her refractory TLE. She had a two-decade history of refractory TLE and was on polytherapy of phenytoin and phenobarbitone. Several types of attack were described. Two types of attack were characterized by behavioral and motor arrest, loss of awareness, and restricted responsiveness. The attacks differed by the presence or absence of manual and oro-alimentary automatisms. They could last anywhere from 1 to 5 minutes, usually with postictal confusion and infrequently dysphasia, and occurred several times per week. The third type was described as “wandering episodes” (sic). Interestingly, no motor arrest was evident, and the patient commonly appeared to be responsive to her surroundings but had no later recollection of the events. The duration of these attacks was longer, possibly in tens of minutes. Also, of late, the patient had developed anxiety-provoked psychogenic nonepileptic attacks (PNES), when she would tend to “go rigid all over her body and scream with closed eyes.” Her overall medical history was otherwise unremarkable, and she was found to be neurologically and neuropsychiatricaly intact, with no clear history of somatizations or dissociative traits apart from her recent pnes. Presurgical evaluation included neuropsychometry, which did not reveal any major discrepancy between her verbal and performance IQ (VIQ: 103; PIQ: 93), although a certain decline was nonetheless suspected in comparison to her premorbid iq estimate. Impaired verbal and visual memory was evident, but her executive functioning was reported to be normal. The intracarotid sodium amytal test showed her to be left-hemisphere dominant for language. Memory function appeared to be supported by both hemispheres, but with strong left preponderance. In the decade before coming to the epilepsy center, Mrs. A underwent several neuroimaging and scalp EEG investigations, with grossly nonconcordant results regarding the side of the abnormalities and the onset of seizures. Namely, two past MRI scans showed the left hippocampus as marginally smaller in volume and of higher T1 and FLAIR signal (Figure 1), suggestive of left mesial temporal sclerosis as a potential source of ictogenic focus. In agreement with this, PET scan showed marked reduction in metabolic activity throughout the left temporal and parieto-occipital lobes, possibly also further indicating the left side as a source. On the other hand, her past interictal sleep EEG showed diffusely slow background, with polymorphic delta over left temporal areas, with independent, bilateral, fronto-temporal sharp waves. Similarly, her scalp telemetry showed interictal prominent spikes over both left and right temporal regions. In contrast, the ictal EEG suggested a right focus: during ictal recording, a rhythmic right anterior temporal seizure onset was seen, followed by bilateral rhythmic ictal transformation. Owing to the discrepancy between neuroimaging and ictal EEG findings, the decision was made to undertake intracranial recordings with bilateral subtemporal 8-contact subdural strips. Single pulse electrical stimulations (SPES), with intensity of 4 mA and pulse duration of 1 msec, were carried out through contiguous contacts of both subtemporal strips.6–8 The SPES results suggested a right epileptogenic focus (Figure 2). In agreement with previous ictal scalp EEG findings, the majority of intracranially-recorded seizures appeared to start from the right mesial temporal regions. In all, five seizures were recorded, starting from the right: two before and three after phenobarbitone reduction was initiated. However, some seizures also showed a clear onset arising from the left mesial temporal region. Two such seizures were recorded, one before and one after phenobarbitone reduction. The decision was made to proceed to intracortical recording, and depth electrodes were inserted symmetrically into the medial temporal structures (e.g., amygdala, anterior hippocampus, and posterior hippocampus) by the orthogonal route, using MRI coordinates. SPES was carried out through pairs of contiguous contacts located on gray matter, with intensity of 5 mA and duration of 1 msec. The interictal recording showed spikes occurring independently in both the medial and lateral electrodes of both temporal lobes, in agreement with previous scalp and subdural recordings. Similarly, SPES suggested both temporal lobes as independently epileptogenic. Finally, four habitual seizures were captured after a prolonged period of recording and reduction of phenobarbitone. It was shown that in each instance there was a clear left-hemisphere onset, starting in the posterior hippocampus with rapid switch (within 20 sec) to the right hippocampus, where it then became the most prominent activity, probably explaining the previously incorrectly lateralized ictal findings. Based on the depth electrode findings, it was decided to perform a left temporal lobectomy. Two years after the neurosurgery, Mrs. A is seizure-free on antiepileptic drug, but with unremitted and continuing PNES. She also additionally developed agoraphobia.
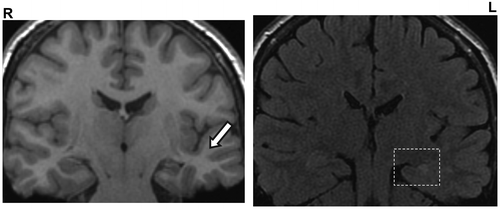
MRI imaging suggested left symptomatic onset: Left hippocampus was shown to be marginally smaller in volume than right and of higher FLAIR and T1 signal, consistent with left mesial temporal sclerosis (square). Associated fornix and thalamic atrophy is present. The grey/white matter differentiation in the left temporal lobe is well preserved, in keeping with the late seizure onset (arrow).
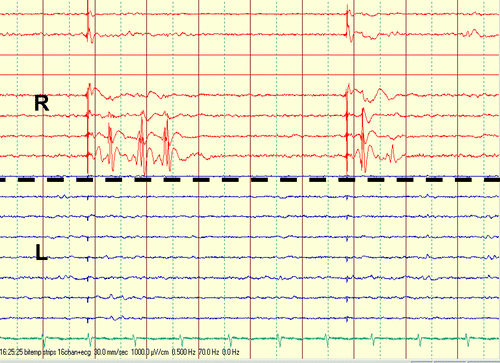
Bilateral subtemporal 8-contact subdural strips were implanted, and SPES with intensity of 4 mA and pulse duration of 1 msec was carried out through contiguous contacts of both subtemporal strips. Clear delayed responses were seen at Contacts 1 and 2 of the right subtemporal strip (R) when stimulation was carried out through Contacts 5 and 6 of the same strip. These findings suggest that the area underneath this contact is epileptogenic. Stimulation of the left subtemporal strip (L) did not elicit clear delayed responses.
Discussion
Phylogenetically, the commissural fibers were present as a major group of extrinsic connections joining the hippocampi. In nonhuman primates, the dorsal commissure carries primarily presubicular and parahippocampal gyrus fibers to the contralateral entorhinal cortex. Gloor et al. in 1993,1 proposed that, in humans, the spread though the dorsal hippocampal commissure is the only likely pathway of contralateral propagation for seizure discharges originating in mesial temporal structures with spread to the contralateral hippocampus. This pathway, according to them, is imperative in cases where the spread between mesial structures occurs before any involvement of the contralateral isocortex. Alternative routes were rejected as unlikely in light of the known primate anatomy of the commissural and other connections of the temporal lobe.1 This was in the direct contradiction to an existing body of work, which consistently refuted presence of hippocampal connections in humans.3–5 More recently, Lacruz et al., 2007,6 using SPES, also showed that, albeit rare, contralateral functional connections between medial temporal cortex and contralateral medial temporal and entorhinal cortices do exist in humans, with recorded latencies between 20 and 40 msec.6 The presence of functional dorsal hippocampal commissure resonates in agreement with findings in our case and may indeed go some way toward explaining repeated false lateralization of seizure onset by extracranial and subdural EEG recordings in our patient. Presumably, only fortuitously placed intracortical depth electrodes could record seizure onset from such deep mesial temporal locus and “catch” it before its rapid (20 msec) switch via the commissure to the contralateral side. Furthermore, this commissural pattern of TLE seizure spread was suggested to be pertinent for the phenomena of pure amnestic seizures.1 Pure amnestic seizures, or transient epileptic amnesia (TEA), sometimes occur in patients with TLE and commonly never represent the only type of seizures in these patients.2,7 Here, the only clinical manifestation is the patients' inability to retain in memory what occurs during the seizure, coupled with the preservation of other cognitive functions and the ability to interact normally with their physical and social environment.2 Interestingly, in our patient, one type of attack (i.e., “wandering episodes”) also arguably shared some of those features. It was postulated that TEA may result from selective ictal inactivation of mesial temporal structures without isocortical involvement.2 Indeed, bilateral selective hippocampal inactivation was shown to induce memory deficits.8 Theoretically, in patients such as Mrs A, whose memory function was supported by both hemispheres, TEA would need to be caused by seizure discharge limited to the MT structures of both temporal lobes; that is, requiring spread through the dorsal commissure. The reported duration of amnestic attacks in literature varies, but is generally accepted as anywhere between 30 and 60 minutes. A deficit in memory encoding or in storage mechanisms has been suggested as an underlying pathophysiological mechanism leading to the anterograde amnesia during the attack.1 Olfactory hallucinations, automatisms, and other seizure types were all reported as additional features in some cases of TEA.7 On the other hand, déjà vu, although associated with TLE and some other psychiatric and neurological conditions, is thought not to be common in TEA.7,9 In this case, the additional presence of SPES abnormalities on the right, bilateral independent interictal EEG findings, and the evident “switching” of seizures further raised the possibility that the contralateral (right) temporal lobe could eventually become independently ictogenic, and a lower probability of a good postoperative seizure control achieved. Fortunately, in our case, the neurosurgery was performed promptly, and the patient rendered seizure-free. Of note is that although all seizure types remitted, the PNES endured. Moreover, the patient additionally developed agoraphobia. Panic disorder with agoraphobia, where the patient has an intense fear related to being in situations from which escape might be difficult or embarrassing, is the most frequently reported of the anxiety disorders in association with or following medical illness (e.g., gastroenteritis, cardiomyopathy, Parkinson's disease, chronic pulmonary disease, chronic pain, post-infarction heart failure, and primary biliary cirrhosis).10 De-novo rise of psychopathology, especially that of depressive, anxious, or psychotic nature, following the epilepsy surgery is a well-recognized problem the exact nature of which merits further research.11
Competing interests: None. Dr Rosenzweig was supported by the EFNS Department to Department Programme grant during her stay at the Department of Neurophysiology, Danish Epilepsy Centre in Dianalund, Denmark.
1. : The human dorsal hippocampal commissure: an anatomically identifiable and functional pathway. Brain 1993; 116:1249–1273Crossref, Medline, Google Scholar
2. : Pure amnestic seizures in temporal lobe epilepsy. definition, clinical symptomatology, and functional anatomical considerations. Brain 1992; 115:749–769Crossref, Medline, Google Scholar
3. : Functional connections in the human temporal lobe, I: analysis of limbic system pathways using neuronal responses evoked by electrical stimulation. Exp Brain Res 1990; 82:279–292Crossref, Medline, Google Scholar
4. : Functional connections in the human temporal lobe, II: evidence for a loss of functional linkage between contralateral limbic structures. Exp Brain Res 1991; 85:174–187Crossref, Medline, Google Scholar
5. : A comparative view of local and interhemispheric limbic pathways in humans: an evoked potential analysis, in Fundamental Mechanisms of Human Brain Function: Opportunities for Direct Investigation in Association With the Surgical Treatment of Epilepsy. Edited by Engel JJOjemann GALüders HO. Stuttgart, Germany, London, UK, Raven, 1987, pp 27–38Google Scholar
6. : Frontal and temporal functional connections of the living human brain. Eur J Neurosci 2007; 26:1357–1370Crossref, Medline, Google Scholar
7. : Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting, and remote memory impairment. Brain 2008; 131:2243–2263Crossref, Medline, Google Scholar
8. : Single pulse electrical stimulation of the hippocampus is sufficient to impair human episodic memory. Neuroscience 2010; 170:623–632Crossref, Medline, Google Scholar
9. : Paroxysmal dyskinesia with déjà vu aura. J Neuropsychiatry Clin Neurosci 2010; 22:E9–E10Link, Google Scholar
10. : Panic disorder with agoraphobia in reaction to gastroenteritis. Psychosomatics 2000; 41:74–75Crossref, Medline, Google Scholar
11. : Obsessive-compulsive disorder after epilepsy surgery. Epilepsy Behav 2004; 5:113–118Crossref, Medline, Google Scholar



