Apathy Is Associated With Increased Amyloid Burden in Mild Cognitive Impairment
Abstract
Apathy is the most common neuropsychiatric symptom in mild cognitive impairment (MCI) and Alzheimer’s disease (AD) dementia. The authors sought to determine whether apathy is associated with cortical amyloid burden, as measured by Pittsburgh Compound B (PiB) positron emission tomography (PET), and regional hypometabolism, measured by 18F-fluorodeoxyglocuse (FDG) PET in MCI. The authors found a significant association between increased apathy (lower Apathy Evaluation Scale score) and greater cortical PiB retention independent of age, but no significant association between apathy and regional FDG metabolism. These results suggest that increased apathy is associated with greater amyloid burden but not regional hypometabolism in MCI.
Apathy is characterized by loss of interest, lack of motivation, and social withdrawal. It is the most common neuropsychiatric symptom in Alzheimer’s disease (AD) dementia and among the most common in amnestic mild cognitive impairment (MCI), and it increases in frequency as the disease progresses.1–3 In AD patients, apathy has been associated with executive dysfunction and impairment in activities of daily living, and it is a major source of frustration for caregivers.4–6 In MCI, apathy has also been shown to herald the development of AD dementia.7,8
It has been hypothesized that apathy is mediated by a frontal–subcortical circuit.9,10 Functional imaging studies have shown that apathy relates most consistently to hypometabolism and hypoperfusion in the anterior cingulate and orbitofrontal region in mild-to-moderate AD dementia.11,12 Apathy has also been associated with increased anterior cingulate neurofibrillary tangle burden in moderate-to-severe AD dementia at postmortem and with elevated cerebrospinal fluid (CSF) total and phospho-tau in vivo earlier in the disease course in mild AD dementia.13,14
To our knowledge, apathy has yet to be linked to amyloid burden. In-vivo investigation of amyloid pathology is now possible using Pittsburgh Compound B (PiB) positron emission tomography (PET).15 Multiple studies in MCI have demonstrated a bimodal distribution of PiB retention, with one group exhibiting PiB binding similar to that seen in AD dementia patients and the other exhibiting nonspecific binding similar to that seen in PiB-negative, clinically normal elderly subjects.16–19 Similar findings have been reported with autopsy studies, as well.20,21 Therefore, further clinical characterization of MCI might help better define individuals who are likely to have underlying AD pathology. Neuropsychiatric symptoms, such as apathy, can serve this purpose.
The objective of this study was to determine whether apathy is associated with cortical amyloid burden, measured by PiB PET, and regional hypometabolism, measured by 18F-fluorodeoxyglocuse (FDG) PET, in MCI.
Methods
Subjects
A group of 24 MCI subjects participating in an investigator-initiated imaging study underwent clinical assessments and PET imaging with PiB and FDG. Subjects were ages 54–85 (inclusive), were medically stable, and did not have significant cofounding neurological conditions, recent substance or alcohol abuse, or current primary psychiatric diagnoses. Subjects had a Modified Hachinski Ischemic Score22 ≤4 and a Geriatric Depression Scale (GDS; long form)23 ≤10. Subjects had a study partner who provided collateral information about their mood, behavior, and daily functioning.
Subjects met criteria for amnestic MCI, single or multiple domain.24 These criteria included a memory complaint (reported by the subject or study partner); objective memory impairment, assessed with the Logical Memory IIa (story recall) of the Wechsler Memory Scale–Revised; essentially intact activities of daily living; and no evidence of dementia. They also had a Mini-Mental State Exam (MMSE)25 score of 24–30 (inclusive), a Clinical Dementia Rating (CDR)26 global score of 0.5, and a Memory Box score ≥0.5.
The study was approved by the Partners (local) Institutional Review Board. Informed consent was obtained from all subjects and their study partners before any of the study procedures were carried out.
Clinical Assessments
The Apathy Evaluation Scale (AES)27 was used to assess apathy severity based on an informant interview. The AES consists of 18 items relating to apathy. Each item was rated on a 4-point, Likert-type scale. Lower scores indicated greater apathy (range: 42–72 in this analysis; full range 18–72). The AES examines apathy in greater depth than the Neuropsychiatric Inventory,28,29 which was used in many previous studies.
PET Imaging
PiB was synthesized, and dynamic PiB PET imaging acquisition was performed as previously described.15,30–34 Data were acquired using a Siemens/CTI (Knoxville, TN) ECAT HR+scanner (3D mode; 63 image planes; 15.2-cm axial field of view; 5.6-mm transaxial resolution, and 2.4-mm slice interval; 69 frames: 12×15 seconds, 57×60 seconds). After a transmission scan, 8.5–15 mCi 11C-PiB was injected as a bolus and followed immediately by a 60-minute dynamic acquisition. PiB PET data were reconstructed with ordered set expectation maximization, corrected for attenuation. Each frame was evaluated to verify adequate count statistics and absence of head motion. The Logan graphical analysis method with cerebellar cortex as the reference tissue input function was used to evaluate specific PiB retention expressed as the distribution volume ratio (DVR).34,35 PiB DVR was calculated in an aggregate cortical region of interest, defined using the Automated Anatomic Labeling (AAL) template following Statistical Parametric Mapping (SPM) spatial transformation, as described previously.36,37 This aggregate of cortical PiB regions consisted of regions that typically have elevated PiB retention in AD dementia; it was used in all analyses.
FDG PET data were acquired according to the Alzheimer’s Disease Neuroimaging Initiative protocol.38 Briefly, a bolus of 5 mCi of FDG was injected, in a quiet, dimly lit room, with subjects in the eyes-open state. FDG acquisition began 30 minutes after injection and lasted 30 minutes. Cortical FDG metabolism was expressed as the standardized uptake value and normalized to the cerebellum for each region of interest. On the basis of previous functional imaging studies in AD dementia,11,12 two regions highly correlated with apathy were selected from the AAL template for these analyses: the anterior cingulate and the orbitofrontal cortices. Three additional regions typically affected in MCI and AD dementia, regardless of symptoms of apathy, were also selected: supramarginal, precuneus, and inferior temporal cortices.
Statistical Analyses
All analyses in this study were carried out with SPSS Version 20.0. The AES distribution was not normal (it was left-skewed). Therefore, nonparametric tests were used. The AES was related to subject demographics and characteristics, using Spearman’s correlations for continuous variables and the Mann-Whitney U test for categorical variables (two-tailed p values are reported).
Partial Spearman’s correlations, controlling for age, were used to assess the association between apathy and the aggregate of cortical PiB regions, as well as the five FDG regions (correlation coefficients and two-tailed p values are reported). For the association of apathy with cortical PiB, additional analyses were done to control for age, as well as disease severity, using either the Rey Auditory Verbal Learning Test (RAVLT)39 Total Learning score (a measure of memory), the MMSE (a measure of global cognition), or the CDR Sum of Boxes (a measure of global functioning).
Exploratory whole-brain, voxel-based analyses were performed with SPM8 (Wellcome Department of Cognitive Neurology), a UNIX-based software package using custom routines in MATLAB (Mathworks, Inc.), to further determine whether there is any regional association between apathy and FDG metabolism. Analyses were adjusted for age, and data were smoothed using an 8-mm kernel.
Results
Subject demographics and characteristics are displayed in Table 1. Increased apathy (lower AES score) was significantly associated with greater global functioning impairment (higher CDR sum of boxes; rs = −0.45; p=0.03). Greater cortical PiB retention was also significantly associated with higher CDR sum of boxes (rs=0.57; p=0.003). Apathy was not significantly associated with any other subject demographics and characteristics.
| MCI Subjects | |
|---|---|
| N | 24 |
| Age, years | 73.6±9.2 (54–85) |
| Sex, % men | 72.0 |
| Education, years | 17.3±2.4 (12–20) |
| RAVLT Total Learning | 32.7±8.9 (18–55) |
| MMSE | 27.4±1.9 (24–30) |
| CDR Sum of Boxes | 1.8±1.0 (0.5–3.5)* |
| AES | 61.4±8.3 (42–72) |
There was a significant association between increased apathy (lower AES score) and greater cortical PiB retention after adjusting for age (prs = −0.46; p=0.03; see Figure 1. After adjusting for age and measures of disease severity, the relationship between apathy and cortical PiB retention was retained with RAVLT Total Learning (prs = −0.44; p=0.05) and MMSE (prs = −0.43; p=0.05), but not with CDR Sum of Boxes (prs = −0.19; NS).
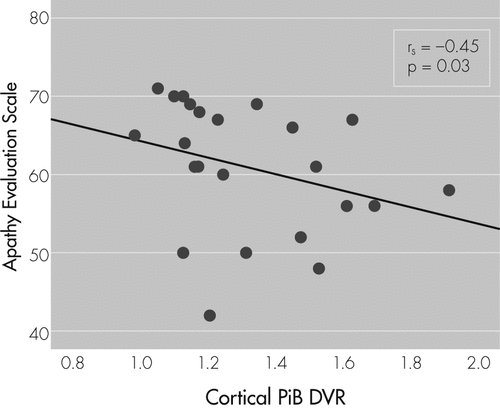
Unadjusted Spearman’s correlation coefficient and p value are reported here, whereas the main analyses used partial Spearman’s correlations adjusted for age. Lower scores on the AES indicate greater apathy.
AES: Apathy Evaluation Scale; PiB DVR: Pittsburgh Compound B distribution volume ratio.
There was no significant association between apathy and any of the FDG regions after adjusting for age: anterior cingulate (prs=0.03; NS), orbitofrontal (prs=0.12; NS), supramarginal (prs=0.11; NS), precuneus (prs=0.14; NS), and inferior temporal (prs=0.23; NS). No significant associations were seen with the SPM analyses even when using an inclusive voxel-level threshold of 0.05 and small volume correction.
Discussion
The results of our study suggest that, after adjusting for age, increased apathy in MCI is associated with greater amyloid burden, but not with regional hypometabolism. To our knowledge, this is the first study to show a relationship between apathy and amyloid pathology in the AD spectrum. It suggests that apathy, which has been shown to be an early and salient symptom of MCI and AD dementia,1,3 can help more accurately characterize the heterogeneous clinical diagnosis of MCI due to AD.40 Impairment in instrumental activities of daily living has been associated with increased amyloid burden in MCI, as well,33 and can similarly help better clinically characterize these individuals that are primarily diagnosed on the basis of their cognitive performance.
Previous studies assessing the relationship between apathy and functional and pathological markers of AD at later stages of AD dementia have found associations with medial-frontal metabolism, perfusion, and tau pathology, rather than amyloid burden.11–14 As such, the lack of association between apathy and anterior cingulate and orbitofrontal hypometabolism in the current study was unexpected. However, this study focused on subjects with MCI, in whom these associations have not been previously explored. It is possible that, at the stage of MCI, decreased cerebral activity is not as prominently linked to apathy. In order to determine whether this lack of association was due to an a priori selection of the wrong regions of interest, we also looked at parietal and temporal regions, which are typically affected in MCI and AD dementia, regardless of the presence of apathy. However, we did not find an association with these regions, either. Finally, we performed an exploratory whole-brain, voxel-based analysis in order to find potentially smaller regional metabolic associations with apathy, but again we did not find a significant association. This question may be more definitively answered if a larger subject population is assessed.
This study had several limitations. First, the sample size of 24 was small. However, this is the first study with MCI subjects and sensitive imaging biomarkers that used a specialized assessment of apathy, the AES, which allowed us to measure numerous apathy-related symptoms with a continuous scale. Second, the primary analyses performed in the current study controlled for age, but no other covariates. Because of the small sample size, we could not afford to control for many covariates. However, of the covariates assessed initially, only CDR Sum of Boxes was significantly related to apathy. This was expected, since the CDR, a measure of global functioning, heavily relies on assessment of executive function and activities of daily living, both of which have been shown to relate to apathy.4–6 We also found that CDR Sum of Boxes was significantly associated with amyloid burden as previously reported with the CDR and other measures of activities of daily living.16,33 Consequently, we repeated the analyses after adjusting for age and CDR Sum of Boxes, and the association between apathy and amyloid burden was no longer significant. The sample size and cross-sectional design of our analyses limited our ability to examine the reciprocal relationships and causal associations between apathy, CDR Sum of Boxes, and amyloid burden. Future larger studies can address these clinically-important associations. Aside from the CDR, we also controlled for other measures of disease severity, including a measure of memory (RAVLT Total Learning) and a measure of global cognition (MMSE). Controlling for these cognitive measures did not influence the relationship between apathy and amyloid burden, which remained significant. Finally, for the association between apathy and regional hypometabolism, we focused on two regions, the anterior cingulate and orbitofrontal cortices, which have been shown to be most closely related to apathy in AD dementia. However, even when we extended our analyses to include other regions typically affected in AD dementia, as well as performed a whole-brain exploratory SPM analyses, we did not find a relationship with apathy. This could be due to the small sample size or due to the milder severity of apathy at the stage of MCI, making it more difficult to detect an association with FDG metabolism.
In conclusion, this initial study, for the first time, showed an association between apathy and increased in-vivo amyloid burden, but not regional hypometabolism in MCI. This association was independent of age, memory, and global cognition, but not global functioning, which has been previously associated with apathy. Future larger cross-sectional and longitudinal studies of subjects across the AD spectrum assessing additional AD biomarkers, such as CSF aβ 1–42 and total and phospho-tau, will help further clarify these associations.
1 : Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord 2008; 25:115–126Crossref, Medline, Google Scholar
2 : The spectrum of behavioral changes in Alzheimer’s disease. Neurology 1996; 46:130–135Crossref, Medline, Google Scholar
3 ;
4 : Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry 2003; 11:214–221Crossref, Medline, Google Scholar
5 ;
6 : Apathy and executive function in Alzheimer’s disease. J Int Neuropsychol Soc 2002; 8:373–381Crossref, Medline, Google Scholar
7 : Neuropsychiatric predictors of progression from amnestic mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis 2010; 20:175–183Crossref, Medline, Google Scholar
8 ;
9 : Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50:873–880Crossref, Medline, Google Scholar
10 : The limbic system: an anatomic, phylogenetic, and clinical perspective. J Neuropsychiatry Clin Neurosci 1997; 9:315–330Link, Google Scholar
11 : Brain perfusion correlates of the apathy inventory dimensions of Alzheimer’s disease. Int J Geriatr Psychiatry 2004; 19:864–869Crossref, Medline, Google Scholar
12 : Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Arch Neurol 2007; 64:1015–1020Crossref, Medline, Google Scholar
13 : Neuropathologic correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord 2006; 21:144–147Crossref, Medline, Google Scholar
14 : Neuropsychiatric correlates of cerebrospinal fluid biomarkers in Alzheimer’s disease. Dement Geriatr Cogn Disord 2008; 25:559–563Crossref, Medline, Google Scholar
15 : Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004; 55:306–319Crossref, Medline, Google Scholar
16 : Imaging beta-amyloid burden in aging and dementia. Neurology 2007; 68:1718–1725Crossref, Medline, Google Scholar
17 : Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 2009; 63:178–188Crossref, Medline, Google Scholar
18 : Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol 2009; 65:557–568Crossref, Medline, Google Scholar
19 : 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 2008; 131:665–680Crossref, Medline, Google Scholar
20 : Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology 2005; 64:834–841Crossref, Medline, Google Scholar
21 : Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006; 63:665–672Crossref, Medline, Google Scholar
22 : Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 1980; 7:486–488Crossref, Medline, Google Scholar
23 : Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982-1983; 17:37–49Crossref, Medline, Google Scholar
24 : Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256:183–194Crossref, Medline, Google Scholar
25 : “Mini-Mental State:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
26 : The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414Crossref, Medline, Google Scholar
27 : Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991; 38:143–162Crossref, Medline, Google Scholar
28 : The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 1997; 48(Suppl 6):S10–S16Crossref, Medline, Google Scholar
29 : The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
30 : Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem 2003; 46:2740–2754Crossref, Medline, Google Scholar
31 : Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol 2007; 62:229–234Crossref, Medline, Google Scholar
32 : Imaging amyloid deposition in Lewy body diseases. Neurology 2008; 71:903–910Crossref, Medline, Google Scholar
33 : Instrumental activities of daily living impairment is associated with increased amyloid burden. Dement Geriatr Cogn Disord 2011; 31:443–450Crossref, Medline, Google Scholar
34 : Amyloid-β associated cortical thinning in clinically normal elderly. Ann Neurol 2011; 69:1032–1042Crossref, Medline, Google Scholar
35 : Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab 2005; 25:1528–1547Crossref, Medline, Google Scholar
36 : Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol 2010; 67:353–364Medline, Google Scholar
37 : Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15:273–289Crossref, Medline, Google Scholar
38 ;
39 : L'examen clinique en psychologie. Paris, France, Presses Universitaires de France, 1964Google Scholar
40 : The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:270–279Crossref, Medline, Google Scholar



