Neuroanatomical Mechanism on the Effect of Distraction in Working Memory Maintenance in Patients With Schizophrenia
Abstract
This study utilized functional magnetic resonance imaging (fMRI) to discriminate brain activation patterns associated with the effect of distraction during working memory (WM) maintenance for human faces in healthy controls and patients with schizophrenia. Event-related fMRI data were obtained while the subjects performed WM maintenance in a delayed-response WM task with task-irrelevant distracters. Compared with healthy controls, patients showed significantly decreased activities in the superior frontal gyrus, dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex, anterior cingulate cortex, inferior parietal gyrus, and fusiform gyrus during the delayed-response WM task with human face distracters. The blood-oxygen-level-dependent signal changes in the DLPFC were negatively correlated with both of the scores of the Positive Subscale and General Psychopathology Subscale under the Positive and Negative Syndrome Scale during the WM maintenance for the human faces in the patients. This study will be helpful in understanding the neural mechanisms in the general impairment of the inhibition control in schizophrenia.
Schizophrenia is a disease of disrupted functional and structural neural connectivity, leading to symptoms that affect various aspects of mental activity, including memory, attention, and emotion. Cognitive impairment predicts poor functional outcome, and particularly, patients with schizophrenia exhibit reduced functions across nearly all social-cognitive factors such as speed of processing, executive processes, attention, working memory (WM), and problem solving.1 Working memory disturbances are a robust correlate of schizophrenia, and the WM deficit in patients with schizophrenia have been implicated as a crucial key in the disorders.2–4 Thus, WM deficits have recently become a major therapeutic target for pharmacological treatments.5 Accordingly, several studies5–7 with patients with schizophrenia have focused on the assessment of the central network related to the general impairment of WM in patients with schizophrenia, reflected as symptoms of poor attention and goal-directed behavior.
Recent advances in the neuroimaging techniques such as functional magnetic resonance imaging (fMRI)8 enable the identification of the neural centers related with the cognitive dysfunction in patients with schizophrenia. Numerous fMRI studies3–7 reported that the cognitive deficits in patients with schizophrenia were associated with dysfunction of the frontal and parietal lobes, and cingulate gyrus, and, particularly, the lateral prefrontal cortex was shown to play an important role in sustained attention, WM, and higher cognitive function. Patients with schizophrenia performed poorly on the cognitive tasks, including the Verbal fluency, N-back, Sternberg and Tower of London, and these results were consistent with decreased activity of the frontal lobe.7,9–11 Moreover, patients with schizophrenia during the stroop tasks showed reduced activation in the dorsolateral prefrontal cortex, anterior cingulated cortex, and parietal regions compared with healthy controls.12 Given the similarity between task accuracy for spatial WM task in both the patients with schizophrenia and healthy controls, the patients showed reduced activation in the frontal lobe during maintenance of WM than healthy controls.6
It is widely recognized that cognitive deficits in patients with schizophrenia are associated with dysfunction of the lateral prefrontal cortex, resulting in difficulties in the high-order information processing in conjunction with problem-solving and executive function. A study13 concerning the cognitive deficits in patients with schizophrenia indicated that dysfunction of the lateral prefrontal cortex was closely related to goal-directed behavior. Goal-directed behavior depends on the cognitive control such as the WM, which allows one to maintain and manipulate information relevant to our current tasks over short periods of time.14,15 Two categories of operations have been identified to contribute to the cognitive control: operations that allow active maintenance of goal-relevant information in mind and operations that allow keeping goal-irrelevant information out of mind.15,16 The effects of distraction by presenting task-irrelevant distracters during delay interval of a WM task have received much less attention and distraction, and, particularly, patients with schizophrenia directly linked to the deficit of their ability to maintain focus on goal-relevant information for confusing distracters.12,17,18 Inhibition control for task-irrelevant information has been proposed to have an important role in the cognitive control, and also was associated with impaired memory performance.12,17,18 Therefore, it appears that inhibition control has become important to understand higher cognitive function of the patients with schizophrenia.
In this study, event-related fMRI data were obtained while the volunteers performed a delayed-response WM task with distracters in which interference was manipulated across trials. A delayed-response WM task is to investigate the nature of distraction upon the neural correlates of WM maintenance operations by presenting task-irrelevant distracters during the interval between encoding and retrieval in delayed-response WM task.15,16,19 This task may provide novel evidence concerning the differential neural mechanism regard to inhibitory processes between healthy controls and patients with schizophrenia. The target faces and distracters in our study included human faces normally experienced in a daily social setting. The ability to infer information such as the presented individual’s age, sex, and emotional status by facial expressions can predict the social cognitive function, including communication skills and interpersonal relation.20 A few functional imaging studies15,16,19 in the healthy controls have been reported to demonstrate the neural mechanisms on the effect of distraction during a delayed-response WM task so far. However, similar studies in patients with schizophrenia have not been reported.
Although there are numerous studies for identifying the neural circuitry on cognitive control such as the WM, the neural mechanisms on WM associated with cognitive inhibition components have not yet been revealed in patients with schizophrenia in particular. For the first time, our study discriminated the differential brain activation patterns associated with the effect of distraction during the delay interval of the WM task with the normal face and scrambled face distracters between the healthy controls and patients with schizophrenia using fMRI. A delayed-response WM paradigm was used in our study to investigate the neural impact of task-irrelevant distracters on WM maintenance during the interval between encoding and retrieval phases in patients with schizophrenia.
Methods
Participants
A total of 18 patients with schizophrenia (mean age=31.0±8.7 years) and 18 healthy controls (mean age=33.5±7.3 years) underwent fMRI on a 3.0 Tesla Magneton Verio MR Scanner (Siemens Medical Solutions, Erlangen, Germany), all of whom were right-handed (Table 1). All volunteers had no history of neurological or psychiatric illness, and had no other psychiatric disorders.
| Characteristic | Schizophrenia Subjects (N=18) | Control Subjects (N=18) | p |
|---|---|---|---|
| Age (years) | 31.0±8.7 | 33.5±7.3 | p=0.267a |
| Gender (male/female) | 9/9 | 9/9 | p=1.000b |
| Handedness (% right) | 100 | 100 | p=1.000b |
| Education (years) | 14.1±2.6 | 15.3±2.1 | p=0.134a |
| Duration of illness (years) | 8.4±5.8 | — | — |
| Age at onset (years) | 22.6±5.8 | — | — |
| Clinical global impression | 3.9±1.0 | — | — |
| Positive and Negative Syndrome Scale score | |||
| Positive symptoms | 16.4±5.5 | — | — |
| Negative symptoms | 19.6±6.5 | — | — |
| General psychopathology | 36.6±6.6 | — | — |
TABLE 1. Demographic and Clinical Characteristics of the Patients With Schizophrenia and Healthy Controls
All patients were inpatients or outpatients of the Chonbuk National University Hospital and were assessed on the basis of an interview of the DSM-IV-TR21 by a psychiatrist. The patients were assessed using the Positive and Negative Syndrome Scale (PANSS). In the PANSS, the patients showed the following results (mean±SD): positive, 16.4±5.5; negative, 19.6±6.5; and global, 36.6±6.6. Seventeen patients with schizophrenia received prescription for multiple psychotropic medications and one psychotropic medication for one patient (Table 2). All volunteers had enough practice to be accustomed to the activation paradigm prior to MR scanning. Moreover, they were asked to notice the procedure and matters that require attention prior to experiment, and written informed consent was obtained from them. This study was approved by institutional review board of the Chonbuk National University Hospital.
| Psychotropic Medicationsa | Dosage Range (1 Day) |
|---|---|
| Amisulpride (N=3) | 1200–1600 mg |
| Benztropine (N=15) | 1–4 mg |
| Fluoxetine (N=3) | 40–80 mg |
| Lorazepam (N=12) | 0.5–2 mg |
| Paliperidone (N=6) | 6–15 mg |
| Propranolol (N=8) | 40–80 mg |
| Risperidone (N=6) | 4–12 mg |
| Trazadone (N=3) | 50 mg |
| Zolpidem (N=3) | 10 mg |
TABLE 2. Psychotropic Medications in Patients With Schizophrenia
Stimuli and Procedure
All volunteers performed a delayed-response WM task with distracters presented during the delay interval. The activation paradigm consisted of a string of memory preparation and encoding (6 seconds); WM maintenance (4 seconds); distracter (6 seconds); button preparation (2 seconds); retrieval (2 seconds); and intertrial interval (ITI) (12 seconds) (Figure 1). As for the encoding task, three different human faces sequentially appear once (1.66 seconds each) on a quartile coordinate. During the delay time following the encoding, the subjects were asked to maintain the WM for the encoded faces. Then, the distracters were presented for a total time of 6 seconds (3 seconds each) in the distracter task, and the subjects were instructed to look at the distracters while maintaining the WM. The distracters consisted of a human face (high-level distraction) and a scrambled face (low-level distraction). In the retrieval task, either of the face presented in the encoding task or a new face was presented for a total time of 2 seconds (50% were presented with an encoding face, and 50% were presented with a new face). The accuracy of the face recognition task was evaluated by the criterion with volunteer’s response within 2 seconds in the retrieval task. The volunteers were asked to make a recognition decision for the retrieval of the human face by pressing one of two response keys.
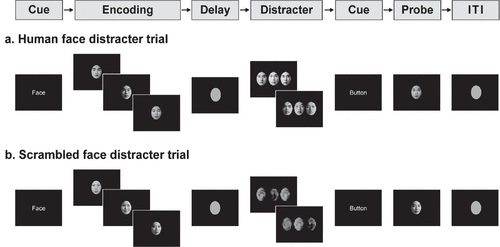
FIGURE 1. Diagrams for the Delayed-Response Working Memory (WM) Tasks With Human Face (a) and Scrambled Face (b) Distractersa
a In the encoding task, three different human faces appear once; the subjects were instructed to encode and maintain the WM for the presented human faces, followed by looking at the distracters with human face (or scrambled face) while maintaining the WM, and then response to the probe for the previously presented human face or a new one. ITI: intertrial interval
The retrieval task consisted of 3 sessions: 10 trials for human face distracters and 10 trials for scrambled face distracters for recognition task of the human face. In the total 20 trials, the order of two types of the distracters were randomly arranged. The human faces were selected from a high school yearbook, which were similarly made as black-and-white pictures of an oval shape with eyes, nose, mouth, and eyebrows. The human faces consisted of male and female in equal ratio. The encoding, distracters, and retrieval stimuli in one trial were created with face pictures of the same sex. The scrambled faces were rotated –200° from the human faces using Photoshop. The gender of the face distracters and retrieval probe always matched the gender of the human faces in the encoding items. The visual stimuli were presented using the SuperLab software (Cedrus Corporation, San Pedro, CA). The average illuminance levels of the each face picture were equivalent. The illuminance levels were measured with a digital illuminance meter (Illuminance Meter; TES-1339, Taipei, Taiwan).
MRI and fMRI
Functional MRI was performed on a Magnetom Verio MR Scanner (Siemens Medical Solutions, Malvern, Pa.) with a bird cage head coil. The functional images were acquired from a total of 25 transverse slices parallel to an AC-PC (anterior commissure to posterior commissure) line using a gradient-echo echo planar pulse sequence with the following parameters: repetition time (TR)/echo time (TE) = 2000ms/30ms, flip angle=90°, field of view (FOV) = 22×22cm, matrix size=64×64, number of excitations (NEX) = 1, and slice thickness=5mm. In addition, two phases of dummy scans were supplemented to circumvent unstable fMRI signals. The high-resolution T1-weighted images (TR/TE=1900ms/2.35ms) were acquired with FOV=22×22cm, matrix size=256×256, NEX=1, slice thickness=5mm.
Data Preprocessing and Analysis
The fMRI data were analyzed by postprocessing and data analysis using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, University College London, London, UK). In statistical analysis, we excluded incorrectly performed trials for the face recognition task in both healthy controls and patients. Prior to statistical analysis, a slice-timing correction was performed on the fMRI data. Then, the images were realigned to match each functional volume to the reference volume and were spatially normalized to the standard EPI template in Montreal Neurological Institute (MNI) space, which is a template created from 152 brain data sets, and resampled to 2×2×2mm resolution. Finally, the images were smoothed with an 8-mm full-width-half-maximum Gaussian filter. After that, activated areas were identified by means of multiple regression analysis of the time series of MR signal intensities in each voxel. Reported coordinates identify the voxel with peak activity within the cluster of activation. Preprocessed data were analyzed using the standard general linear model (GLM) in SPM8. To analyze the individual blood-oxygen-level-dependent (BOLD) signal in a voxel with dimension of 2×2×2mm, an independent t-test was performed in the rest and activation conditions (normal shape of human faces and scrambled face distracters). For the between-group analysis of the healthy controls and the patients, the differential activation maps, which are correspondent to the contract of human faces versus scrambled faces, were obtained from the paired t test (p<0.0005). Two-sample t-test (uncorrected, p<0.005) was used to compare the differential brain activation patterns between the healthy controls and the patients. We reported activations including more than 10 contiguous voxel for cluster size.
Based on findings from previously studies15,16,19 investigating the effect of distraction during the WM maintenance for the human faces, we identified seven brain regions-of-interest (ROIs) such as superior frontal gyrus (SFG), dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), anterior cingulate cortex (ACC), superior parietal gyrus (SPG), inferior parietal gyrus (IPG), and fusiform gyrus (FG). The DLPFC (otherwise known as the middle frontal gyrus) comprises the Brodmann areas 9, 10, and 46, and the VLPFC (otherwise known as the inferior frontal gyrus) comprises the Brodmann areas 44, 45, and 47.22
Results
Clinical Evaluation
Table 1 shows demographic and clinical characteristics of the patients with schizophrenia and healthy controls. There were no significant group differences on age, gender, handedness, and education between two groups (Table 1). Table 2 shows the psychotropic medications for all patients.
Face Recognition Task
Scores for the face recognition task with normal human face distracters (10 trials) were 69.1±8.6% and 56.8±10.4% in the healthy controls and the patients with schizophrenia, respectively, whereas the scores for the scramble face distracters (10 trials) were 78.6±14.5% and 67.6±13.5%, respectively. The reaction times for the face recognition task with normal human face distracters were 1285±173ms and 1491±179ms in the healthy controls and the patients with schizophrenia, respectively, whereas reaction times for the scramble face distracters were 1235±170ms and 1460±220ms, respectively. The patients performed worse with face and scrambled face distracters compared with healthy controls.
Differential Activation Patterns Between Human Face and Scrambled Face Distracters
In the healthy controls, the human face distracters showed significantly stronger activities compared with the scrambled face distracters in the brain areas including the superior frontal gyrus, DLPFC, VLPFC, ACC, superior/inferior parietal gyri, and fusiform gyrus during the delayed-response WM task (p<0.0005) (Figures 2–4). Similar to the healthy controls, the patients showed significantly stronger activities with the human face distracters over the scramble face distracters in the same brain areas except the ACC (p<0.0005) (Figures 2–4). However, these brain areas showed decreased activities in the patients over healthy controls, which are supposed to be involved in a specific role for impairment of the inhibition control in the patients.
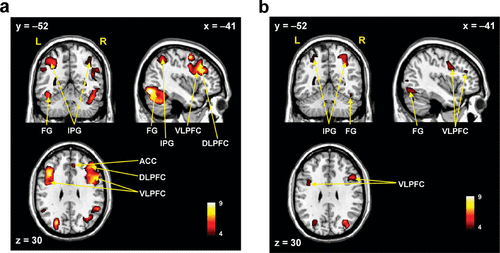
FIGURE 2. Brain Activation Mapsa
a Brain activation maps on the sagittal (x), coronal (y), and axial (z) planes demonstrating full details of the predominance of the human face over the scrambled face distracters during the working memory for the target faces in healthy controls (a) and patients with schizophrenia (b), resulting from the paired t test (p<0.0005).
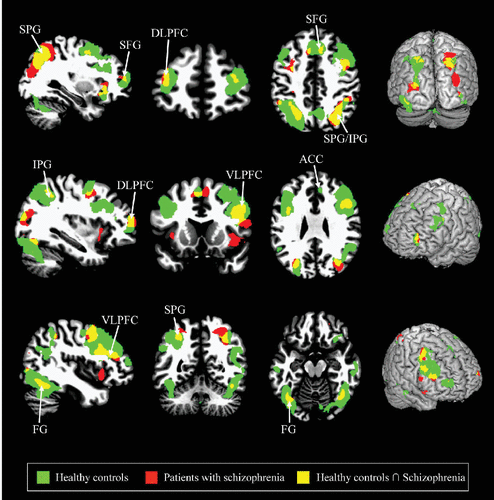
FIGURE 3. Regional Activation Mapsa
a Regional activation maps demonstrating the activation patterns derived from the healthy controls (green) and patients with schizophrenia (red), in which yellow activation indicates the overlapping areas between two groups, resulting from the paired t test (p<0.0005).
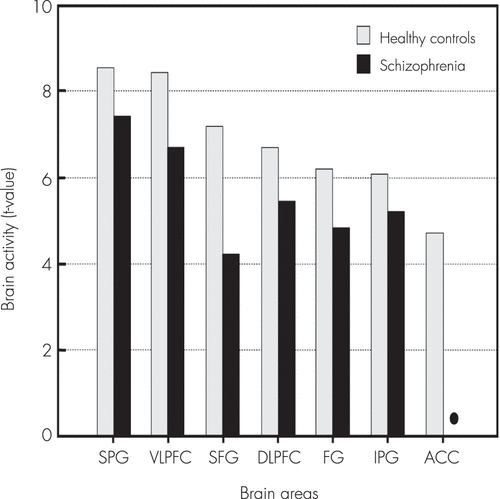
FIGURE 4. Comparison of Localized Brain Activitiesa
a Comparison of the localized brain activities (maximum t-value) resulting from the contrast of the distracters with the human face over the scrambled face while maintaining the working memory (WM) of the target faces in healthy controls and patients with schizophrenia: paired t test (p<0.0005).
Differential Activation Patterns Associated With Human Face Distracters Between Two Groups
Compared with the healthy controls, the patients with schizophrenia showed significantly decreased activities in the superior frontal gyrus, DLPFC, VLPFC, ACC, inferior parietal gyrus, and fusiform gyrus during the delayed-response WM with normal human face distracters (p<0.005) (Table 3, Figure 5). These brain areas are potentially considered to be related with an impairment of the inhibition control in the patients. The BOLD signal changes of the DLPFC (x, y, z=38, 50, 20) were negatively correlated with the score of the PANSS-Positive Subscale (Spearman’s rho=–0.73, p<0.005) during the WM maintenance for the human faces in the patients (Figure 6). Moreover, the scores of the PANSS-General Psychopathology Subscale (Spearman’s rho=–0.66, p<0.01) were negatively correlated with the BOLD signal changes of the DLPFC (x, y, z=36, 48, 20) (Figure 6).
| Brain Areas | BA | t-Value | Montreal Neurological Institute Coordinates (x, y, z) | Cluster Size | |||
|---|---|---|---|---|---|---|---|
| Healthy controls over schizophrenia | |||||||
| L | VLPFC (IFG) | 45 | 4.51 | –42 | 34 | 18 | 435 |
| R | Fusiform gyrus (FG) | 37 | 3.75 | 44 | –56 | –18 | 126 |
| R | DLPFC (MFG) | 9 | 3.70 | 50 | 14 | 42 | 467 |
| L | Inferior parietal gyrus (IPG) | 40 | 3.63 | –30 | –52 | 44 | 88 |
| R | Superior frontal gyrus (SFG) | 8 | 3.15 | 4 | 28 | 44 | 178 |
| R | Anterior cingulate cortex (ACC) | 32 | 3.14 | 16 | 30 | 26 | 28 |
TABLE 3. Brain Activities (Maximum t-Value) Resulting From the Predominance of Healthy Controls Over Patients While Maintaining the Working Memory (WM) of the Target Faces With Human Face Distractersa
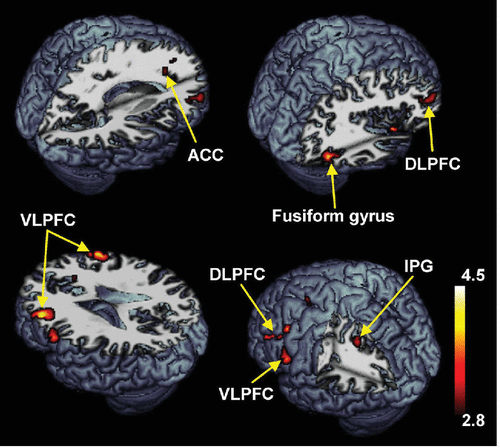
FIGURE 5. Brain Areas Predominantly Activated in Healthy Controls as Contrasted With Schizophrenia Patientsa
a Brain areas activated during working memory (WM) maintenance of the target faces with human face distracters, which were analyzed with two-sample t tests (p<0.005).
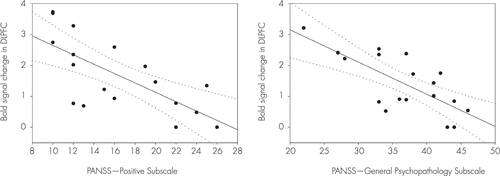
FIGURE 6. The Blood-Oxygen-Level-Dependent (BOLD) Signal Changes in the Dorsolateral Prefrontal Cortex (DLPFC)a
a The BOLD signal changes in the DLPFC (x, y, z=38, 50, 20) were negatively correlated with the scores of the Positive and Negative Syndrome Scale (PANSS)-Positive Subscale (Spearman’s rho=–0.73, p<0.005) during the working memory (WM) maintenance for the human faces in patients with schizophrenia, in which the dotted lines shows 95% confidence interval. The scores of the PANSS-General Psychopathology Subscale (rho=–0.66, p<0.01) were negatively correlated with the BOLD signal changes in the DLPFC (x, y, z=36, 48, 20).
Discussion
The ability to maintain one’s focus on goal-relevant information while ignoring goal-irrelevant and potent distracting information depends on the coordination of a set of operations that contributes to the cognitive control.16 Symptoms in schizophrenia may be directly linked to the deficit of the patient’s ability to maintain focus on goal-relevant information in the presence of distracters. Scores for the face recognition task of the human face and scrambled face distracters in the healthy controls were 69.1±8.6% and 78.6±14.5%, respectively, whereas scores in the patients were 56.8±10.4% and 68.1±12.4%, respectively. Patients may have difficulties in WM maintenance for confusing face distracters in delayed-response WM task compared with healthy controls.
Compared with the healthy controls, the patients with schizophrenia showed significantly decreased activities in the superior frontal gyrus, DLPFC, VLPFC, ACC, inferior parietal gyrus, and fusiform gyrus during the delayed-response WM with human face distracters. These brain areas showed decreased activities in the patients over healthy controls, which are supposed to be involved in a specific role for impairment of the inhibition control in the patients. It is very important to note that the ACC was significantly activated during the WM for the target faces with the human face distracters as contrasted with the scramble face in the healthy controls only. These results provided evidence that the ACC may play an important role in rational cognitive functions such as decision-making and conflict detection during WM tasks associated with confusing distraction.
A couple of neuroimaging studies16,19 used a similar paradigm to our finding revealed the brain areas, which were significantly activated by viewing the human face distracters in the healthy controls, included the superior frontal gyrus, DLPFC, VLPFC, superior/inferior parietal gyri, and fusiform gyrus. An interesting finding in our study is significant activities in the dorsal part of the ACC during the WM for the target faces with face distracters in the healthy controls only. It has been proposed that the ACC is a part of a circuit involved in a form of attention that serves to regulate both cognitive and affective processing. The ACC is divided into two parts: cognitive and affective division. The dorsal cognitive division is part of a distributed attentional network, and the ventral affective division is primarily involved in assessing the salience of emotional and motivational information and the regulation of emotional responses.23 The confusing distracters may induce a conflict and need the attention, and therefore, significant activities in the dorsal part of the ACC in our study are associated with increased WM monitoring and cognitive control.24,25 Moreover, the ACC is known to be linked to attention and inhibitory processes, showing enhanced responses in particular to high conflicts rather than low conflict conditions.26 The absence of the ACC activity in the patients may be due to lack of attention and inhibitory processes for the confusing face distracters while maintaining the target faces.
Healthy controls showed higher activities in the frontal lobe for human face distracter compared with patients. Decreased activities in the WM maintenance for target faces with the confusing face distracters in the patients may be associated with the functional deficit in high-order cognitive function including attention, executive function, and problem-solving. Focusing on the DLPFC and VLPFC, which are respectively involved in the WM maintenance and inhibition control of the effect of distraction, the patients showed significantly lower activities in both DLPFC and VLPFC compared with healthy controls. The VLPFC plays an important role in rational recognition and decision during inhibitory processes.27,28 Kross et al29 suggested that rejection sensitivity is associated with a significant activity in the VLPFC. Decreased activation of the VLPFC in patients with schizophrenia may reflect impairment of the inhibition control during WM task. It should be noted the VLPFC plays the most important role in cognitive control for confusing distracter. Jha et al30 suggested that the VLPFC was associated with the interference resolution as well as inhibitory processes, while the DLPFC has been linked to WM maintenance. The DLPFC is critical to the active maintenance of goal-relevant information in WM, and the highest level of distraction showed higher activities in the DLPFC than low level of distraction.16,19 In addition, the high-level encoded items in the encoding task during the WM maintenance were observed bilaterally in the DLPFC.31 Similar to the interpretation by Dolcos and McCarthy,15 we suggest that decreased activation of the DLPFC in the patients as contrasted with the healthy controls reflects reduced resistance against the distracters. Note that the BOLD signal changes of the DLPFC were negatively correlated with the scores of the PANSS-Positive Subscale and PANSS-General Psychopathology Subscale during the WM maintenance for the human faces in the patients. The higher symptom severity in the patients with schizophrenia, the activity of the DLPFC associated with WM maintenance decreased during the WM maintenance for the human faces. Patients with higher scores of the PANSS-Positive Subscale and PANSS-General Psychopathology Subscale may have difficulties in the WM maintenance during encoding the target faces that were distorted by the normal human faces. The superior frontal gyrus showed increased activities during the WM for the target faces in the healthy controls over patients; this finding is consistent with results of an fMRI study16 that high-level of distraction produced the greatest increase in the superior frontal gyrus compared with the low-level of distraction. Given that decreased activation of the VLPFC, DLPFC, and superior frontal gyrus in patients may be due to the decreased ability to maintain target faces while ignoring confusing face distracters.
The healthy controls showed increased activity in the superior/inferior parietal gyri and fusiform gyrus during a delayed-response WM with face distracters than the patients. Assuming that viewing the face distracters leads to increased visual and perceptual processing, activation of these areas could be related to higher cognitive function. The superior/inferior parietal gyri are implicated in attention to the visual objects and sustained attention to maintenance WM.16,32 Especially, these areas showed increased activity during the face (high trial) over scrambled (low trial) for distracters. These results suggest that the patients did not pay attention to highly visual processing for confusing distracters during the delay interval of the WM task. Therefore, these areas may play an important role in visual information perception. In particular, the fusiform gyrus has long been known to be involved in the perception of faces.33 Given the increased activities in this area in response to the face distracters and the lack of activation in the absence of distracters suggests, activation of this area is related to perceptual rather than WM maintenance.16 Decreased activation of the fusiform gyrus in the patients suggests that they did not pay attention to target faces for confusing distracters during WM maintenance. Such evidence supports that that the lower brain activation in patients over healthy controls may be assumed to be because of the decreased visually perceptual processing caused by the distracters that need a higher cognitive function.
Based on the results mentioned above, the task-irrelevant distracters during WM maintenance induce conflict and need attention for which the subjects initialize the behavior and decide on the special significance. This series of behaviors should not be limited by only the frontal lobe, but with interconnected activation of the frontal lobe and ACC. The frontal lobe plays a pivotal role in the maintenance of the target faces, inhibiting goal-irrelevant information and solving problems, and the ACC is involved in rational cognitive functions such decision-making and conflict detection during maintenance processes of WM with distracters. In addition, the superior/inferior parietal gyri and fusiform gyrus are associated with visually perceptual processing caused by the distracters. It is summarized that the impairment of the inhibition control during WM task in patients with schizophrenia may potentially correlate with the brain areas mentioned above.
There are some limitations in this study. First, the number of trials for the WM task used in our study was limited because the patients with schizophrenia moved their heads with increasing trial time during the fMRI examination. Second, the accuracy of the face recognition task was evaluated by the criterion with volunteer’s response within 2 seconds in the retrieval task, but this time limit is too short to accurately respond to the task for the patients. Third, because of the close relationship between negative symptoms of patients with schizophrenia and executive function deficits, objective interpretation of positive or negative symptoms of the patients may be compromised. We were unable to specifically divide and analyze the individual differences or symptoms of patients. Finally, the possibility of drug effects for brain activation patterns associated with distraction during the delay interval of the WM task could not be excluded because the patients have been taking the psychotropic medication.
Conclusions
Our findings are the first, to our knowledge, to provide evidence for the differential brain activation patterns associated with the effect of distraction between healthy controls and patients with schizophrenia during the delay interval of the WM task. This study will be helpful in understanding the neural mechanisms in the general impairment of the inhibition control in schizophrenia.
1 : Identification of separable cognitive factors in schizophrenia. Schizophr Res 2004; 72:29–39Crossref, Medline, Google Scholar
2 : Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry 2006; 67(Suppl 9):3–8, discussion 36–42Crossref, Medline, Google Scholar
3 : Executive-frontal lobe cognitive dysfunction in schizophrenia: a symptom subtype analysis. Psychiatry Res 1998; 79:139–149Crossref, Medline, Google Scholar
4 : Evidence for sustained attention and working memory in schizophrenia sharing a common mechanism. J Neuropsychiatry Clin Neurosci 2005; 17:391–398Link, Google Scholar
5 : An event-related FMRI study of phonological verbal working memory in schizophrenia. PLoS ONE 2010; 5:e12068Crossref, Medline, Google Scholar
6 : Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol Psychiatry 2008; 64:1026–1034Crossref, Medline, Google Scholar
7 : Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry 2001; 158:1105–1113Crossref, Medline, Google Scholar
8 : Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med 1990; 14:68–78Crossref, Medline, Google Scholar
9 : Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia. Assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49:943–958Crossref, Medline, Google Scholar
10 : Effects of psychotic state and task demand on prefrontal function in schizophrenia: an fMRI study of overt verbal fluency. Am J Psychiatry 2005; 162:485–494Crossref, Medline, Google Scholar
11 : Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage 2003; 18:247–256Crossref, Medline, Google Scholar
12 : Brain activation patterns during a selective attention test—a functional MRI study in healthy volunteers and unmedicated patients during an acute episode of schizophrenia. Psychiatry Res 2007; 154:31–40Crossref, Medline, Google Scholar
13 : Reduced neural activity of the prefrontal cognitive control circuitry during response inhibition to negative words in people with schizophrenia. J Psychiatry Neurosci 2012; 37:379–388Crossref, Medline, Google Scholar
14 : Working memory. Oxford, Oxford UP, 1986Google Scholar
15 : Brain systems mediating cognitive interference by emotional distraction. J Neurosci 2006; 26:2072–2079Crossref, Medline, Google Scholar
16 : Regional brain differences in the effect of distraction during the delay interval of a working memory task. Brain Res 2007; 1152:171–181Crossref, Medline, Google Scholar
17 : Dysfunction of the left dorsolateral prefrontal cortex is primarily responsible for impaired attentional processing in schizophrenia. Psychiatry Invest 2008; 5:52–59Google Scholar
18 : Brain activation patterns during a selective attention test-a functional MRI study in healthy volunteers and patients with schizophrenia. Psychiatry Res 2003; 123:1–15Crossref, Medline, Google Scholar
19 : Opposing influences of emotional and non-emotional distracters upon sustained prefrontal cortex activity during a delayed-response working memory task. Neuropsychologia 2008; 46:326–335Crossref, Medline, Google Scholar
20 : Social cognition in schizophrenia: a review of face processing. Br Med Bull 2008; 88:43–58Crossref, Medline, Google Scholar
21 : Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC, American Psychiatric Publishing, 2000Google Scholar
22 : Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychol Bull 2009; 135:638–677Crossref, Medline, Google Scholar
23 : Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000; 4:215–222Crossref, Medline, Google Scholar
24 : Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage 2003; 18:483–493Crossref, Medline, Google Scholar
25 : Anterior cingulate metabolism correlates with stroop errors in paranoid schizophrenia patients. Neuropsychopharmacology 2001; 25:139–148Crossref, Medline, Google Scholar
26 : Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 2000; 97:1944–1948Crossref, Medline, Google Scholar
27 : Inhibition and the right inferior frontal cortex. Trends Cogn Sci 2004; 8:170–177Crossref, Medline, Google Scholar
28 : The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 2010; 50:1313–1319Crossref, Medline, Google Scholar
29 : Neural dynamics of rejection sensitivity. J Cogn Neurosci 2007; 19:945–956Crossref, Medline, Google Scholar
30 : The role of prefrontal cortex in resolving distractor interference. Cogn Affect Behav Neurosci 2004; 4:517–527Crossref, Medline, Google Scholar
31 : Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto-parietal network. Neuroimage 2003; 20:1518–1530Crossref, Medline, Google Scholar
32 : Functional neuroanatomy of sustained attention in schizophrenia: contribution of parietal cortices. Hum Brain Mapp 2002; 17:116–130Crossref, Medline, Google Scholar
33 : The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 1997; 17:4302–4311Crossref, Medline, Google Scholar



