Enhanced Olfactory Cortex Connectivity in a Patient With PTSD With Olfactory Hallucinations
To the Editor: Previous reports described the presence of olfactory hallucinations in 10.3% of patients affected by post-traumatic stress disorder (PTSD),1 although there is limited understanding of this phenomenon. We present the case of a woman experiencing olfactory hallucinations with PTSD, and we discuss potential neural mechanisms. Using functional MRI during the resting state, we found enhanced resting-state functional connectivity between the olfactory cortex and brain regions involved in emotion.
Case Report
A 53-year-old, white, non-Hispanic woman self-referred to a clinical trial of an experimental medication for PTSD. She complained of daily intrusive memories, nightmares, and flashbacks of a traumatic event, as well as avoidance, anhedonia, and difficulty concentrating. She had no history of psychiatric treatment. Psychiatric assessment using the SCID-I2 revealed diagnoses of PTSD and major depressive disorder, and a clinical rating assessment3 revealed PTSD symptoms within the “extreme” category. The patient did not meet diagnostic criteria for any personality disorder or psychotic disorder. She was medically cleared for participation in the trial, with only mild hypertension that was treated with metoprolol.
The patient’s PTSD stemmed from witnessing her mother’s murder at age 5 years. Although the patient had no memory of the event, she experienced intense flashbacks that coincided with olfactory hallucinations. These episodes included experiencing “the overwhelming scent of blood” and lasted up to 30 minutes. These episodes were triggered by seeing someone who reminded the patient of her mother’s murderer. These particular olfactory hallucinations disappeared at age 40 years; however, the patient continued to experience additional olfactory hallucinations related to subsequent traumas (including extensive sexual abuse), specifically the smell of “stale urine” and tobacco. She denied having a generally enhanced sense of smell.
We used resting-state functional connectivity MRI (CONN software4) to study the connectivity between the olfactory cortex and areas related to emotion (inferior frontal gyri [iFG], medial frontal gyri, superior frontal gyri, anterior cingulate cortex [ACC], amygdala, and insula). As controls, we used data previously collected from 12 matched patients with depression (all white women, aged 27–51 years; six controls had comorbid anxiety disorders). We found that our patient had markedly stronger functional connectivity between the olfactory cortex and the insula, ACC, and iFG, but not in an unrelated region (supplementary motor area) compared with controls. Assuming a Gaussian distribution, we calculated the probability of obtaining these results as 0.02 for the ACC and 0.08 for the insula and iFG. To study white matter tracts, we performed diffusion tensor imaging tractography on the patient and controls (TrackVis software5). We observed a normal number of streamlines and normal fractional anisotropy in the olfactory cortex (Figure 1).
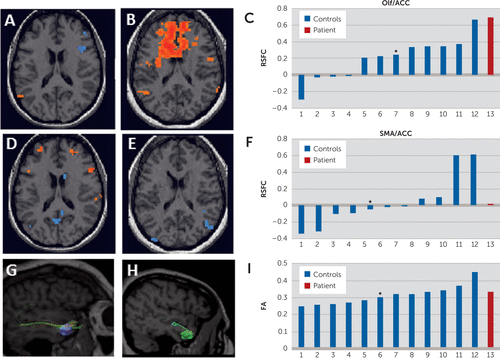
FIGURE 1. Resting-State Functional Connectivity and Diffusion Tensor Imaging of the Patient and 12 Matched Controlsa
a[A, B, D, and E] Resting-state functional connectivity (only one representative transversal section is shown) in a single control [A and D] and in the patient [B and E] when the olfactory cortex [A and B] or the supplementary motor area [D and E] was used as the seed. [C and F]: Resting-state functional connectivity between the olfactory cortex and anterior cingulate [C] and between the supplementary motor area and anterior cingulate [F] in all subjects studied. Asterisks indicate the subject that was used as an example in both [A] and [D]. [G and H]: Tractography in a single control [G] and in the patient [H] when the olfactory cortex was used as the seed. [I]: Fractional anisotropy of 12 controls (blue) and the patient (red) as measured with diffusion tensor imaging. The control shown in [G] is marked with an asterisk. In [C], [F], and [I], controls are arranged according to the size of parameter; therefore, each patient may be in a different position in each panel. ACC, anterior cingulate; DTI, diffusion tensor imaging; FA, fractional anisotropy; Olf, olfactory cortex; RSFC, resting-state functional connectivity; SMA, supplementary motor area.
Discussion
We postulate that a traumatic event at age 5 years affected the patient’s brain development, resulting in enhanced connectivity between the olfactory cortex and brain regions that integrate emotion-related information, such as the insula, iFG, and ACC. It is possible that the development of a second olfactory hallucination associated with subsequent trauma was related to an already hyperconnected olfactory cortex. Although additional subjects are needed to reach statistically significant conclusions, we did not find any overt white matter differences between the patient and matched controls, which suggests that the event-related changes may be functional and not anatomical. Further study is clearly needed to determine the relationship between olfactory hallucinations and PTSD.
1 : Co-occurrence of posttraumatic stress disorder with positive psychotic symptoms in a nationally representative sample. J Trauma Stress 2005; 18:313–322Crossref, Medline, Google Scholar
2 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN). New York, New York State Psychiatric Institute, 2002Google Scholar
3 : The development of a clinician-administered PTSD scale. J Trauma Stress 1995; 8:75–90Crossref, Medline, Google Scholar
4 : Statistical Parametric Mapping: The Analysis of Functional Brain Images. London, Elsevier/Academic Press, 2007Crossref, Google Scholar
5 : Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc Int Soc Magn Reson Med 2007; 15:36–37Google Scholar



