Outcome of Non-Invasive Brain Stimulation in Substance Use Disorders: A Review of Randomized Sham-Controlled Clinical Trials
Abstract
Non-invasive brain stimulation (NIBS) might be a new approach to treat substance use disorders (SUD). A systematic review and critical analysis was performed to identify potential therapeutic effects of NIBS on addictions. A search of the Medline database was conducted for randomized sham-controlled trials using NIBS in the field of addiction and published until August 2016. Twenty-six studies in various SUD met the inclusion criteria. Converging evidence indicates that NIBS might be a promising mean to treat patients with alcohol and tobacco use disorders, by acting on craving reduction and other mechanisms such as improvement in cognitive dysfunctions.
In the science of addiction, the concept of craving has been the key therapeutic target of numerous experiments over the past 20 years and has been integrated in several theoretical models.1,2 Although there have always been questions regarding the definition of craving and its utility, it is now a DSM-5 diagnosis criterion for defining substance use disorders (SUD).3 The recognition of craving as a clinical symptom was one of the most significant changes introduced by this manual compared with previous versions. An “intensive desire or urge” defines craving in the DSM-5 and appears as a key symptom of SUD.4 According to the Work Group, the rationale of including craving as a criterion was guided by psychometric research.5 Human brain imaging studies reporting the neural architecture of craving may have played a major role in the validation of the criterion.4,6 In addition, clinical considerations are in line with the inclusion of craving as a symptom of SUD, as it is usually assumed to promote and maintain substance dependence, to compound the severity of the addictive disorders, and to be an important risk factor for relapse, even though research on the subject has not always been conclusive.7,8
Craving is also a prime therapeutic target. Numerous medications have proved to be effective in the treatment of SUD thanks to their ability to reduce craving levels (e.g., naltrexone for alcohol, bupropion for nicotine),9 and research to find new anticraving medication is ongoing for stimulant and cannabis use disorders (e.g., disulfiram, topiramate, cannabidiol, N-acetylcysteine, etc.).10 Following the example of these medications, new noninvasive brain stimulation (NIBS) techniques have a defined immediate goal of reducing substance craving.8 The principle of NIBS is first to act focally on superficial brain regions, then secondarily on the deeper structures thanks to brain connectivity. Most NIBS studied have targeted the dorsolateral prefrontal cortex (DLPFC), as this region has been associated with the development of craving in SUD. A meta-analysis of the effects of NIBS on the DLPFC, which included 17 of these studies, provided evidence that stimulation can decrease craving levels in various SUD.8 In this meta-analysis, random effects analysis revealed a pooled standardized effect size (Hedge’s g) of 0.476, indicating a medium effect size favoring active NIBS over sham NIBS in the reduction of craving. No significant differences were found between NIBS techniques, namely repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), even though the mechanisms of action of these techniques are probably different11:
rTMS is both a neurostimulation and neuromodulation technique: a metallic coil placed against the patient’s scalp generates brief magnetic pulses that traverse the patient’s cranium to induce brief electric currents in the cortical tissue. The cortical neurons are thus depolarized and, depending on the frequency of the pulses, the excitability of the targeted cortical area is either increased or decreased: low frequency (≤ 1 Hz) rTMS reduces neuronal activity and cortical excitability, while higher frequency rTMS increases neuronal activity and cortical excitability.12
tDCS is purely a neuromodulation technique: a pair of saline-soaked surface sponge electrodes are placed on the head and directly deliver a small electrical current (usually 1–2 mA) for around 10–30 minutes to different cortical areas. The weak electrical current modulates neuronal excitability by tonic depolarization or hyperpolarization of the resting membrane potential, without inducing action potentials in axons13–15: anodal stimulation increases cortical excitability, while cathodal stimulation decreases it.16
To localize the DLPFC for rTMS, the most-often used method is the “5 cm” or “6 cm” empirical method, which involves moving the coil 5 cm or 6 cm anterior to the motor cortex. The other studies with rTMS used neuronavigation systems with three-dimensional MRI or the International 10–20 system for EEG electrode placement.17 The latter method is usually used with tDCS, by placing the sponges above F3 and F4 to be over the DLPFC, in order to act remotely on deeper structures.
Although NIBS may be perceived as a promising tool to treat SUD with craving as the main therapeutic target, this outcome raises a number of questions. Indeed, there is no consensus on the definition of craving. There are also diverse forms of craving (withdrawal or cue-elicited craving), and there is no single accepted measure of craving.1,18,19 In clinical research, cue–induced craving is most often used even though there is little evidence that this method has any clinical predictive utility: a link between cue-induced craving and a clear prediction of relapse risk or any other important index of dependence should be demonstrated.18
In addition, another important characteristic in patients with SUD is the continuous consumption of abused substances, despite a rise in negative consequences, including medical, social, and legal problems.20 This “myopia for the future” and the compulsive drug seeking as a result may involve impairment in decision-making processes.20 The DLPFC is believed to influence decision making by exerting an inhibitory influence on emotionally charged, impulsive, and/or immediately rewarding choice options.21 As the DLPFC is the classic cortical target for NIBS, it is possible that neuromodulation of this cortical region may affect decision-making processes.22,23 Improvements in decision making may explain why in some studies NIBS with both rTMS and tDCS has led to the reduced consumption of various substances despite no significant reduction in craving.24–26
In order to provide an overview of the clinical potential of NIBS techniques overall, in the present review, we set out to identify all of the clinical effects that can be observed using NIBS in SUD and behavioral addictions. Thus, contrary to previous reviews on the subject, we did not focus our research on only one clinical symptom, such as craving, or on only one NIBS technique, such as rTMS.12,27,28 Moreover, given that further studies on the subject have recently been published, it was interesting to update the results of previous reviews that included studies up to December 2013.17,29,30
Methods
This systematic review was conducted according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions, and the present report follows PRISMA [Preferred Reporting Items for Systematic Reviews and Meta-Analyses] guidelines.31,32
Search Strategy
Among biomedical bibliographic databases, MEDLINE is the largest and the most widely used in the world.33 Therefore, a comprehensive literature search was conducted on MEDLINE via the PubMed database up to 1st August 2016. All papers excluding editorials, reviews, practice guidelines, meta-analyses, and papers on infants and animals were evaluated using a set of inclusion criteria.
First, we used a free-text search of all relevant fields to retrieve the studies of interest. The text-word terms selected were based on the names of the most usual substances (and their derivatives) and behaviors encountered in the field of addiction. For the NIBS, our review focused on tDCS and rTMS, including the new form of rTMS called theta burst stimulation (TBS), as well as the application of electroconvulsive therapy (ECT), since some authors regard this nonfocal technique as NIBS.34 We thus applied a broad search strategy including the above terms and the following formula: (“electroconvulsive therapy” OR “transcranial magnetic stimulation” OR “Theta-Burst Stimulation” OR “transcranial direct current stimulation”) AND (addict* OR “alcohol” OR “nicotine” OR “tobacco” OR “smoking” OR “opiate” OR “cocaine” OR “amphetamine” OR “cannabis” OR “hallucinogen” OR “inhalant” OR “gambling” OR “Internet”). As SUD does not apply to caffeine, we did not include this substance in the present formula (DSM-5).
Secondly, we used the PubMed filter (article types, species) to select only “clinical trials” or “randomized controlled trials” involving “humans.”
Thirdly, two independent reviewers (B.T. and S.K.) read the abstracts obtained and included studies according to their contents to check inclusion criteria. In case of disagreement, a third reviewer (S.A.) made the decision after reading the title and abstract.
Finally, the full manuscripts of the selected abstracts were read in order to confirm that these studies were in line with the inclusion criteria.
Inclusion Criteria
Studies had to meet the following criteria to be included:
A clinical trial in humans
Sham-controlled trial methodology, either parallel or crossover design
With patients blinded to the treatment arm
In the field of addiction: SUD, gambling disorder, and including Internet gaming disorder, which has been proposed for inclusion in DSM-5's chapter on addictive disorders3
Using single or multiple sessions of NIBS: tDCS, rTMS, TBS, and ECT
In the clinical field (assessment of at least one clinical symptom)
In English, French, Spanish or German
As a sham-controlled condition was one of the inclusion criteria, for each of the selected studies we also determined whether the reliability of the sham conditions was tested.
Results
Sample Characteristics and Qualitative Findings
The electronic text-word search on PubMed retrieved 592 articles. In addition, one additional study not selected using PubMed filter but meeting the inclusion criteria of our review was added.35 After selecting only studies according to our selection criteria, 26 articles were identified. Among these articles, the studies used rTMS, tDCS, and TBS, but none used ECT (Figure 1).
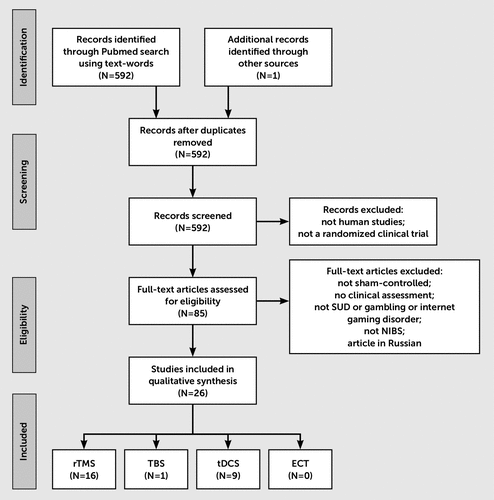
FIGURE 1. Flow Chart for the Selection Process
The cortical target for all the selected studies was the DLPFC. With tDCS, the 10–20 system was used to supposedly reach the DLPFC by placing the electrodes above F3 and F4.36
The largest studies were related to tobacco use disorders (N=16) and alcohol use disorders (N=7), with smaller numbers of studies for methamphetamine (N=2) and marijuana (N=1) and no studies for inhalant, hallucinogen, gambling, and Internet gaming disorders (Tables 1 and 2). No studies examined the concomitant effects of NIBS on two addictive disorders at the same time, although it is usual to find the consumption of two drugs together in clinical practice, especially the combined use of alcohol and tobacco. It should be noted that in the majority of these studies, the patients involved continued to consume (N=16) or had become abstinent (N=10). This means that the therapeutic results achieved in these studies suggest that NIBS may be considered either in a reduction or an abstinence strategy. It should also be noted that the number of stimulation sessions was very low for the vast majority of these identified studies.
| Study | Clinical Trial Registry (Registration Number) | Study Design | Targeted Brain Region | Stimulation Frequency/Motor Threshold | Number of Sessions/Total Pulses | Number of Patients (+Healthy Controls) | Substances (Patients’ Status) | Target Symptom (Scales) | Follow-Up | Results (Compared With Sham) |
|---|---|---|---|---|---|---|---|---|---|---|
| Trojak49 | NCT02812810* | Parallel | Right-DLPFC | 1 Hz | 10/3,600 | 37 | Tobacco (abstinent) | Relapse rates | 3 months | Reduction in the number of relapses (during stimulation period only) |
| 120% MT | Baseline craving (VAS, FTCQ–12, QSU) | Reduction in compulsivity | ||||||||
| Dieler50 | — | Parallel (add-on to CBT) | Right-DLPFC | 3 pulses/50 Hz every 200 ms 80% MT | 4/2,400 | 74 | Tobacco (abstinent) | Relapse rates | 12 months | Reduction in the number of relapses (up to 3 months) |
| Nicotine dependence (FTND) | ||||||||||
| Baseline craving (QSU) | ||||||||||
| Self-efficacy (SER) | ||||||||||
| Prikryl48 | — | Parallel | Left-DLPFC | 10 Hz | 21/42,000 | 35 | Tobacco (consumers) | Number of cigarettes smoked | 1 week | Reduction in cigarettes smoked |
| 110% MT | Schizophrenia symptoms (PANSS) | |||||||||
| Depression symptoms (MADRS, CDSS) | ||||||||||
| Dinur-Klein24 | NCT00951782* | Parallel | PFC (H-coil) bilaterally | 10 Hz or 1 Hz 120% MT | 13/12,870 or 7,800 | 115 | Tobacco (consumers) | Number of cigarettes smoked | 6 months | With 10 Hz-rTMS: |
| Response (–50%) and abstinence rates | Reduction in cigarettes smoked (up to 6 months) | |||||||||
| Nicotine dependence (FTND) | Reduction in nicotine dependence | |||||||||
| Baseline and cue-induced craving (sTCQ) | Reduction in urine cotinine levels | |||||||||
| Urine cotinine levels | Higher response and abstinence rates (up to 6 months) | |||||||||
| Pripfl35 | — | Crossover | Left-DLPFC | 10 Hz | 1/1,200 | 14 | Tobacco (consumers) | Cue-induced craving (VAS) | <1 day | Reduction in craving |
| 90% MT | Delta EEG activity (resting-state EEG) | Reduction in EEG delta power | ||||||||
| Sheffer21 | NCT00973622* | Crossover | Left-DLPFC | 10–20 Hz | 2/1,800 | 47 (+19) | Tobacco (consumers) | Decision making (DDT, RT) | 1 day (24 hours) | Decrease in delay discounting of monetary gains; increase in |
| 110% MT | Number of cigarettes smoked | discounting of monetary losses | ||||||||
| Li47 | NCT01690130* | Crossover | Left-DLPFC | 10 Hz | 1/3,000 | 16 | Tobacco (consumers) | Cue-provoked craving (adapted QSU-B) | No | Reduction in craving |
| 100% MT | ||||||||||
| Hayashi45 | — | Parallel | Left-DLPFC | 1 Hz | 1/1,800 | 10 | Tobacco (consumers) | Cue-provoked craving (VAS) | <1 day | Transient reduction in craving |
| 110% MT | ||||||||||
| Wing15 | NCT01523730* | Parallel | DLPFC bilaterally | 20 Hz | 20/30,000 | 15 schizophrenia patients | Tobacco (smokers) | Baseline cravingb (TQSU) | 9 weeks | Reduction in craving only at short-term (up to 1 week) |
| 90% MT | Withdrawal (MNWS) | |||||||||
| Abstinence (CO) | ||||||||||
| Psychiatric (PANSS) | ||||||||||
| Amiaz43 | — | Parallel | Left-DLPFC | 10 Hz | 10/20,000 | 48 | Tobacco (consumers) | Cue-induced craving (VAS, sTCQ) | 6 months | Reduction in craving during stimulation period |
| 100% MT | Nicotine dependence (FTND) | Reduction in cigarettes smoked (but not at 6 months) | ||||||||
| Number of cigarettes smoked | Reduction in nicotine dependence (but not at 6 months) | |||||||||
| Urine cotinine levels | Reduction in urine cotinine levels | |||||||||
| Johann41 | — | Crossover | Left-DLPFC | 20 Hz | 1/1,000 | 11 | Tobacco (consumer) | Baseline craving (VAS) | No | Reduction in craving |
| 90% MT | ||||||||||
| Eichhammer25 | — | Crossover | left-DLPFC | 20 Hz | 2/2,000 | 14 | Tobacco (consumer) | Number of cigarettes smoked | <1 day | Reduction in cigarettes smoked |
| 90% MT | Baseline craving (VAS) | |||||||||
| Herremans52 | — | Crossover | Right-DLPFC | 20 Hz, | 1/1,560 | 29 | Alcohol (abstinent) | Inhibitory control (Go-NoGo task) | <1 day | |
| 110% MT | Baseline craving (OCDS) | |||||||||
| Herremans66 | — | Parallel / Crossover | Right-DLPFC | 20 Hz | 1/1,560 | 36 | Alcohol (abstinent) | Baseline and habitual alcohol-related cue-induced craving (OCDS) | 1 week | |
| 110% MT | ||||||||||
| Höppner57 | — | Parallel | Left-DLPFC | 20 Hz | 10/10,000 | 19 | Alcohol (abstinent) | Baseline craving (OCDS); | No | Increase in the attentional blink effect |
| 90% MT | Depression (HAM-D, BDI) | |||||||||
| Attentional blink paradigm | ||||||||||
| Mishra42 | NCT01685463c | Parallel | Right-DLPFC | 10 Hz | 10/9,800 | 45 | Alcohol (abstinent) | Baseline craving (ACQ) | 4 weeks | Reduction in craving |
| 110% MT | ||||||||||
| Li47 | — | Crossover | Left-DLPFC | 1 Hz | 1/900 | 10 (+8) | Methamphetamine (consumers) | Cue-induced craving (VAS) | <1 day | Increase in craving |
| 100% MT |
TABLE 1. Repetitive Transcranial Magnetic Stimulation (rTMS) and Theta Burst Stimulation (TBS) in Substance-Related and Addictive Disorders: Randomized, Sham-Controlled Studies With Patients Blinded to the Treatment Arm
| Study | Clinical Trial Registry (Registration Number) | Study Design | Parameters/Electrode Positioningb | Number of Active Sessions | Number of Patients | Substances (Patients’ Status) | Target Symptoms (Measurement Tools) | Follow-Up (Long-Term Effects) | Active tDCS (Compared With Sham) |
|---|---|---|---|---|---|---|---|---|---|
| Smith40 | — | Parallel | 2 mA; 20 min Anode over F3 | 5 | 37 | Tobacco (consumers) | Number of cigarettes | 1 week | |
| Baseline and cue-induced craving (QSU, VAS) | |||||||||
| Carbon monoxide levels (CO) | |||||||||
| Fecteau26 | — | Cross-over | 2 mA; 30 min Anode over F4 | 5 | 20 | Tobacco (consumers) | Number of cigarettes (DC) | 4 days | Reduction in consumption |
| Carbon monoxide levels (CO) | Reduction in the item “desire to smoke” | ||||||||
| Cue-induced craving (QSU) | Modulation of decision-making process | ||||||||
| Decision making (UG, RT) | |||||||||
| Boggio37 | — | Parallel | 2 mA; 20 min; Anode over F3 | 5 | 27 | Tobacco (consumer) | Number of cigarettes (DC) | No | Reduction in consumption |
| Baseline and cue-induced craving (VAS) | Reduction in craving (both baseline and cue-induced) | ||||||||
| Mood (VAS) | |||||||||
| Fregni38 | — | Cross-over | 2 mA; 20 min; Anode over both F3 and F4 | 2 | 24 | Tobacco (consumer) | Baseline and cue-induced craving (VAS) | No | Reduction in craving (both baseline and cue-induced) |
| Mood (VAS) | |||||||||
| Klauss51 | NCT01330394b | Parallel | 2 mA; 2×13 min; Anode over F4 | 5 | 33 | Alcohol (abstinent) | Relapse rates | 6 months | Reduction in the number of relapses (up to 6 months) |
| Baseline craving (OCDS) | Improvement in quality of life (perception) | ||||||||
| Executive functions (FAB, MMSE) | |||||||||
| Mood (HAM-D) | |||||||||
| Anxiety (HAM-A) | |||||||||
| Quality of life (WHOQOL-BREF) | |||||||||
| Da Silva44 | — | Parallel | 2 mA; 20 min Anode over F3 | 5 | 13 | Alcohol (abstinent) | Relapse rates | 4 weeks | Reduction in craving |
| Cue-induced craving (OCDS, AUQ) | Reduction in depressive symptoms | ||||||||
| Quality of life (WHOQOL-BREF) | Increase in relapse (trend) | ||||||||
| Mood (HAM-D) | |||||||||
| Anxiety (HAM-A) | |||||||||
| Executive functions (FAB) | |||||||||
| Cognitive functions (MMSE) | |||||||||
| Boggio16 | — | Cross-over | 2 mA; 20 min Anode over both F3 and F4 | 2 | 13 | Alcohol (abstinent) | Cue-induced craving (AUQ) | No | Reduction in craving |
| Mood (VAS) | |||||||||
| Boggio22 | — | Parallel | 2 mA; 10 min Anode over both F3 or F4 | 1 | 25 | Marijuana (24-hour of abstinence) | Baseline craving (VAS) | No | Modulation of decision-making process |
| Decision making (RT) | Reduction in craving (anode F4) | ||||||||
| Shahbabaie39 | IRCT2012102311234N1c | Cross-over | 2 mA; 20 min; Anode over F4 | 1 | 32 | Methamphetamine (1 week of abstinence) | Baseline craving (VAS) and cue-induced craving (CICT) | No | Reduction in baseline craving but increase in cue-induced craving |
| Mood (PANAS) |
TABLE 2. Transcranial Direct Current Stimulation (tDCS) in Substance-Related and Addictive Disorders: Randomized, Sham-Controlled Studies With Patients Blinded to the Treatment Arma
Efficacy of NIBS to Decrease Craving
In terms of target symptoms, craving reduction was the most commonly used efficacy endpoint assessed in the studies (N=24). The effect of NIBS on craving levels was measured by comparing the scores before and after NIBS, either in the change from baseline without exposure to cues associated with a given drug (N=10), or in the change in the variation of craving level after exposure to cues (N=9). Five studies included a baseline assessment of craving followed by exposure to a drug cue designed to increase or elicit craving.24,37–40
Regarding the outcomes of the 24 studies that assessed the effect of NIBS on craving, more than half of them found a reduction in craving (N=13): five studies found a reduction in baseline craving (in state, at rest),15,25,39,41,42 six studies a reduction in cue-induced craving,16,35,43–46 and two a concomitant reduction for the two craving assessments.37,38 Studies that found no craving reduction with NIBS (N=11) used both methods to measure craving. Thus, it seems that the manner in which craving was measured did not significantly influence the results. In contrast and surprisingly, two studies in methamphetamine-dependent users found that low-frequency (LF)-rTMS over the left DLPFC and anodal tDCS over F4 increased self-reported craving during exposure to methamphetamine-related pictures.39,47
Efficacy of NIBS to Reduce Consumption or Prevent Relapse
Eight of the selected studies assessed the effects of NIBS on tobacco consumption (Tables 1 and 2). Except for Sheffer et al.’s study, which reported no significant effect of high-frequency (HF)-rTMS on cigarette consumption,21 and for Smith et al.’s study, which reported no significant effect of anodal tDCS on the number of cigarettes smoked in patients with schizophrenia,40 all of the other studies found a reduction in the number of cigarettes smoked following HF-rTMS or tDCS.24–26,37,43,48 Three studies also found a significant reduction in the number of relapses in abstinent patients with tobacco or alcohol disorders, up to 3 or 6 months after the brain stimulation according to two of these studies.49–51 All these results are very encouraging because whatever the mechanism implicated in the therapeutic effects of NIBS, reduced consumption and maintenance of abstinence are the ultimate therapeutic goals for patients with SUD.
Efficacy of NIBS to Improve Cognitive Function
In the present review, four studies examined the effects of NIBS on different tasks used to assess risk-taking impulsive behaviors and attention21,22,26,52:
Sheffer et al. assessed the effects of NIBS on delay discounting, a cognitive process that allows the individual to compare values between the immediate and delayed consumption of a determined commodity: delay discounting is the degree to which one de-values delayed outcomes.21,53,54 Its assessment is useful to study impulsive decision making associated with SUD and may explain self-control failure in addictive behavior. Using a delay discounting task, Sheffer et al. found that HF-rTMS in smokers decreased delay discounting of monetary gains, suggesting that neuromodulation of the DLPFC can enable individuals to make less impulsive and/or more future-oriented decisions.21
The risk task, a binary decision-making exercise useful to measure both impulse control and risk-taking behavior, was used in two tDCS studies.26,55 In this task, which uses colored boxes on a screen to which an amount of money has been attributed, participants must decide whether to select the unlikely option, which could generate sizeable rewards, or the likely option, which could generate modest rewards.56 In the first study, marijuana users demonstrated an increased likelihood to select the more-risky prospect during tDCS of the DLPFC, even though the stimulation significantly decreased craving for marijuana. In the second study, active tDCS did not significantly influence performance on the risk task in smokers, regardless of whether rewards were money or cigarettes.26 However, using the ultimatum game, in which subjects can accept or reject offers of money or cigarettes, the authors found that smokers were more likely to reject offers of cigarettes after they received active tDCS than after sham.26 The authors also found a significant decrease in the number of cigarettes smoked when participants received active tDCS compared with sham, suggesting that stimulation with tDCS may help smokers to control their behavior by acting on the decision-making process with cigarettes as rewards.
A Go-NoGo task was used in the latest study.52 This task is used to measure a participant's capacity for sustained attention and response control. In the Go-NoGo task, while participants see a salvo of stimuli presented in a continuous stream, they have to make a binary decision for each stimulus and press a button to sate it. Accuracy and reaction time are measured for each decision. Using this technique, Herremans et al. found in detoxified alcohol-dependent patients that active HF-rTMS compared with sham decreased intraindividual reaction time variability (dispersion of the reaction times), reflecting greater stability in attentional mechanisms.52
Höppner et al. studied the influence of rTMS on an attentional blink (AB) paradigm to emotional and alcohol-related pictures in patients with alcohol use disorder.57 The AB paradigm tests the limits of attentional capacities and the role of emotions on these, when rapid succession of visual stimuli are seen at the same spatial location on a screen. It is based on the phenomenon that, within a brief period after presentation of a first target stimulus (T1), attentional resources cannot be allocated adequately to a subsequent second target stimulus (T2), and thus the AB is lengthened. However, if T2 is emotionally relevant, the AB is reduced. In their study, Höppner et al. used alcohol-related pictures as T2 target stimuli.57 They found that real rTMS compared with sham increased the AB effect, namely that patients were less able to detect the alcohol-related pictures after the real HF rTMS session above the left DLPFC, thus suggesting that the rTMS may modulate emotional regulation and attentional abilities in SUD.
Parameters That Seem to be Less Influenced by NIBS
Among the studies included in the present review, eight assessed the effects of NIBS using rTMS or tDCS on mood and one on anxiety.16,35,37–39,44,51,57 This is relevant because compared with the general population, people addicted to drugs are roughly twice as likely to suffer from mood and anxiety disorders.58 Moreover, rTMS and tDCS may be efficacious for the treatment of depressive disorders and anxiety disorders.59–61 Da Silva et al. found significant decreases in depression scores in detoxified alcohol-dependent patients treated with tDCS. However, in all of the other studies, no changes in depression or anxiety symptoms were observed.16,37,38,48,57
Da Silva et al. and Klauss et al. performed cognitive assessments before and after tDCS in patients with alcohol use disorders, but there was no statistical difference between active and sham stimulation.44,51 In these two studies, one but not both also found an improvement in perceived quality of life.
Stimulation Parameters and Efficacy
In the majority of rTMS studies, the treatment was focused on the left-DLPFC using HF stimulation 10–20 Hz (N=8) (Table 1). This stimulation parameter seems to be particularly interesting because it leads to a large clinical effect, ranging from a decrease in craving to a reduction in consumption. We must note, however, no significant improvement in craving symptoms in patients with alcohol use disorder even though they underwent 10 HF-rTMS sessions that included up to 10,000 pulses.57 Using the other stimulation parameters for rTMS, such as LF over the left DLPFC, and HF or LF over the right DLFPC, the results are more nuanced. In fact, even when the latest studies are included, it is difficult to identify the most effective stimulation parameter, as these studies are extremely heterogeneous with regard to TMS characteristics (i.e., number of sessions, number of pulses, laterality of treatment, percentage of motor threshold). Moreover, clinical assessments vary substantially from one study to another, in terms of the number of symptoms studied, the way they were assessed, and the duration of the follow-up (Table 1).
In tDCS studies, the issues raised are similar to those found in rTMS studies (Table 1). Studies differed, for example, for the duration of the stimulation session (10–30 minutes), the number of sessions delivered (1–5 sessions), and, above all, the laterality of the anode (F3 or F4), which revealed contradictions about the outcome on craving: some studies reported no significant difference using anodal stimulation over F4,26,51 whereas those that compared anodal stimulation to F3 and F4 found either that only the latter was able to significantly reduce the craving55 or that both stimulation conditions were effective in decreasing craving.16,38
Among the 26 articles selected, only three tDCS studies tested the effectiveness of their blinding condition.26,40,51 No rTMS studies assessed the reliability of their sham condition.
Discussion
Assessment of the Performance of Craving Reduction
The measurement of craving is widely used by clinicians and researchers. This is also true for research using NIBS to treat SUD, as in the present review it was the most-often used therapeutic target and experimental outcome. In one-half to two-thirds of the studies, NIBS led to craving reduction (Tables 1 and 2). However, there are a number of issues that need to be addressed regarding the assessment of craving. First, the threshold at which a desire becomes a craving was not clearly defined in the studies selected in our review. Secondly, the time frame to gauge the craving experience varies across studies. While some investigators considered craving a relatively stable experience, others viewed it as a pulsatile state with transient urges, thus raising the question of the most appropriate moment to assess it.19 According to the second perspective, the time at which craving is measured may prove to be a critical determinant of its validity, since we may miss it by infrequent measurements.1 One way to avoid this issue is to elicit craving using experimental settings. In more than half of the studies selected for this review, participants with SUD were exposed to cues associated with drugs to elicit craving. But the natural progression of craving remains poorly understood. According to some authors, craving may be sustained for 30 minutes following exposure to cues, with a gradual decrease in intensity.62 It has been hypothesized that under certain conditions (for example, during an excessively long session of NIBS), craving intensity may actually diminish naturally over the course of a few minutes, and the decrease may be incorrectly attributed to the specific effects of NIBS.17 We tried to limit this bias in our review by selecting only randomized controlled trials with sham as the control group. Thirdly, although it is common in clinical research to assess levels of craving after exposure to cues, little is known about the value of this cue-reactivity to predict increases in addictive behavior or relapse.18,63,64 Fourthly, none of these studies took into account the severity of the SUD, although a positive correlation was reported between reduced craving and the severity of dependence, in particular in nicotine dependence.46 Fifthly, two different ways were used to assess craving in the selected articles. The first, based on single-item rating using Visual Analog Scales (VAS), was used in nearly half of the selected studies (Tables 1 and 2). Even though this method is easy to administer and score, and is suitable for frequent and repeated measurements, it does not reflect the multidimensional nature of craving. Moreover, this subjective measurement fluctuates over time and is subject to the influence of other variables.17 The second, based on multi-item self-report questionnaires, remedies this drawback by including multiple content domains, and the fact that it contains multiple items tends to boost the reliability of the scale.19 Multiple-item scales also have the advantage of being specifically suited to each type of drug. Different multi-item self-report questionnaires were used in 14 of the 23 selected studies and, in two cases, in combination with VAS.43,49 However, although multiple-item questionnaires have the advantage of providing a craving assessment by the type of drug with better sensitivity than single-item measurements, most of them have been made over the past two decades with the dominant concept at that time. They thus represent different conceptualizations of craving.19,65 The Obsessive-Compulsive Drinking Scale, which reflects compulsive characteristics of drinking-related thought and was used in three of the selected studies, is one such example.44,57,66 Moreover, using multi-item questionnaires can be disadvantageous if we posit that certain items are more accurate reflections of craving than others, since the global calculated score would be lower by including items that may prove to be a weak indicator of craving.19 In addition, the time required to complete the questionnaire should also be taken into account: long self-report craving questionnaires compared with the “immediate” response in single-item methods may exacerbate or even create a craving experience in patients deprived of a drug.19 Finally, there are numerous types of self-report craving questionnaires that differ in several parameters. It is still unknown whether a reliable correlation exists between self-report single-item rating and multi-item questionnaires to measure craving and which one is the more useful to predict improvements in the addictive behavior.19 The present review shows great diversity in the instruments used to measure craving and in the use of cues to elicit craving. On the one hand, this may be a limitation of our study because we cannot objectively compile all the results in order to draw conclusions on an overall effect of NIBS in craving reduction. On the other hand, as there is no perfect way to measure craving, the broad variety of the methods used in these studies suggests that NIBS has the potential to reduce craving in many of its dimensions and components.
Alternative Outcome to Craving Reduction
The outcomes measured in the selected studies were not limited to craving reduction. Among the other assessments, substance consumption was the most widely studied outcome, as were cognitive functions and decision-making processes. Indeed, several impaired decision-making patterns, especially risk taking and impulsivity, have been observed in patients with SUD, and they may represent one of the key symptoms of addiction.67 Furthermore, converging evidence from neuropsychological, neuroimaging, and animal studies suggests that decision-making processes are supported by a complex neural network that includes the orbitofrontal cortex, the anterior cingulate cortex, the thalamus, parietal cortices, the caudate nucleus, and more particularly, the DLPFC, identified as a key element of this network.68,69 It is uncertain whether the neural impairment of these regions identified by neuroimaging studies is a preexisting condition that led to the SUD or whether they are a consequence of repeated drug use.68 In any event, data from the selected studies indicate that both rTMS and tDCS, by modulating cortical excitability of the DLPFC, can transiently modulate processes involved in decision making for SUD.21,22,26
In the same way, a number of studies using behavioral, neurobiological, and imaging techniques have confirmed a strong association between impulsivity and addictive behaviors.70 DLPFC disruption in SUD could affect self-control and behavior-monitoring processes and may lead to impulsivity, compulsivity, risk taking, and impaired self-monitoring,71 hence the idea to act on compulsive drug taking by using NIBS with the intention to reverse activity alterations in this cortical region. For clinical studies using NIBS, delay discounting can be used to assess changes in impulsivity in patients after NIBS, as observed in Sheffer et al.’s study and in other studies that could not be included in the present review.21,72,73
How to Explain Convergent and Divergent Results?
Four SUDs were studied in the selected articles of the present review (tobacco, alcohol, methamphetamine, and marijuana). All of the studies obtained a concrete clinical response to the brain stimulation. This result is in line with our finding of common dysfunctions involving brain reward, motivation, and memory among the various SUDs. The shared mechanisms that underlie drug addictions involve the mesolimbic dopamine pathway, which includes dopaminergic neurons in the ventral tegmental area and their targets in the limbic forebrain, as well as several addiction-related brain areas that interact with this dopamine circuitry, such as the amygdala, hippocampus, hypothalamus, and several regions of the frontal cortex, the latter being the usual targets for NIBS.74 However, even though we can argue that as all drugs involved in abuse implicate similar neural mechanisms during the addiction process, and as a consequence we can expect brain stimulation to be effective in various drug addictions, we must recognize that the results obtained in NIBS studies in addictions are unclear. Indeed, therapeutic results were obtained for both rTMS and tDCS with different stimulation parameters, and sometimes these results appear contradictory (i.e., reduction in symptoms with an increase or a decrease in the excitability of the cortical target). Moreover, some results are nonreproducible from one study to another even though the stimulation parameters are similar, suggesting that other factors should be considered when interpreting findings. Among these parameters, we can evoke the accuracy of the targeting methods used, as well as intensity levels of the stimulation and, a parameter that is often ignored in NIBS, the scalp to cortex distance, which may affect the efficacy of brain stimulations.12,75,76 In addition, although the most common target for therapeutic NIBS is the DLPFC, other cortical areas, such as the dorsal anterior cingulate cortex and the insula, may be of interest for SUD and potentially accessible to NIBS. Specific cell types of the cortico-striatal circuitry could also be targeted in future research.77,78
Independently of the stimulation parameters and the methods used to assess the outcomes, stages of addiction also deserve to be considered in NIBS studies. Indeed, the development of addiction can be perceived as a succession of neuroadaptive changes in the brain: it begins with changes in the mesolimbic dopamine system and continues with a cascade of neuroadaptations from the ventral striatum to the dorsal striatum and orbitofrontal cortex and eventually dysregulation of the prefrontal cortex, cingulate gyrus, and extended amygdala.79 As the neurobiology varies during the addiction process, the impact of NIBS on the brain may also vary over time, depending on the underlying brain changes at the moment of the treatment. We can suggest this hypothesis to explain the various results with NIBS in addiction.
Limitations
Some caution is warranted in interpreting the results of this review. First, we limited our review by selecting only randomized controlled trials in order to have the highest level of evidence. However, numerous studies with relevant results in subjects have been done, even though the level of evidence was lower. Secondly, a large majority of the selected studies were preliminary, with small sample sizes, without testing their sham condition and without follow-up of the patients, and there was considerable heterogeneity in terms of sample population, study design, TMS or tDCS parameters, and outcome measurements. This field is still recent, and most studies delivered a small number of NIBS sessions, since 12 of the 21 selected studies estimated the efficacy of NIBS from only one or two stimulation sessions with both rTMS and tDCS, which encouragingly showed no serious adverse events. Future studies should now include a greater number of NIBS sessions in order to induce greater and more sustained changes in brain activity to reduce craving and consumption and to improve cognitive functions. Thirdly, although NIBS has been studied in different SUDs, not all types of addiction were taken into account.
Conclusions
Interesting results using NIBS were obtained to treat patients with tobacco and alcohol use disorders. Converging evidence indicates that craving reduction with NIBS is an objective of interest, with the exception of methamphetamine use disorders. Other therapeutic goals need to be explored, especially substance consumption and cognitive functions. Indeed, craving is not the only component that leads to the development, continuation, and relapse in addictive disorders. Risk taking and impulsive behavior are also implicated in the history of SUD, and neuromodulation of the DLPFC using NIBS may assist individuals with SUD by making them more future-oriented toward their goal to quit, or to reduce their consumption as has been observed in some studies.
1 : What does cue-reactivity have to offer clinical research? Addiction 2000; 95(Suppl 2):S129–S144Crossref, Medline, Google Scholar
2 : The clinical neurobiology of drug craving. Curr Opin Neurobiol 2013; 23:649–654Crossref, Medline, Google Scholar
3 : Desk Reference to the Diagnostic Criteria From DSM-5. Washington, DC, American Psychiatric Publishing, 2013,Google Scholar
4 : Neuroimaging craving: urge intensity matters. Addiction 2015; 110:195–203Crossref, Medline, Google Scholar
5 : Commentary: craving diagnostic validity in DSM-5 substance use disorders. J Am Acad Psychiatry Law 2014; 42:453–458Medline, Google Scholar
6 : Performance of a craving criterion in DSM alcohol use disorders. J Stud Alcohol Drugs 2010; 71:674–684Crossref, Medline, Google Scholar
7 : The craving withdrawal model for alcoholism: towards the DSM-V. Improving the discriminant validity of alcohol use disorder diagnosis. Alcohol Alcohol 2005; 40:314–322Crossref, Medline, Google Scholar
8 : Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci Biobehav Rev 2013; 37:2472–2480Crossref, Medline, Google Scholar
9 : Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry 2005; 162:1423–1431Crossref, Medline, Google Scholar
10 : Pharmacological treatments for drug misuse and dependence. Expert Opin Pharmacother 2015; 16:325–333Crossref, Medline, Google Scholar
11 : Neurostimulation, neuromodulation, and the treatment of epilepsies. J Epileptol 2015. http://www.degruyter.com/view/j/joepi.2015.23.issue-1/joepi-2015-0022/joepi-2015-0022.xmlGoogle Scholar
12 : Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci 2014; 1327:79–93Medline, Google Scholar
13 : Action mechanisms of transcranial direct current stimulation in Alzheimer’s disease and memory loss. Front Psychiatry 2012; 3:48Crossref, Medline, Google Scholar
14 : Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimulat 2009; 2:241–245Crossref, Medline, Google Scholar
15 : High frequency repetitive transcranial magnetic stimulation reduces tobacco craving in schizophrenia. Schizophr Res 2012; 139:264–266Crossref, Medline, Google Scholar
16 : Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend 2008; 92:55–60Crossref, Medline, Google Scholar
17 : The use of repetitive transcranial magnetic stimulation for modulating craving and addictive behaviours: a critical literature review of efficacy, technical and methodological considerations. Neurosci Biobehav Rev 2014; 47:592–613Crossref, Medline, Google Scholar
18 : Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction 2009; 104:1610–1616Crossref, Medline, Google Scholar
19 : The measurement of drug craving. Addiction 2000; 95(Suppl 2):S189–S210Crossref, Medline, Google Scholar
20 : Emotion, decision-making and substance dependence: a somatic-marker model of addiction. Curr Neuropharmacol 2006; 4:17–31Crossref, Medline, Google Scholar
21 : Neuromodulation of delay discounting, the reflection effect, and cigarette consumption. J Subst Abuse Treat 2013; 45:206–214Crossref, Medline, Google Scholar
22 : Modulation of decision-making in a gambling task in older adults with transcranial direct current stimulation. Eur J Neurosci 2010; 31:593–597Crossref, Medline, Google Scholar
23 : Neuromodulation of decision-making in the addictive brain. Subst Use Misuse 2010; 45:1766–1786Crossref, Medline, Google Scholar
24 : Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry 2014; 76:742–749Crossref, Medline, Google Scholar
25 : High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J Clin Psychiatry 2003; 64:951–953Crossref, Medline, Google Scholar
26 : Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study. Drug Alcohol Depend 2014; 140:78–84Crossref, Medline, Google Scholar
27 : rTMS in the treatment of drug addiction: an update about human studies. Behav Neurol 2014; 2014:815215Crossref, Medline, Google Scholar
28 : Noninvasive brain stimulation to suppress craving in substance use disorders: Review of human evidence and methodological considerations for future work. Neurosci Biobehav Rev 2015; 59:184–200Crossref, Medline, Google Scholar
29 : Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev 2010; 34:559–574Crossref, Medline, Google Scholar
30 : Brain stimulation methods to treat tobacco addiction. Brain Stimulat 2013; 6:221–230Crossref, Medline, Google Scholar
31 Higgins J, Green S: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (online edition). Cochrane Collab 2011. www.cochrane-handbook.orgGoogle Scholar
32 : Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg 2010; 8:336–341Crossref, Medline, Google Scholar
33 : Comparison of medical subject headings and text-word searches in MEDLINE to retrieve studies on sleep in healthy individuals. J Med Libr Assoc 2004; 92:349–353Medline, Google Scholar
34 : Non-invasive brain stimulation therapy in multiple sclerosis: a review of tDCS, rTMS and ECT results. Brain Stimulat 2014; 7:849–854Crossref, Medline, Google Scholar
35 : Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimulat 2014; 7:226–233Crossref, Medline, Google Scholar
36 : Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr 2003; 16:95–99Crossref, Medline, Google Scholar
37 : Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett 2009; 463:82–86Crossref, Medline, Google Scholar
38 : Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry 2008; 69:32–40Crossref, Medline, Google Scholar
39 : State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int J Neuropsychopharmacol 2014; 17:1591–1598Crossref, Medline, Google Scholar
40 : Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: A randomized controlled study. Schizophr Res 2015; 168:260–266Crossref, Medline, Google Scholar
41 : Transcranial magnetic stimulation for nicotine dependence. Psychiatr Prax 2003; 30(Suppl 2):S129–S131Crossref, Medline, Google Scholar
42 : Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction 2010; 105:49–55Crossref, Medline, Google Scholar
43 : Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 2009; 104:653–660Crossref, Medline, Google Scholar
44 : Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol Paris 2013; 107:493–502Crossref, Medline, Google Scholar
45 : Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc Natl Acad Sci USA 2013; 110:4422–4427Crossref, Medline, Google Scholar
46 : Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry 2013; 73:714–720Crossref, Medline, Google Scholar
47 : Low frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex transiently increases cue-induced craving for methamphetamine: a preliminary study. Drug Alcohol Depend 2013; 133:641–646Crossref, Medline, Google Scholar
48 : Repetitive transcranial magnetic stimulation reduces cigarette consumption in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry 2014; 49:30–35Crossref, Medline, Google Scholar
49 : Transcranial magnetic stimulation combined with nicotine replacement therapy for smoking cessation: a randomized controlled trial. Brain Stimulat 2015; 8:1168–1174Crossref, Medline, Google Scholar
50 : Can intermittent theta burst stimulation as add-on to psychotherapy improve nicotine abstinence? Results from a pilot study. Eur Addict Res 2014; 20:248–253Crossref, Medline, Google Scholar
51 : A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int J Neuropsychopharmacol 2014; 17:1793–1803Crossref, Medline, Google Scholar
52 : Reduced intra-individual reaction time variability during a Go-NoGo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol 2013; 48:552–557Crossref, Google Scholar
53 : Delay discounting: Concepts and measures. Psychol Neurosci 2012; 5:135–146Crossref, Google Scholar
54 : Frames of mind in intertemporal choice. Manage Sci 1988; 34:200–2014Crossref, Google Scholar
55 : Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend 2010; 112:220–225Crossref, Medline, Google Scholar
56 : Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 1999; 19:9029–9038Crossref, Medline, Google Scholar
57 : Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J Biol Psychiatry 2011; 12(Suppl 1):57–62Crossref, Medline, Google Scholar
58 : Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 2006; 67:247–257Crossref, Medline, Google Scholar
59 : Durability of the antidepressant effect of the high-frequency repetitive transcranial magnetic stimulation (rTMS) In the absence of maintenance treatment in major depression: a systematic review and meta-analysis of 16 double-blind, randomized, sham-controlled trials. Depress Anxiety 2015; 32:193–203Crossref, Medline, Google Scholar
60 : Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014; 125:2150–2206Crossref, Medline, Google Scholar
61 : Transcranial direct current stimulation (tDCS) in the treatment of depression: Systematic review and meta-analysis of efficacy and tolerability. Neurosci Biobehav Rev 2015; 57:46–62Crossref, Medline, Google Scholar
62 : Prolonged duration of craving, mood, and autonomic responses elicited by cues and imagery in smokers: Effects of tobacco deprivation and sex. Exp Clin Psychopharmacol 2010; 18:245–256Crossref, Medline, Google Scholar
63 : Laboratory-based, cue-elicited craving and cue reactivity as predictors of naturally occurring smoking behavior. Addict Behav 2009; 34:536–541Crossref, Medline, Google Scholar
64 : Examining the relationship between cue-induced craving and actual smoking. Exp Clin Psychopharmacol 2015; 23:90–96Crossref, Medline, Google Scholar
65 : Clinical and laboratory assessment of the subjective experience of drug craving. Clin Psychol Rev 2009; 29:519–534Crossref, Medline, Google Scholar
66 : No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: results of a naturalistic study. Drug Alcohol Depend 2012; 120:209–213Crossref, Medline, Google Scholar
67 : Decision-making for risky gains and losses among college students with Internet gaming disorder. PLoS One 2015; 10:e0116471Medline, Google Scholar
68 : Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry 2005; 58:597–604Crossref, Medline, Google Scholar
69 : Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage 2006; 32:477–484Crossref, Medline, Google Scholar
70 : The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008; 200:1–26Crossref, Medline, Google Scholar
71 : Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 2011; 12:652–669Crossref, Medline, Google Scholar
72 : Investing in the future: stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacology 2015; 40:546–553Crossref, Medline, Google Scholar
73 : Effect of continuous theta burst stimulation of the right dorsolateral prefrontal cortex on cerebral blood flow changes during decision making. Brain Stimulat 2012; 5:116–123Crossref, Medline, Google Scholar
74 : Is there a common molecular pathway for addiction? Nat Neurosci 2005; 8:1445–1449Crossref, Medline, Google Scholar
75 : A comprehensive study of sensorimotor cortex excitability in chronic cocaine users: Integrating TMS and functional MRI data. Drug Alcohol Depend 2015; 157:28–35Crossref, Medline, Google Scholar
76 : Should we consider the depth of the cortex for the use of rTMS? J Neuropsychiatry Clin Neurosci 2012; 24:E3–E4Link, Google Scholar
77 : The Neural Crossroads of Psychiatric Illness: An Emerging Target for Brain Stimulation. Trends Cogn Sci 2016; 20:107–120Crossref, Medline, Google Scholar
78 : Cortico-striatal circuits: Novel therapeutic targets for substance use disorders. Brain Res 2015; 1628(Pt A):186–198Crossref, Medline, Google Scholar
79 : Neurocircuitry of addiction. Neuropsychopharmacology 2010; 35:217–238Crossref, Medline, Google Scholar



