Guanfacine Attenuates Adverse Effects of Dronabinol (THC) on Working Memory in Adolescent-Onset Heavy Cannabis Users: A Pilot Study
Abstract
The cannabinoid-1 receptor (CB1R) agonist Δ9-tetrahydrocannabinol (THC), the main psychoactive constituent of cannabis, adversely effects working memory performance in humans. The α2A-adrenoceptor (AR) agonist guanfacine improves working memory performance in humans. The authors aimed to determine the effects of short-term (6 days) treatment with guanfacine on adverse cognitive effects produced by THC. Employing a double-blind, placebo-controlled crossover design, the cognitive, subjective, and cardiovascular effects produced by oral THC (20 mg) administration were determined twice in the same cannabis users: once after treatment with placebo and once after treatment with guanfacine (3 mg/day). Compared with performance at baseline, THC negatively affected accuracy on spatial working memory trials while participants were maintained on placebo (p=0.012) but not guanfacine (p=0.497); compared with placebo, accuracy was significantly (p=0.003, Cohen’s d=–0.640) improved while individuals were treated with guanfacine. Similarly, compared with baseline, THC increased omission errors on an attentional task while participants were maintained on placebo (p=0.017) but not on guanfacine (p=0.709); compared with placebo, there were significantly (p=0.034, Cohen’s d=0.838) fewer omissions while individuals were maintained on guanfacine. Although THC increased visual analog scores of subjective effects and heart rate, these increases were similar during treatment with placebo and guanfacine. THC did not significantly affect performance of a recognition memory task or blood pressure while individuals were maintained on either treatment. Although preliminary, these results suggest that guanfacine warrants further testing as a potential treatment for cannabis-induced cognitive deficits.
According to national estimates, the proportion of Americans reporting current cannabis use,1 and the proportion seeking treatment for primary cannabis use,2 are both increasing. Despite these trends, there are no U.S. Food and Drug Administration (FDA)-approved medications for treating cannabis use disorders (CBUD). Medication development efforts have primarily focused on attenuation of cannabis withdrawal symptoms. Many theories of addictions, however, also suggest that by disrupting executive control functions of the prefrontal cortex (PFC), repeated drug use can produce behavioral changes that ultimately underlie the difficulty that many individuals have in resisting habitual drug use once established.3 Consistent with this, among cannabis users, impairments of PFC functions have been shown to predict poor treatment outcomes in pharmacotherapy4 and psychotherapy5,6 studies. In fact, numerous epidemiological studies support the idea that cannabis use onset at an early age increases risk for the later development of cannabis use problems.7–10
An age-dependent, dose-response relationship exists between prior cannabis use and impaired executive functions.11 For example, longitudinal12–14 and cross-sectional studies15 suggest that in comparison with adult-onset users, adolescent-onset cannabis users exhibit more severe and more persistent cognitive impairments. Neuroanatomical and neurophysiological studies in adolescent-onset cannabis users,16–19 and well-controlled studies in adolescent monkeys,20,21 provide additional evidence that the still-maturing PFC22–24 is particularly vulnerable to the adverse effects produced by cannabis. Furthermore, that the majority (81%) of admissions to substance abuse treatment services for primary cannabis use initiated by age 162 also supports the hypothesis that cannabis use during the protracted developmental period of adolescence increases risk for the later development of cannabis use problems by disrupting normal maturation of the PFC. These studies collectively support the idea that normalizing PFC-mediated functions affected by cannabis is a reasonable pharmacological strategy for improving CBUD treatment outcomes.
While cannabinoid-1 receptors colocalize with,25 and cause functional desensitization of, α-2A adrenoceptors26,27 in PFC, stimulation of postsynaptic α-2A adrenoceptors normalizes PFC functions.28 Guanfacine, which preferentially stimulates α-2A receptors in PFC,29 has been shown to improve executive functions in healthy volunteers,30 individuals with schizophrenia,31 and children with attention-deficit hyperactivity disorder.32 Medications targeting α-2A receptors have been proposed as a promising pharmacotherapy target for addiction, not only because of beneficial effects on cognitive functioning related to drug use behavior but also because of the ability of this medication class to decrease noradrenergic hyperactivity and symptomatology associated with cannabis withdrawal and relapse.33–39 We therefore conducted a double-blind, placebo-controlled crossover pilot study to examine the effects of short-term treatment with guanfacine on cognitive task performance following acute administrations of ∆-9-tetrahydrocannabiol (THC) and on laboratory-induced withdrawal in adolescent-onset, cannabis-dependent individuals (withdrawal data will be published separately from the data presented in this study). We focused here on evaluating the influence of guanfacine on cognitive processes that are adversely affected by acute THC administration and hypothesized that guanfacine would attenuate THC-induced adverse effects on cognitive functions mediated by the PFC.
Methods
This double-blind, placebo-controlled crossover study was approved by the Baylor College of Medicine and the Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) institutional review boards. Additionally, this material is the result of work supported with resources from, and the use of facilities at, the MEDVAMC (Houston, Tex.). All volunteers provided written informed consent after being apprised of the potential risks of study participation.
Participants
Recruited through advertisements, participants were nontreatment-seeking, English-speaking volunteers between 18- and 55-years-old. Study volunteers self-reported a history of using cannabis prior to age 18, provided urine samples that were positive for carboxy-THC (but negative for other illicit drugs), and met criteria for CBUD. Potential participants were excluded if they had a history of seizure disorder, head trauma, drug dependence (except nicotine), adverse reactions to study medications, psychoactive medication use, or other axis I psychiatric disorders. Serious medical conditions, including heart disease, symptomatic HIV-related disease, and asthma were exclusionary. Women were excluded if they were pregnant, breastfeeding, or not using a reliable form of birth control. Participants were paid for their participation.
General Procedures
The FDA approved an investigational new drug application for the use of dronabinol and guanfacine in this study. Participants were housed on the Research Commons at the MEDVAMC during each of the two 8-day study periods. Participants completed identical procedures during both study periods, which were separated by 2–4 weeks. On the day of admission (day 1) at approximately 1400 hours, participants completed the cognitive battery. On day 2, participants received 10-mg THC capsules at 0900, 1100, and 1300 hours, to standardize final THC exposure before the onset of abstinence39 and allow for comparative assessment of withdrawal data during abstinence. On days 3–8, participants received either guanfacine or matching placebo (masked by encapsulation) with the order counterbalanced across participants. Guanfacine was titrated up by 1 mg per day; hence, participants received 1 mg on day 3, 2 mg on day 4, and 3 mg on days 5–8. On day 7, THC (0, 10, and 20 mg) capsules were administered, single-blinded, in ascending order at 0900, 1100, and 1300 hours; participants completed the cognitive battery, 1 hour after the 20-mg dose. Figure 1 provides an overview of the crossover design and timeline of day 7 events.
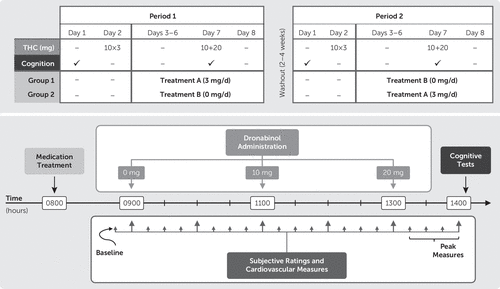
FIGURE 1. Overview of the Crossover Study Design and Timing of Day 7 Study Events
Cognitive Tests
Participants completed the revised Hopkins Verbal Learning Test (HVLT), the dual n-back task, and the Continuous Performance Test-II (CPT), as previously described.40,41 Standardized instructions were provided, both oral and written, prior to administration, and participants were instructed to respond as quickly and as accurately as possible.
HVLT.
Immediate recall, short-term learning, and delayed free recall were measured with the HVLT.42 For this study, after the three consecutive, immediate free-recall trials of a 12-item, semantically categorized list, the dual n-back test was completed. Following the dual n-back task (approximately 25 minutes), the delayed free-recall trials of the HVLT were completed. To minimize practice effects, we administered six different but parallel versions of the HVLT.
Dual n-back.
Working memory was measured using the dual n-back task.43 In this task, spatial locations were cued successively at eight different locations on a computer monitor at a frequency of one cue per 3 seconds (stimulus duration of 500 ms; interstimulus interval of 2500 ms). Simultaneously, auditory cues (one of eight consonants) were presented through headphones, in sequence with the presentation of spatial locations. There were 20 blocks, of 20 trials each, presented over approximately 25 minutes. Responses, made on a standard keyboard, were required whenever one or both stimuli matched a stimulus presented n-positions back in the sequence. No responses were required for nonmatching stimuli. The level of difficulty (i.e., n-value) was always the same for both stimulus modalities. The n-value varied per an individual’s performance after each block of 20 trials.44 That is, performance was determined after each 20-trial block by the program, and the n-value was adjusted per the following criteria: (1)if a participant responded correctly on ≥85% of the trials, the n-value increased by 1; (2)if a participant responded correctly on ≤70% of the trials, the n-value decreased by 1; otherwise, (3) the n-value remained unchanged.
CPT.
Following the delayed free-recall trials of the HVLT, we measured vigilance, distractibility, and impulsivity using the CPT.45 In this task, participants attended to letters presented (250 ms) sequentially on a monitor at intervals of 1, 2, and 4 seconds. Participants were instructed to press the space bar on a standard keyboard as quickly as possible after each letter, except when the letter “X” appeared.
Subjective and Cardiovascular Effects
Participants completed Visual Analog Scale (VAS) forms,46–48 modified for cannabis, to rate the subjective effects produced by THC. VAS ratings, which were measured on a continuous scale digitized between 0 (not at all) and 100 (strongest ever), included ratings of “anxious,” “any drug effect,” “bad effects,” “depressed,” “desire (for cannabis),” “drug liking,” “good effects,” “high,” “likely to use (cannabis if accessible),” and “stimulated.” VAS ratings and cardiovascular measures (i.e., heart rate and blood pressure) were collected before and at 15-minute intervals for 1 hour after THC administrations.
Data Analyses
Cognitive data collected on day 1, and VAS and cardiovascular data collected 15 minutes before administration of the first THC dose on day 2, were averaged across the two treatment periods to establish a baseline for each participant before analyses. Data were subjected to repeated- measures analyses of variance (ANOVAs) with planned comparisons to contrast the effects of THC during treatment with placebo and guanfacine. To compare the means of each treatment on the effects produced by THC, we conducted pairwise multiple comparisons using Fisher’s least significant difference test.
For the HVLT, ANOVAs were calculated on 1) total recall (the total number of words recalled correctly on learning trials 1–3); 2) learning slope (the average number of new correct words recalled per trial); 3) delayed recall (the number of words correctly recalled on the delayed recall trial); and 4) percent retained (delayed-recall score divided by the higher score from trial 2 or 3). For the dual n-back task, ANOVAs were calculated on 1) auditory and 2) visual trial accuracies; 3) auditory and 4) visual trial reaction times (RTs); and 5) mean and 6) maximum n-levels achieved. For the CPT, ANOVAs were calculated on errors of 1) commission, 2) omission, and 3) perseveration, and 4) RT. For VAS ratings and cardiovascular data, ANOVAs were calculated on maximum or peak ratings and measures. Data are presented as means±standard deviations, except as displayed in graphic illustrations in which data are presented as means±standard errors. Cohen’s d effect sizes, to compare the means of each treatment on the effects produced by THC, are included for significant findings. Statistical significance was set at p<0.05, and analyses were conducted with SigmaPlot 12.0 software.
Results
Participants
Forty subjects completed the in-person screen, of whom 20 were ineligible and nine never initiated the study. Eleven subjects, all of whom reported ongoing cannabis use (confirmed by urine toxicology) since before the age of 18, initiated at least one study day. One subject dropped out prior to randomization. Ten subjects completed at least one study period, two subjects never returned to complete the second study period, and one subject was discharged on day 6 of the second period. Analyses were calculated with data from the seven completers.
Demographic Data
On average, the seven study completers were 35 (±12.5) years old with 11.1 (±3.3) years of education. On average, participants reported initiating cannabis use when they were 13.4 (±2.0) years old, using cannabis 8.0 (±5.7) times per day, on 27.9 (±0.4) of the last 28 days. Demographic and drug use means, standard deviations, and ranges are provided in Table 1.
| Characteristic | Means±SD (Range) or N |
|---|---|
| Demographic | |
| Age (years) | 35.0±12.5 (22–55) |
| Education (years) | 11.1±3.3 (4–14) |
| Cannabis use | |
| Years of use | 21.6±13.9 (5–44) |
| Age at onset of use | 13.4±2.0 (11–17) |
| Days used of last 28 | 27.9±0.4 (27–28) |
| Times per day | 8.0±5.7 (2–20) |
| Grams per week | 25.0±16.6 (7–56) |
| Nicotine use | |
| Current user | N=4 |
| Years of use | 14.4±18.1 (0–44) |
| FTND scorea | 6.4±2.3 (0–9.5) |
| Cigarettes per day | 9.0±10.7 (0–25) |
| Alcohol use | |
| Current user | N=4 |
| Years of use | 11.1±12.6 (0–35) |
| Days used of last 28 | 3.7±5.1 (0–12) |
| Drinks per day | 1.0±1.2 (0–3) |
TABLE 1. Demographic Data and Drug Use
Cognition
HVLT.
ANOVAs revealed nonsignificant effects of THC and guanfacine on total recall (p=0.246), learning slope (p=0.550), delayed recall (p=0.802), and percentage retained (p=0.491). Planned comparisons also revealed nonsignificant (p≥0.145) pairwise differences on the HVLT.
Dual n-back.
As is displayed in Figure 2, there was a significant (F[2,12]=4.206, p=0.041) effect of treatment on auditory accuracy; post hoc comparisons revealed that THC association with (p=0.054) decreased auditory accuracy fell short of statistical significance, though guanfacine significantly (p=0.017; Cohen’s d=–0.743) improved THC-induced adverse effects on accuracy. There was also a significant (F[2,12]=7.487, p=0.008) effect of treatment on spatial accuracy; post hoc comparisons revealed that THC significantly (p=0.012) decreased accuracy, and guanfacine significantly (p=0.003; Cohen’s d=–0.640) improved THC-induced adverse effects. There were nonsignificant (p≥0.138) effects of treatment and nonsignificant (p≥0.081) pairwise differences on RTs for both auditory and spatial trials. Similarly, there were nonsignificant (p≥0.949) effects of treatment and nonsignificant (p≥0.752) pairwise differences on mean and maximum n-levels achieved.
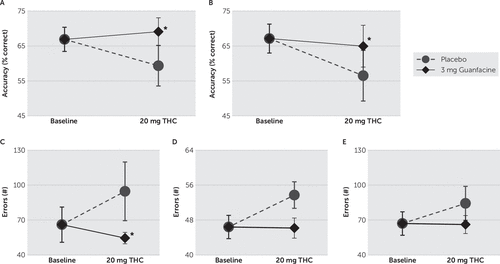
FIGURE 2. Dual N-Back Task-Measured Auditory and Spatial Working-Memory Accuracy and Continuous Performance Test-Measured Omission, Commission, and Perseveration Errorsa
a A) Auditory and B) spatial working-memory accuracy measured by the dual n-back task. ∆-9-Tetrahydrocannabiol (THC) impaired spatial working memory, while guanfacine improved both spatial (p=0.003, Cohen’s d=–0.640) and auditory (p=0.017, Cohen’s d=–0.743) working memory compared with placebo. C) Omission, D) commission, and E) perseveration errors were measured using the Continuous Performance Test-II. THC increased omission errors, while guanfacine reduced the number of THC-induced omissions (p=0.034, Cohen’s d=0.838). Means±standard errors.
CPT.
As displayed in Figure 2, there was a significant (F[2,12]=4.518; p=0.034) effect of treatment on errors of omission, but not on errors of commission (p=0.110) or perseveration (p=0.151). Post hoc comparisons demonstrated that THC significantly (p=0.017) increased errors of omission, while guanfacine significantly (p=0.034; Cohen’s d=0.838) reduced the number of THC-induced omission errors. Planned comparisons revealed nonsignificant pairwise differences on errors of commission (p≥0.065) and perseveration (p≥0.085). There was a nonsignificant (p=0.908) effect of treatment, and nonsignificant (p≥0.676) pairwise differences, on RT.
Cognitive data for HVLT, dual n-back, and CPT are presented in Table 2 as z-transformed scores, along with Cohen’s d values. Accuracy and RTs on visual and auditory tasks are shown for the dual n-back task in Table 3. Participants’ omissions, commissions, perseverations, and RTs on the CPT are summarized in Table 4.
| Participant | Short-Term Recall | Delayed Recall | Learning Slope | Percent Retained | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | PLB | GFC | BL | PLB | GFC | BL | PLB | GFC | BL | PLB | GFC | |
| 2 | 0.91 | 2.18 | –0.03 | –0.14 | 1.94 | 0.58 | 1.35 | 0.33 | 0.46 | –0.85 | 0.97 | 0.59 |
| 3 | –1.74 | –0.63 | –1.19 | –1.54 | –0.12 | –1.30 | –1.28 | –0.13 | –0.42 | –1.26 | 0.97 | –1.29 |
| 10 | 0.17 | –0.23 | –0.81 | 0.06 | –0.53 | –0.67 | 1.09 | 1.02 | 0.17 | –0.40 | –0.71 | –0.75 |
| 16 | 0.80 | –0.03 | 1.33 | 0.65 | 0.29 | 1.21 | 0.30 | –0.82 | –1.01 | 0.13 | 0.41 | 1.64 |
| 19 | 0.17 | –0.83 | –0.03 | 0.45 | –1.35 | –0.36 | –0.49 | 1.02 | 0.46 | 0.67 | –1.82 | –0.59 |
| 29 | 0.69 | –0.23 | 1.33 | 1.45 | –0.12 | 1.21 | 0.04 | –1.74 | 1.63 | 1.75 | 0.34 | 0.59 |
| 30 | –1.00 | –0.23 | –0.61 | –0.94 | –0.12 | –0.67 | –1.01 | 0.33 | –1.30 | –0.03 | –0.15 | –0.19 |
| PLB vs. GFC | Cohen’s d=0.595 | Cohen’s d=0.051 | Cohen’s d=–0.326 | Cohen’s d=0.551 | ||||||||
TABLE 2. Z-Transformed Scores and Cohen's d Effect Sizes for the Hopkins Verbal Learning Taska
| Participant | Accuracy | Reaction Time | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Auditory | Visual | Auditory | Visual | Block Average | Block Maximum | ||||||||||||||
| BL | PLB | GFC | BL | PLB | GFC | BL | PLB | GFC | BL | PLB | GFC | BL | PLB | GFC | BL | PLB | GFC | ||
| 2 | –0.36 | 0.39 | 0.07 | –1.02 | –0.05 | –0.25 | –1.40 | –0.53 | –0.99 | –0.81 | –0.68 | –1.71 | 0.61 | 0.72 | 0.17 | –0.12 | 0.54 | 0.44 | |
| 3 | –1.99 | –1.93 | –1.92 | –1.50 | –1.95 | –2.10 | –0.35 | –0.96 | 1.91 | 1.93 | 1.79 | –0.48 | –0.77 | –1.17 | –1.15 | –0.12 | –0.73 | –1.61 | |
| 10 | –0.19 | –0.52 | 1.01 | 0.30 | 0.02 | 0.30 | 1.32 | 2.03 | 0.51 | 0.12 | 0.92 | 1.25 | –0.11 | –1.00 | –0.79 | –0.12 | –0.73 | –0.59 | |
| 16 | 0.80 | 0.69 | 1.03 | 0.05 | 0.58 | 0.92 | –0.34 | 0.33 | –0.77 | –0.87 | –0.29 | –0.44 | 1.41 | 1.59 | 1.93 | 1.53 | 1.82 | 1.46 | |
| 19 | 0.37 | 1.14 | 0.09 | 1.57 | 1.09 | 0.07 | 1.35 | –0.17 | 0.27 | 0.29 | –0.45 | 0.83 | –1.42 | –0.43 | –0.49 | –1.76 | –0.73 | –0.59 | |
| 29 | 0.41 | 0.00 | –0.44 | 0.26 | 0.70 | 0.48 | –0.12 | 0.03 | –0.44 | 0.20 | –0.20 | –0.05 | 0.83 | 0.56 | 0.17 | 0.71 | 0.54 | 0.44 | |
| 30 | 0.95 | 0.24 | 0.18 | 0.34 | –0.39 | 0.59 | –0.47 | –0.73 | –0.50 | –0.86 | –1.08 | 0.62 | –0.55 | –0.27 | 0.17 | –0.12 | –0.73 | 0.44 | |
| PLB vs. GFC | Cohen’s d=–0.743 | Cohen’s d=–0.640 | Cohen’s d=–0.766 | Cohen’s d=0.098 | Cohen’s d=–0.037 | Cohen’s d=0.000 | |||||||||||||
TABLE 3. Dual N-Back Taska
| Participant | Omissions | Commissions | Perseverations | Reaction Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | PLB | GFC | BL | PLB | GFC | BL | PLB | GFC | BL | PLB | GFC | |
| 2 | –0.59 | 0.01 | –0.82 | –1.00 | 0.29 | –0.49 | –0.84 | –0.53 | –0.74 | –1.35 | –0.14 | –0.16 |
| 3 | 2.23 | 2.19 | 0.33 | 1.89 | –0.40 | 0.96 | 1.98 | 1.58 | 2.10 | 0.94 | 1.36 | –0.67 |
| 10 | 0.02 | –0.22 | 1.97 | –0.55 | –1.15 | –0.28 | 0.15 | –0.53 | –0.12 | 0.64 | 0.79 | 1.12 |
| 16 | –0.48 | –0.78 | –0.61 | –0.20 | 1.76 | –1.44 | –0.79 | 0.03 | –0.99 | –0.89 | –1.39 | –0.56 |
| 19 | –0.39 | –0.19 | –0.70 | –0.85 | –0.65 | –0.76 | 0.39 | 1.19 | 0.04 | 0.49 | 0.71 | 1.31 |
| 29 | –0.35 | –0.49 | 0.39 | 0.58 | –0.59 | 1.22 | –0.14 | –0.76 | –0.14 | 1.05 | –0.37 | 0.41 |
| 30 | –0.44 | –0.53 | –0.56 | 0.14 | 0.75 | 0.79 | –0.75 | –0.97 | –0.14 | –0.88 | –0.96 | –1.44 |
| PLB vs. GFC | Cohen’s d= 0.838 | Cohen’s d= 1.065 | Cohen’s d= 0.590 | Cohen’s d= 0.042 | ||||||||
TABLE 4. Continuous Performance Taska
Subjective and Cardiovascular Data
VAS and cardiovascular data from one participant’s guanfacine treatment were missing; analyses were conducted on the remaining data.
As displayed in Figure 3, there were significant treatment effects on ratings of “any drug effect” (F[2,11]=7.163; p=0.010), “good effect,” (F[2,11]=8.706; p=0.005), “high” (F[2,11]=7.683; p=0.008), “stimulated” (F[2,11]=6.073; p=0.017), and “drug liking” (F[2,11]=8.550; p=0.006), as well as for crossover points (F(2,11)=5.061; p=0.028). Post hoc comparisons demonstrated that THC significantly increased ratings and crossover points during treatment with placebo (p≤0.011) and guanfacine (p≤0.046). There were nonsignificant (p≥0.192) treatment effects, and nonsignificant (p≥0.084) pairwise differences, on other VAS ratings.
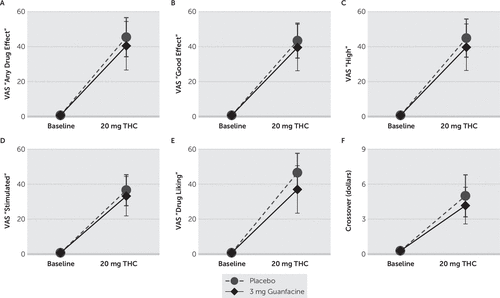
FIGURE 3. Treatment Effect Ratings Measured With the Visual Analog Scalea
a A) “Any drug effect,” B) “good effect,” C) “high,” D) “stimulated,” and E) “drug liking” ratings measured by the Visual Analog Scale, as well as F) crossover points. ∆-9-Tetrahydrocannabiol (THC) significantly increased ratings and crossover points during treatment with both placebo and guanfacine. Pairwise differences between placebo and guanfacine were nonsignificant. Means±standard errors.
As displayed in Figure 4, there was a significant (F[2,11]=5.392; p=0.023) treatment effect on heart rate; post hoc comparisons revealed that THC significantly increased heart rate during treatment with both placebo (p=0.010) and guanfacine (p=0.033). There were nonsignificant (p≥0.512) treatment effects, and nonsignificant (p≥0.268) pairwise differences on systolic and diastolic blood pressure.
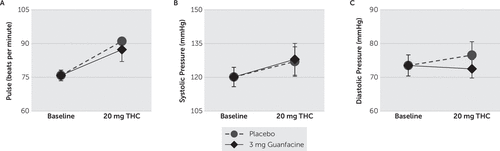
FIGURE 4. Treatment Effects on Heart Rate and Diastolic Blood Pressurea
a A) Heart rate, B) systolic, and C) diastolic blood pressure were measured using the Visual Analog Scale. ∆-9-Tetrahydrocannabiol (THC) significantly increased heart rate but not blood pressure during coadministration with placebo and guanfacine. Pairwise differences between placebo and guanfacine were nonsignificant for all cardiovascular parameters. Means±standard errors.
Discussion
This double-blind, placebo-controlled human laboratory study assessed the impact of guanfacine treatment on the cognitive, cardiovascular, and subjective effects produced by oral THC administration in volunteers who began using cannabis during adolescence. As was expected, THC (20 mg) alone decreased accuracy on working memory and attentional tasks and increased positive subjective ratings. The most consistent treatment effects were observed on working memory. Specifically, in comparison with treatment with placebo, treatment with 3 mg of guanfacine significantly reduced THC-induced adverse effects on spatial working memory performance.
We found that THC had a greater negative impact on spatial working memory than on attention or verbal recall. Indeed, cannabinoids have been reported to acutely impair spatial working memory in rodents,49 healthy adults,50 and adolescent cannabis users.51 Furthermore, that spatial but not auditory working memory was affected in these adolescent-onset cannabis users is consistent with findings that acute THC administration impairs spatial working memory at doses that do not significantly impair object working memory in adolescent rhesus monkeys.21 The adverse effect of THC on attention reported here is consistent with a large body of evidence for various, often dose-dependent, impairments in attention related to acute cannabinoid exposure.52–59 On the other hand, the lack of effect of THC on HVLT performance is consistent with one report of minimal acute effects of cannabinoids in cannabis users60 but not another.54 Overall evidence regarding the acute effects of cannabinoids on certain cognitive domains has been mixed [reviewed by Broyd et al.61]; however, a number of factors likely contribute to the inconsistent findings between studies, such as the different tasks used, and differences between study subjects, including frequency and quantity of cannabis use, and duration of abstinence. Of note, some data suggest that frequent cannabis users may be less sensitive to THC-induced impairment than are occasional smokers, despite achieving similar THC concentrations and subjective ratings.62 This apparent tolerance to THC impairment may be a result of pharmacological adaptation63 or behavioral compensatory mechanisms for deficits. For example, there is evidence that despite similar scores on cognitive tasks, cannabis smokers demonstrate significantly more brain activation and recruitment of alternative neural networks.64–66 Our findings further indicate that acute adverse effects in chronic cannabis users may be underestimated and may require additional forms of assessment alongside traditional cognitive tests. Nonetheless, we found that both auditory and visual memory along with aspects of attention were more accurate during short-term treatment with guanfacine. Furthermore, beyond demonstrating statistical significance, the treatment effects presented here meet the threshold of a minimum clinically important difference (MCID) based on a distribution-based method involving effect size with a cut-off value of d=0.2.67,68 On the basis of convention,69 the effect sizes we report are considered moderate for both spatial and auditory working memory performance and are considered large for errors of omission. Because neither treatment-related changes to functional status nor laboratory-based assessments of real-world function were completed in this study, it is important to note that these observed changes still meet MCID criteria by a variety of effect sizes and merit further consideration.
The beneficial effects of guanfacine on spatial working memory and attention described here are consistent with a report that guanfacine (29 μg/kg) improved both spatial working memory and attention in healthy subjects.30 Although the nonselective α2-adrenergic receptor agonist, lofexidine, has been reported to impair attention in cannabis users,39 differential effects of α2-adrenergic receptor agonists on cognitive functions have been consistently reported in both humans30,70 and monkeys.71 These studies collectively suggest that the greater selectivity of guanfacine for the α2A receptor subtype underlies its potency for enhancing attention and working memory. Furthermore, although acute THC administration increased VAS subjective ratings in a manner consistent with previous studies,72–76 no significant differences in ratings were observed between when THC was administered with either guanfacine or placebo. Thus, in contrast to other therapeutic agents such as modafinil, which has been associated with increases in anxiety, aggression, and negative subjective scores,77–79 and lofexidine, which has been associated with increases in subjective ratings of “good drug effect” and “sedated” in cannabis users,39 the beneficial effects of guanfacine on cognition reported here do not appear to be the result of secondary subjective effects.
As far as therapeutic targets are involved, most medications studied for treatment of CBUD have focused on managing withdrawal symptoms.80 Indeed, these symptoms are common upon abrupt cessation of cannabis use and cause significant distress, thereby contributing to relapse.81 On the other hand, behavioral therapies, such as cognitive-behavioral therapy, focus on building cognitive and emotional skills, to strengthen the ability of individuals to manage cravings, as well as to cope with withdrawal symptoms that might trigger drug use. Thinking of CBUD as a cluster of problems that reflect impaired cognition might be critical for addressing the persistent risk of relapse to cannabis use; 1-year abstinence rates range between 19% ανδ 29% across behavioral treatment studies.82 Evidence that the adolescent PFC is particularly vulnerable to the adverse effects produced by cannabis,16–19 coupled with the negative impact cognitive deficits have on treatment outcomes for CBUD,4,5 supports targeting PFC-mediated cognitive functions to improve treatment outcomes. The potency of guanfacine to selectively improve cognitive functions dependent upon PFC function,83,84 in both adolescent-onset cannabis users and abstinent cocaine-dependent individuals,85 supports the potential utility of guanfacine for improving treatment outcomes by targeting cognitive functions in cannabis users.37 Results from the current study provide preliminary support for the idea that by mitigating the acute adverse effects of THC on PFC-mediated cognitive abilities, guanfacine treatment might help prevent a lapse or a single incident of cannabis use from resulting in a relapse to sustained cannabis use.
There are several limitations of the current study. As the term of our study was relatively short and the sample of participants was small, the findings presented here will require further support. It is also important to note that we used a single (3 mg) dose of guanfacine, and ongoing study will be needed to determine optimal dosing and scheduling of medication delivery, especially because the beneficial effects of treatments on PFC-mediated cognitive functions display inverted, U-shaped dose-response curves.86 Furthermore, it is unclear to what extent acute THC adverse effects or reversal of these effects contribute to more persistent cognitive changes and deficits, and this will be an important area for future investigation regarding the utility of medications such as guanfacine that target cognitive processes implicated in addiction. In addition, certain general limitations are associated with effective modeling of cannabis usage and outcomes in the community. Dronabinol, while well-suited to characterize the effects of THC, may not fully capture the heterogeneous neurocognitive effects of cannabis use, especially those that may be mediated by non-THC constituents of cannabis. For example, there is some evidence that cannabidiol, another major constituent of cannabis, may protect users from acute, THC-induced memory impairments.87,88 Furthermore, in the present study, there was environment control for otherwise heavy patterns of cannabis use ranging from 2 to 20 times per day, and it is unclear whether the medication effect that was observed in this investigation would translate to more frequent patterns of use. Similarly, quantitative THC levels were not measured throughout this study, and there may be differences in dronabinol effect on measures of interest secondary to interindividual pharmacokinetic variability, especially given a range of cannabis use patterns prior to testing. Finally, although guanfacine was safely combined with THC in our study, and no unanticipated adverse effects were reported, further investigation is warranted to monitor for long-term adverse outcomes. Nonetheless, the findings presented here support that treatment with 3-mg guanfacine reduces acute THC-induced adverse effects on cognition in adolescent-onset, heavy cannabis users meeting CBUD criteria. Given concerning trends in cannabis use and CBUD, medications like guanfacine that stabilize PFC function and strengthen cognition merit further exploration, for minimizing acute adverse effects associated with THC intoxication, as initially demonstrated here, but perhaps also for mitigating the severity of relapse, enhancing cannabis treatment responses, and attenuating chronic processes that contribute to problematic use in adolescent-onset, heavy cannabis users.
1 SAMHSA: Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings (NSDUH Series H-48, HHS Pub no SMA-14-4863). Rockville, Md, Substance Abuse and Mental Health Services Administration, 2014Google Scholar
2 SAMHSA: Treatment Episode Data Set (TEDS): 2002-2012. National Admissions to Substance Abuse Treatment Services (BHSIS Series S-71, HHS Pub no SMA-14-4850). Rockville, Md, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2014Google Scholar
3 : Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 2011; 12:652–669Crossref, Medline, Google Scholar
4 : A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology 2012; 37:1689–1698Crossref, Medline, Google Scholar
5 : Cognitive deficits in marijuana users: effects on motivational enhancement therapy plus cognitive behavioral therapy treatment outcome. Drug Alcohol Depend 2008; 95:279–283Crossref, Medline, Google Scholar
6 : Situational determinants of use and treatment outcomes in marijuana dependent adults. Addict Behav 2014; 39:546–552Crossref, Medline, Google Scholar
7 : Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol 1994; 2:244–268Crossref, Google Scholar
8 : Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA 2003; 289:427–433Crossref, Medline, Google Scholar
9 : Early initiation of cannabis use: a cross-national European perspective. J Adolesc Health 2006; 39:712–719Crossref, Medline, Google Scholar
10 : Early-onset drug use and risk for drug dependence problems. Addict Behav 2009; 34:319–322Crossref, Medline, Google Scholar
11 : Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999; 142:295–301Crossref, Medline, Google Scholar
12 : Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc 2002; 8:873–883Crossref, Medline, Google Scholar
13 : Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction 2011; 106:2195–2203Crossref, Medline, Google Scholar
14 : Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA 2012; 109:E2657–E2664Crossref, Medline, Google Scholar
15 : Cannabis and adolescent brain development. Pharmacol Ther 2015; 148:1–16Crossref, Medline, Google Scholar
16 : The impact of early-onset cannabis use on functional brain correlates of working memory. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34:837–845Crossref, Medline, Google Scholar
17 : Neuroimaging in cannabis use: a systematic review of the literature. Psychol Med 2010; 40:383–398Crossref, Medline, Google Scholar
18 : Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol Psychiatry 2012; 71:684–692Crossref, Medline, Google Scholar
19 : Alcohol and drug use and the developing brain. Curr Psychiatry Rep 2016; 18:46Crossref, Medline, Google Scholar
20 : Repeated Δ9-tetrahydrocannabinol exposure in adolescent monkeys: persistent effects selective for spatial working memory. Am J Psychiatry 2014; 171:416–425Crossref, Medline, Google Scholar
21 : Delay- and dose-dependent effects of Δ9-tetrahydrocannabinol administration on spatial and object working memory tasks in adolescent rhesus monkeys. Neuropsychopharmacology 2012; 37:1357–1366Crossref, Medline, Google Scholar
22 : Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 2004; 101:8174–8179Crossref, Medline, Google Scholar
23 : Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci USA 2006; 103:9315–9320Crossref, Medline, Google Scholar
24 : Prediction of individual brain maturity using fMRI. Science 2010; 329:1358–1361Crossref, Medline, Google Scholar
25 : Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res 2007; 1127:36–44Crossref, Medline, Google Scholar
26 : Cannabinoid modulation of alpha2 adrenergic receptor function in rodent medial prefrontal cortex. Eur J Neurosci 2014; 40:3202–3214Crossref, Medline, Google Scholar
27 : Stress-induced sensitization of cortical adrenergic receptors following a history of cannabinoid exposure. Exp Neurol 2012; 236:327–335Crossref, Medline, Google Scholar
28 : Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry 2011; 69:e89–e99Crossref, Medline, Google Scholar
29 : Comparison of the binding activities of some drugs on alpha 2A, alpha 2B and alpha 2C-adrenoceptors and non-adrenergic imidazoline sites in the guinea pig. Pharmacol Toxicol 1995; 76:353–364Crossref, Medline, Google Scholar
30 : Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology 1999; 20:460–470Crossref, Medline, Google Scholar
31 : Guanfacine treatment of cognitive impairment in schizophrenia. Neuropsychopharmacology 2001; 25:402–409Crossref, Medline, Google Scholar
32 : Alpha-2 adrenergic agonists in children with inattention, hyperactivity and impulsiveness. CNS Drugs 2009; 23(Suppl 1):43–49Crossref, Medline, Google Scholar
33 : Increasing treatment options for cannabis dependence: a review of potential pharmacotherapies. Drug Alcohol Depend 2005; 80:147–159Crossref, Medline, Google Scholar
34 : Hippocampal noradrenaline release in awake, freely moving rats is regulated by alpha-2 adrenoceptors but not by adenosine receptors. J Pharmacol Exp Ther 1997; 281:648–654Medline, Google Scholar
35 : Precipitated cannabinoid withdrawal is reversed by delta(9)-tetrahydrocannabinol or clonidine. Pharmacol Biochem Behav 2001; 69:181–188Crossref, Medline, Google Scholar
36 : Prostaglandin E2 attenuates SR141716A-precipitated withdrawal in tetrahydrocannabinol-dependent mice. Brain Res 2003; 966:47–53Crossref, Medline, Google Scholar
37 : Cognitive function as an emerging treatment target for marijuana addiction. Exp Clin Psychopharmacol 2010; 18:109–119Crossref, Medline, Google Scholar
38 : Future pharmacological treatments for substance use disorders. Br J Clin Pharmacol 2014; 77:382–400Crossref, Medline, Google Scholar
39 : Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2008; 197:157–168Crossref, Medline, Google Scholar
40 : Preliminary findings of the effects of rivastigmine, an acetylcholinesterase inhibitor, on working memory in cocaine-dependent volunteers. Prog Neuropsychopharmacol Biol Psychiatry 2014; 50:137–142Crossref, Medline, Google Scholar
41 : Short-term, low-dose varenicline administration enhances information processing speed in methamphetamine-dependent users. Neuropharmacology 2014; 85:493–498Crossref, Medline, Google Scholar
42 : Construct and concurrent validity of the Hopkins Verbal Learning Test-Revised. Clin Neuropsychol 1999; 13:348–358Crossref, Medline, Google Scholar
43 : On how high performers keep cool brains in situations of cognitive overload. Cogn Affect Behav Neurosci 2007; 7:75–89Crossref, Medline, Google Scholar
44 : Improving fluid intelligence with training on working memory. Proc Natl Acad Sci USA 2008; 105:6829–6833Crossref, Medline, Google Scholar
45 Conners CK: Conners’ Continuous Performance Test-II (CPT-II). Toronto, Ontario, Canada, Multi-Health Systems, 2002Google Scholar
46 : Treatment with modafinil and escitalopram, alone and in combination, on cocaine-induced effects: a randomized, double blind, placebo-controlled human laboratory study. Drug Alcohol Depend 2014; 141:72–78Crossref, Medline, Google Scholar
47 : Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol 2014; 17(2):223–233Crossref, Medline, Google Scholar
48 : Subjective and cardiovascular effects of intravenous methamphetamine during perindopril maintenance: a randomized, double-blind, placebo-controlled human laboratory study. Int J Neuropsychopharmacol 2016; 19(7)Crossref, Medline, Google Scholar
49 : Delta 9-tetrahydrocannabinol increases prefrontal cortical catecholaminergic utilization and impairs spatial working memory in the rat: blockade of dopaminergic effects with HA966. Neuropsychopharmacology 1997; 16:426–432Crossref, Medline, Google Scholar
50 : Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of delta-9-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 2008; 198:587–603Crossref, Medline, Google Scholar
51 : The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev 2007; 26:309–319Crossref, Medline, Google Scholar
52 : The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 2004; 29:1558–1572Crossref, Medline, Google Scholar
53 : Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology 2008; 33:2505–2516Crossref, Medline, Google Scholar
54 : Sex, drugs, and cognition: effects of marijuana. J Psychoactive Drugs 2010; 42:413–424Crossref, Medline, Google Scholar
55 : Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addict Biol 2013; 18:872–881Crossref, Medline, Google Scholar
56 : Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC). Psychopharmacology (Berl) 2009; 204:85–94Crossref, Medline, Google Scholar
57 : An exploratory study of the combined effects of orally administered methylphenidate and delta-9-tetrahydrocannabinol (THC) on cardiovascular function, subjective effects, and performance in healthy adults. J Subst Abuse Treat 2015; 48:96–103Crossref, Medline, Google Scholar
58 : Rivastigmine but not vardenafil reverses cannabis-induced impairment of verbal memory in healthy humans. Psychopharmacology (Berl) 2015; 232:343–353Crossref, Medline, Google Scholar
59 : Nabilone produces marked impairments to cognitive function and changes in subjective state in healthy volunteers. J Psychopharmacol 2010; 24:1659–1669Crossref, Medline, Google Scholar
60 : Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacol Biochem Behav 2010; 96:333–341Crossref, Medline, Google Scholar
61 : Acute and chronic effects of cannabinoids on human cognition: A systematic review. Biol Psychiatry 2016; 79:557–567Crossref, Medline, Google Scholar
62 : Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology (Berl) 2011; 214:391–401Crossref, Medline, Google Scholar
63 : Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav 2005; 81:300–318Crossref, Medline, Google Scholar
64 : Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage 2004; 23:914–920Crossref, Medline, Google Scholar
65 : Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004; 176:239–247Crossref, Medline, Google Scholar
66 : Effects of marijuana on visuospatial working memory: an fMRI study in young adults. Psychopharmacology (Berl) 2010; 210:429–438Crossref, Medline, Google Scholar
67 : Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics 1999; 15:141–155Crossref, Medline, Google Scholar
68 : The concept of clinically meaningful difference in health-related quality-of-life research. How meaningful is it? Pharmacoeconomics 2000; 18:419–423Crossref, Medline, Google Scholar
69 : Statistical Power Analysis for the Behavioral Sciences. Abingdon, United Kingdom, Taylor and Francis, 2013Crossref, Google Scholar
70 : Clonidine, but not guanfacine, impairs choice reaction time performance in young healthy volunteers. Neuropsychopharmacology 1999; 21:495–502Crossref, Medline, Google Scholar
71 : The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J Neurosci 1988; 8:4287–4298Crossref, Medline, Google Scholar
72 : Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology 2003; 28:1356–1365Crossref, Medline, Google Scholar
73 : Comparison of the subjective effects of delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002; 161:331–339Crossref, Medline, Google Scholar
74 : Low doses of delta-9 tetrahydrocannabinol (THC) have divergent effects on short-term spatial memory in young, healthy adults. Neuropsychopharmacology 2006; 31:462–470Crossref, Medline, Google Scholar
75 : Responses to oral delta-9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacol Biochem Behav 1999; 63:137–142Crossref, Medline, Google Scholar
76 : The effects of orally administered delta 9-tetrahydrocannabinol in man on mood and performance measures: a dose-response study. Pharmacol Biochem Behav 1990; 35:861–864Crossref, Medline, Google Scholar
77 : Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug Alcohol Depend 2002; 67:311–322Crossref, Medline, Google Scholar
78 : A randomized, double-blind, crossover trial of modafinil on mood. J Clin Psychopharmacol 2007; 27:76–79Crossref, Medline, Google Scholar
79 : Effects of modafinil on cognitive performance and alertness during sleep deprivation. Curr Pharm Des 2006; 12:2457–2471Crossref, Medline, Google Scholar
80 : Pharmacological treatment of cannabis dependence. Curr Pharm Des 2011; 17:1351–1358Crossref, Medline, Google Scholar
81 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Publishing, 2013Crossref, Google Scholar
82 : Pharmacological and psychosocial interventions for cannabis use disorders. Rev Bras Psiquiatr 2010; 32(Suppl 1):S46–S55Medline, Google Scholar
83 : The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology 2000; 23:240–249Crossref, Medline, Google Scholar
84 : The alpha-2A noradrenergic receptor agonist guanfacine improves visual object discrimination reversal performance in aged rhesus monkeys. Behav Neurosci 1997; 111:883–891Crossref, Medline, Google Scholar
85 : Guanfacine enhances inhibitory control and attentional shifting in early abstinent cocaine-dependent individuals. J Psychopharmacol 2015; 29:312–323Crossref, Medline, Google Scholar
86 : Targeting prefrontal cortical systems for drug development: potential therapies for cognitive disorders. Annu Rev Pharmacol Toxicol 2016; 56:339–360Crossref, Medline, Google Scholar
87 : Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected]. Br J Psychiatry 2010; 197:285–290Crossref, Medline, Google Scholar
88 : Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol 2013; 27:19–27Crossref, Medline, Google Scholar



