A Neuropsychiatric Analysis of the Cotard Delusion
Abstract
Cotard’s syndrome, a condition in which the patient denies his or her own existence or the existence of body parts, is a rare illness that has been reported in association with several neuropsychiatric diagnoses. The majority of published literature on the topic is in the form of case reports, many of which are several years old. The authors evaluated associated diagnoses, neuroimaging, and treatments recorded in patients diagnosed with Cotard’s syndrome at their institution. A search of the Mayo Clinic database for patients with mention of signs and symptoms associated with Cotard’s in their records between 1996 and 2016 was conducted. The electronic medical records of the identified patients were then reviewed for evidence of a true diagnosis of Cotard’s. Clinical and neuroimaging data were also recorded for these patients. The search identified 18 patients, 14 of whom had Cotard delusions. Two of the 14 were excluded due to them being under age 18. The resulting 12 patients had a median age of 52 years (range: 30–85 years). On neuroimaging, four patients exhibited frontal lobe changes, four demonstrated generalized volume loss, and five had ischemic changes; seven patients demonstrated right-sided or bilateral hemisphere lesions. Treatments included ECT, pharmacotherapy, behavioral therapy, psychotherapy, rehydration, and removal of offending drugs. To conclude, Cotard delusions occur in the context of a relatively wide spectrum of neurological, psychiatric, and medical disorders and present with various neural changes. Nondominant hemisphere lesions may play a role in the pathophysiology. A number of effective treatments are available.
Cotard’s syndrome is a rare neuropsychiatric condition that manifests as nihilistic delusions ranging from denial of the existence of body parts to negation of self-existence.1 Though described initially in 1880, very little is understood about this disorder, and its inclusion in the DSM-5 as a specific listing has been avoided. The current status of Cotard’s syndrome is its conceptualization as a manifestation of an underlying disorder. While Cotard’s is often described as being a psychiatric syndrome, published studies have shown a strong correlation between a preexisting neurological disease and the condition.1
A variety of associated neurological conditions have been described, including migraines,2 subdural hemorrhage,3 cerebral atrophy,4 cerebral arterio-venous malformation,5 multiple sclerosis,6 cerebral infarction,7 superior sagittal sinus thrombosis,8 parietal lobe tumor,9 temporal lobe epilepsy,5 Parkinson’s disease,10 encephalopathy,1 and brain injury.11 Understanding Cotard’s syndrome and its connection to other neurological conditions is essential to identifying appropriate treatment modalities.
The goal of this study thus was to identify patients with Cotard’s syndrome seen at our institution within the last two decades and identify those patients in whom the Cotard’s was felt to be secondary to another psychiatric or neurologic illness or insult. In doing so, we hoped to describe the association between Cotard’s and other conditions affecting the brain in order to better define possible pathophysiologic mechanisms underlying the syndrome.
Methods
Patients were identified by searching the Mayo Clinic computerized clinical database (1996–2016) for all individuals for whom any of the following terms had been mentioned in the medical records, with variations: Cotard(’s) syndrome, delusion(s) of negation, nihilistic delusion(s), walking corpse syndrome, delusion(s) of missing organs, denial of existence, idea(s) of damnation.
The search identified 18 patients. The medical records of all 18 patients were reviewed in detail to determine whether there was evidence of Cotard delusions. Inclusion criteria for Cotard’s syndrome included descriptions of negation of the existence or belief in the destruction of internal organs, denial of being alive, or denial of the existence of one’s soul, with nihilistic delusions being necessary for inclusion. Of the 18, three patients were excluded after a review of the medical records revealed no evidence of Cotard’s. These three patients were captured in the original 18 because of comments in the medical records, such as “denies Cotard delusions.” Two additional patients were excluded, as they were under the age of 18. One patient was removed because he showed signs of somatoparaphrenia (limb denial) rather than Cotard’s, although he was initially diagnosed with Cotard delusions. Hence, a total of 12 adult patients were identified as having been diagnosed with Cotard’s syndrome and were included in this study based on the inclusion criteria. Demographic, clinical, neuroimaging, and treatment data were then abstracted for these 12 patients.
The study was approved by the Mayo Clinic Institutional Review Board. All patients in this study gave written informed consent for their data to be used for research purposes.
Results
Of the 12 patients identified as having evidence of Cotard delusions, eight (67%) were male and four (33%) were female. The median age at presentation was 52 years (range: 30–85 years). Four of the 12 patients exhibited other unusual behaviors, and eight of the 12 were described as having other delusions or psychoses (Table 1).
| Patient | Sex and Age at Onset of Cotard Delusions | Description of Cotard Delusions | Cotard Delusions Occurring in the Context of the Following Signs/Symptoms/Diagnoses | Other Delusions or Psychoses | Other Unusual Behaviors | Treatments Tried and Response |
|---|---|---|---|---|---|---|
| 1 | 44 M | Described feeling as though he was no longer alive and that he did not think that anyone could kill him because he was not alive anyway. | Worsening of his mood symptoms, hallucinations, delusional thinking, and self-injurious behaviors over the last few weeks; PMH of bipolar disorder, borderline personality disorder, polysubstance dependence (alcohol and opiates) in full sustained remission, and cluster headaches. | Visual hallucinations of rats running across the baseboard, spiders on the wall, and people’s faces melting. | None described | Dialectical-behavioral therapy plus buspirone (30 mg t.i.d.) plus lithium (900 mg b.i.d.) plus lamotrigine (200 mg b.i.d.) plus quetiapine (500 mg q.d.) plus clonazepam (1.5 mg q.d. for 5 days) resulted in complete resolution of symptoms. |
| 2 | 85 F | Became increasingly preoccupied with morbid thoughts about her own life and was convinced she was not alive and her life “was a lie.” | Subacute onset of depressive symptoms, including fragmented sleep; decreased energy; decreased interest in eating; depressed mood; and positive helplessness, hopelessness, and worthlessness. | Felt that people were trying to confiscate money from her and to steal her identity. | None described | Eight treatments of ECT plus quetiapine (titrated from 25 mg to 100 mg q.d.) plus lorazepam (0.5 mg PRN) plus citalopram (20 mg q.d.) resulted in resolution of symptoms within 21 days of presentation. |
| 3 | 74 M | Reported that he had been stabbed and killed at his nursing home. Referred to his bed as his “casket.” | Altered mental status with concern for psychosis and/or delirium. | Noted hearing the voice of “almighty God” telling him that people are dying on the hospital unit. | None described | Risperidone (1.5 mg q.d.) for 10 days resulted in complete resolution of symptoms. |
| Also convinced that wife was an imposter and an alien (Capgras syndrome). | ||||||
| Claimed that he was being fed cat food for dinner and that once they put “pins in the hamburgers,” because somebody “sabotaged them.” | ||||||
| 4 | 66 M | Believed he died in the hospital and had risen again on Easter Sunday; continued to have a fixed delusion over his “death” for several days until treated. | Steroid-induced psychosis | None described | None described | Removal of corticosteroid treatment resulted in complete resolution of symptoms after 20 days. |
| 5 | 65 M | Believed he died on the operating table during surgery. His proof was that the television screen light “keeps fading” and that this was the afterlife and he was being tested. | Encephalopathy and seizures in the postoperative setting after sedation in the ICU and without sleep for at least 48 hours | Hallucinating that other people were in the room | “Swatting” at staff members | Lacosamide (150 mg) for 4 days resulted in resolution of symptoms. |
| 6 | 35 F | Believed that her brain was rotting and that there were worms in her brain that she could feel. | Dehydration-induced psychosis in the setting of pyelonephritis, chronic paranoid schizophrenia, delusional parasitosis, and a history of opiate and benzodiazepam addiction. | Auditory hallucinations | None described | Fluid resuscitation and hydration resulted in alleviation of symptoms and return to baseline at 2 weeks. |
| 7 | 39 F | Insisted that she had died from an overdose given by the hospital staff; noted that she wants to sleep “in my coffin.” | Seizures, mild hyponatremia; PMH of paranoid schizophrenia, schizoaffective disorder, bipolar I disorder, and cluster B personality traits. | Paranoia | Intense eye contact | Lorazepam (2 mg q.d.) plus valproic acid (1000 mg q.d.) plus risperidone (8 mg q.d.) plus milieu therapy resulted in resolution of symptoms after 1 month. |
| 8 | 50 M | Believed that his arm had been cut off and his fingers were being ground up and that he was dying. | Medication-resistant psychotic disorder not otherwise specified, with history of polysubstance abuse and narcissistic personality traits. | Believed that the television was talking to him and this was when “the voices are the clearest.” | None described | One treatment of ECT drastically improved symptoms. |
| 9 | 30 M | Believed that he had died and was now in an in-between world, between the living and the dead. | Frontal lobe dysfunction and cognitive impairment (confirmed by neuropsychometric testing) and polysubstance abuse (marijuana and alcohol). | Paranoid behavior; for example, he would put on socks and ask, “Who put these on me?” | None described | Escitalopram (20 mg b.i.d.) plus risperidone (varying doses going as high as 4 mg q.h.s.) plus lurasidone (80 mg q.d.) for 1 month resulted in improvement of symptoms. |
| 10 | 39 F | Despite reassurance that she was not dead, she perseverated over the idea that she was not alive. | Sagittal sinus thrombosis; PMH of depression. | None described | Breath-holding spells (“Dead people don't breathe”), repetitive tongue protrusion-retraction movements for 1–2 hours, and mutism (possibly caused by suggestions told to her that dead people would not speak). | Lorazepam (2 mg cumulative) plus clonazepam (1.5 mg q.d.) for 22 days resulted in symptom resolution. |
| 11 | 67 M | Thought that there were bugs in his eyes, in his stomach, and in his bottom. Wanted to go to the doctor to have these removed, as he thought that the bugs were “killing him.” | Amantadine-induced psychosis in the setting supranuclear palsy diagnosis; PMH of depression. | None described | Standing in front of the mirror to brush his teeth and not doing anything for an hour. | Removal of amantadine resulted in slight improvement of symptoms, although follow-up data are limited. |
| 12 | 30 M | Believed that his internal body organs were being eaten by a virus and that he was going to die soon because of it. | No associated psychological or neurological diagnoses, but complaints of autonomic dysfunction and muscle twitching of undetermined etiology. | None described | None described | No treatments were pursued, and no follow-up data are available. |
TABLE 1. Patient Demographic and Clinical Dataa
Eight of the 12 patients described feeling that they had died, and four patients claimed that they were dying. Three of the eight patients described being killed by healthcare workers. Three of the four patients who claimed to be dying described the destruction of their internal organs and body parts by either “worms,” “viruses,” or “bugs,” with one of these patients being previously diagnosed with delusional parasitosis (patient 6).
Eight patients had a documented psychiatric history, with two (17%) carrying a diagnosis of schizophrenia, three (25%) having been diagnosed with depression, and four (33%) having a history of polysubstance abuse (Table 1). One patient (patient 3) presented with co-occurring Capgras syndrome; another patient (patient 10) showed signs of catatonia.
Five individuals (42%) presented with neurological symptoms at the time of their Cotard diagnosis. The neurological diagnoses included seizures, cluster headaches, sagittal sinus thrombosis, encephalopathy and seizures, supranuclear palsy, and frontal lobe dysfunction (Table 1). Non-neuropsychiatric conditions copresenting with the Cotard delusions included steroid-induced psychosis, dehydration, and amantadine-induced psychosis (Table 1).
Electroencephalogram (EEG) data were available for four of the 12 patients. All four patients with EEGs showed some degree of nonspecific slowing (Table 2).
| Patient | Imaging Type | Neuroimaging Findings | EEG Available | EEG Findings |
|---|---|---|---|---|
| 1 | MRI head | Few scattered foci of increased T2 signal in the white matter of the right frontal lobe. | No | N/A |
| 2 | MRI head | Moderate diffuse leukoaraiosis and parenchymal volume loss, more pronounced in both frontal lobes. | No | N/A |
| 3 | MRI and CT head | Chronic right MCA territory infarction with frontal-temporal lobe encephalomalacia and gliosis and Wallerian degeneration of the brainstem. Small amounts of associated blood products in the region of chronic infarct. Mild scattered leukoaraiosis. Moderate-prominent cerebral parenchymal volume loss. Right subinsular encephalomalacia periventricular hypoattenuation and dystrophic calcification. | Yes | EEG shows mild diffuse nonspecific background slowing (grade 1 dysrhythmia). No potentially epileptogenic activity was present during the recording. |
| 4 | MRI head | Negative | Yes | EEG shows some mild diffuse nonspecific slowing of the background and excessive beta. No potentially epileptogenic activity was present during the awake or sleep recordings. Dysrhythmia grade 1 generalized. The recording during wakefulness contains 9 Hz alpha activity over the posterior head regions. Some mild generalized slowing and increase in beta is seen. No abnormal activity occurred during photic stimulation. |
| 5 | MRI head | Chronic right corona radiata lacunar infarct with associated hemosiderin. Mild leukoaraiosis. Mild generalized cerebral and cerebellar volume loss with commensurate dilatation of the ventricular system. | Yes | EEG shows some mild diffuse nonspecific slowing of the background. Although the patient was intermittently drowsy throughout the recording, the mild slowing was also present during times of relative alertness. No potentially epileptogenic activity was present during the awake or sleep recordings. |
| 6 | MRI head | 2–3 small foci of T2 signal within the subcortical white matter of both cerebral hemispheres, likely ischemic or degenerative. | No | N/A |
| 7 | CT head | Negative | No | N/A |
| 8 | None | N/A | No | N/A |
| 9 | CT head | Negative | No | N/A |
| 10 | MRI head | Progression of previous bilateral cerebral hemisphere infarcts involving both frontal lobes anteriorly, both occipital lobes, and the right posterior frontal-anterior parietal lobes. Complete thrombosis of the superior sagittal sinus. MRI also demonstrates irregularity of both transverse sinuses, left greater than right, which may represent extension of the thrombus into the transverse and perhaps sigmoid sinuses. New cortical enhancement in a gyriform pattern present in the frontal and occipital lobes bilaterally and in both parietal lobes. | Yes | The portable EEG shows a moderate degree of nonspecific slowing over the bitemporal head regions. These findings are consistent with a focal disturbance of cerebral function or focal lesion involving these regions. |
| 11 | MRI head | Ischemic small-vessel disease in both cerebral hemispheres, without focal restricted diffusion, nor intracranial paramagnetic susceptibility effect. Generalized cerebral and cerebellar volume loss. | No | N/A |
| 12 | MRI head | Normal mild lateral ventricular asymmetry. | No | N/A |
TABLE 2. Neuroimaging and Electroencephalogram Findingsa
Neuroimaging data were available for 11 out of the 12 patients (Table 2). Seven of the 11 (64%) patients with neuroimaging showed evidence of bilateral or right-sided hemisphere lesions. Four patients (36%) exhibited changes in their frontal lobes, including bilateral leukoaraiosis and volume loss, right-sided white matter hyperintensities on T2, right-sided encephalomalacia, and right-sided infarcts. Four (36%) demonstrated generalized volume loss on imaging. Three (27%) individuals had large vascular lesions, including one right chronic middle cerebral artery (MCA) infarct, one chronic right corona radiata infarct, and one with superior sagittal sinus thrombosis with resultant bilateral frontal and occipital lobe infarcts (Figure 1).
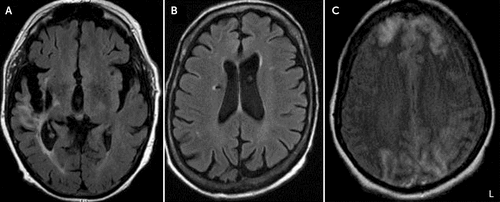
FIGURE 1. Axial Brain Scans Highlighting Areas of Large Vascular Lesions in Three Patients With Cotard Delusionsa
a The image shows large vascular lesions in three patients with Cotard delusion, including a chronic right-middle cerebral artery territory infarction with frontal-temporal lobe encephalomalacia and gliosis in patient 3 (A), a chronic right corona radiata lacunar infarct with associated hemosiderin in patient 5 (B), and bilateral cerebral hemisphere infarcts involving both frontal lobes anteriorly and both occipital lobes in patient 10 (C). L=Left side of the image.
Eleven out of 12 patients received treatment, and all 11 experienced an improvement and/or resolution of Cotard delusions (Table 1). Treatments utilized in the patients included electroconvulsive therapy (ECT), psychotherapy, behavioral therapy, anxiolytics (buspirone), antidepressants (citalopram), antipsychotics (risperidone, quetiapine, lurasidone, and lithium) anticonvulsants (valproic acid, lacosamide, and lamotrigine), sedatives (clonazepam and lorazepam), rehydration therapy, and removal of offending drugs. Response to ECT was variable in terms of number of treatments (range: 1–8) and addition of pharmacotherapy (Table 1). In a majority of the patients, pharmacologic treatment involved multiple classes of drugs (for example, lorazepam plus valproic acid plus risperidone) (see Table 1).
Discussion
Cotard delusions were reported in patients across a relatively wide spectrum of neurological, psychiatric, and medical disorders in this study. Interestingly, there was a gender predilection for males. The median age of affected individuals was 52, which contrasts with the previously proposed idea that Cotard’s syndrome is primarily a condition of the elderly.12
Previous publications on the topic of Cotard’s have shown an association with headaches,2 seizures,5 sagittal sinus thrombosis,8 cerebral atrophy,4 encephalopathy,1 infarction,7 catatonia,13,14 and Capgras syndrome,7,11 which we have confirmed. Additionally, we identified other potential diagnostic associations not previously reported, including dehydration, steroid psychosis, and supranuclear palsy and/or amantadine psychosis. However, it is important to note that in the patient presenting with Cotard delusions in the setting of dehydration-induced psychosis, there had been previously documented diagnoses of paranoid schizophrenia and delusional parasitosis, and a history of opiate and benzodiazepam addiction. Nevertheless, the patient’s delusions did respond to fluid resuscitation, strengthening the idea of its possible involvement in the pathophysiology.
Amantadine has been shown to induce psychosis and mania in the literature, but it has not previously been reported in those presenting with Cotard delusions.15,16 The same goes for dehydration-related hyponatremia17 and corticosteroid use.18
A majority (64%) of the patients presented with right-sided or bilateral changes. This is an important finding that adds to the growing literature on delusional misidentification syndromes (DMSs), under which some posit that Cotard’s syndrome falls.19 Additionally, studies have demonstrated trends of increased bilateral cerebral atrophy and median frontal lobe atrophy in particular in patients diagnosed with Cotard’s.20 We also found the most commonly affected region was the frontal lobe (36% bilaterally or right-sided), although other areas were involved, as well. Bilateral cerebral atrophy was found in four out of 11 patients (36%). Vascular lesions seemed to be the most common change seen, especially on the right side; this aligns with the findings of several reports.3,7,8
Right frontal damage has been reported in those with delusional misidentification.19,21,22 Cotard’s syndrome falls under the category of hypofamiliarity delusions of self in terms of DMSs.19 A “two-hit” hypothesis has been suggested for DMSs, in which the first hit produces abnormal perception and the second hit leads to persistence of the abnormal perception despite being presented evidence to the contrary.19 The pathophysiology of some of these identified syndromes involves disconnections in the neural circuits involved in perception of self, with potential explanations citing that DMSs, in particular hypofamiliarity delusions, result from an inability to link perceptions of external or environmental stimuli with internally generated autobiographical memories.19,23 Studies cite that a deep right frontal lesion could disrupt the anatomic connection between temporal and limbic regions and the damaged frontal lobe; this disconnection could result in a disturbance in familiarity of self, people, places, objects, and so on, and the occurrence of frontal lobe injury could lead to a problem with resolving the cognitive conflict.22 Lesions specifically of the nondominant hemisphere have also been implicated in Cotard’s syndrome,24 indicating that the findings of nondominant hemisphere changes may indeed be significant. Furthermore, DMSs have been shown to simultaneously co-occur in previously published literature.19 We also saw this in our patient who presented with both Capgras and Cotard delusions, both of which are classified as hypofamiliarity syndromes.19
In terms of treatment modalities, both ECT and pharmacotherapy have been shown to be effective in combatting Cotard’s syndrome in the literature.3,21,25 One previous study demonstrated increased dopamine receptor binding in a patient presenting with Cotard’s,26 which bolstered the association between schizophrenia and Cotard’s syndrome via the dopamine hypothesis. Thus, the effectiveness of dopamine antagonists such as risperidone and quetiapine and selective serotonin reuptake inhibitors (SSRIs) such as citalopram (which also has been shown to decrease dopamine in the brain) in this condition can be readily explained.27 In a similar vein, amantadine is a dopamine receptor agonist, which could potentially explain the resulting psychosis and delusions in patient 11.
The general approach for treatment involved treating the underlying medical or neuropsychiatric illness in all cases. Our study also points to the potential benefit of behavioral and psychotherapy in the treatment of Cotard’s syndrome when used in conjunction with pharmacotherapy. The numerous treatment modalities shown to be effective in alleviating symptoms of Cotard’s syndrome in this study are promising in that they confirm that the delusion is often easily combatable with readily available treatments.
Limitations of this study include a relatively small cohort, although this is a rare delusion, and incomplete follow-up information for some patients. Also, in terms of neuroimaging, three of the 12 patients did not undergo MRI, which is unfortunately an unavoidable limitation in a retrospective study such as this. Therefore, multicenter, prospective, clinico-anatomic, and functional imaging studies are needed to determine the anatomic or network disruption that accounts for this uncommon but interesting delusion.
1 : Cotard syndrome in neurological and psychiatric patients. J Neuropsychiatry Clin Neurosci 2010; 22:409–416Link, Google Scholar
2 : Cotard’s syndrome in migraine (a case report). Indian J Med Sci 1993; 47:152–153Medline, Google Scholar
3 : A case of Cotard syndrome in a woman with a right subdural hemorrhage. J Neuropsychiatry Clin Neurosci 2014; 26:E29–E30Link, Google Scholar
4 : “I do not exist”—Cotard syndrome in insular cortex atrophy. Biol Psychiatry 2015; 77:e52–e53Crossref, Medline, Google Scholar
5 : Cotard’s syndrome and temporal lobe epilepsy. Psychiatr J Univ Ott 1988; 13:36–39Medline, Google Scholar
6 : The Cotard syndrome. Report of two patients: with a review of the extended spectrum of “délire des négations.” Eur J Neurol 2004; 11:563–566Crossref, Medline, Google Scholar
7 : Cotard and Capgras syndrome after ischemic stroke. J Stroke Cerebrovasc Dis 2015; 24:e103–e104Crossref, Medline, Google Scholar
8 : Cotard's syndrome in a patient with superior sagittal sinus thrombosis. Biol Psychiatry 2006; 56:263SGoogle Scholar
9 : Cotard’s syndrome in parietal lobe tumor. Indian Pediatr 1993; 30:1019–1021Medline, Google Scholar
10 : Threatening auditory hallucinations and Cotard syndrome in Parkinson disease. Clin Neuropharmacol 2004; 27:205–207Crossref, Medline, Google Scholar
11 : Diurnal variation in Cotard’s syndrome (copresent with Capgras delusion) following traumatic brain injury. Aust N Z J Psychiatry 2000; 34:684–687Crossref, Medline, Google Scholar
12 : Cotard’s delusion or syndrome? a conceptual history. Compr Psychiatry 1995; 36:218–223Crossref, Medline, Google Scholar
13 : Cotard syndrome with catatonia: unique combination. Indian J Psychol Med 2013; 35:314–316Crossref, Medline, Google Scholar
14 : Catatonia, neuroleptic malignant syndrome, and Cotard syndrome in a 22-year-old woman: a case report. Case Report Psychiatry 2013; 2013:452646Crossref, Medline, Google Scholar
15 : Amantadine-induced psychosis in a young healthy patient. Am J Psychiatry 2008; 165:1613Crossref, Medline, Google Scholar
16 : Amantadine-induced psychosis in a geriatric patient with renal disease. Am J Psychiatry 1979; 136:111–112Crossref, Medline, Google Scholar
17 : Hyponatremia-induced psychosis in an industrial setting. Ind Psychiatry J 2009; 18:137–138Crossref, Medline, Google Scholar
18 : Quetiapine therapy for corticosteroid-induced mania. Can J Psychiatry 2005; 50:77–78Crossref, Medline, Google Scholar
19 : Lesion-related delusional misidentification syndromes: a comprehensive review of reported cases. J Neuropsychiatry Clin Neurosci 2016; 28:217–222Link, Google Scholar
20 : Brain atrophy and interhemispheric fissure enlargement in Cotard’s syndrome. J Clin Psychiatry 1986; 47:518–520Medline, Google Scholar
21 Debruyne HPM, Peremans K, Audenaert K: Cotard’s syndrome. Mind Brain 2011; 2:67–72Google Scholar
22 : The Lost Self: Pathologies of the Brain and Identity. Oxford, United Kingdom, Oxford University Press, 2005Crossref, Google Scholar
23 : “Cat-gras” delusion: a unique misidentification syndrome and a novel explanation. Neurocase 2016; 22:251–256Crossref, Medline, Google Scholar
24 : Attributional style in a case of Cotard delusion. Conscious Cogn 2007; 16:349–359Crossref, Medline, Google Scholar
25 : Electroconvulsive therapy for lycanthropy and Cotard syndrome: a case report. J ECT 2010; 26:280–281Crossref, Medline, Google Scholar
26 : A case of Cotard syndrome: (123)I-IBZM SPECT imaging of striatal D(2) receptor binding. Psychiatry Res 2004; 130:109–112Crossref, Medline, Google Scholar
27 : Long-term citalopram treatment alters the stress responses of the cortical dopamine and noradrenaline systems: the role of cortical 5-HT1A receptors. Int J Neuropsychopharmacol 2016; 19:pyw026Crossref, Medline, Google Scholar



