Cognitive Correlates of the Negative, Disorganized, and Psychotic Symptom Dimensions of Schizophrenia
Abstract
Knowledge of the relationship between specific cognitive abnormalities and the clinical symptoms of schizophrenia could give insight into the nature of their underlying pathophysiology. Composite scores were generated for negative, disorganized, and psychotic symptom ratings in 134 patients with schizophrenia (DSM-IV criteria). Partial correlations (each composite corrected for the others) were computed with neuropsychological measures. Negative symptoms were related to poor performance on tests of verbal learning and memory, verbal fluency, visual memory, and visual-motor sequencing. Disorganized symptoms were correlated with lower verbal IQ and poor concept attainment. Psychotic symptoms had no significant relationship with cognitive deficit.
Abnormal cognitive performance has been observed in individuals with schizophrenia on tasks ranging from early attentional gating to full-scale IQ,1–3 suggesting that schizophrenia is associated with a broad impairment of cognitive function. However, several studies have suggested that more specific or primary deficits exist within the pattern of global impairment.4,5 There is at present, therefore, no consensus as to whether the multiple cognitive abnormalities that have been noted in individuals with schizophrenia reflect generalized cognitive impairment, whether more focal and primary deficits are masked by the clinical heterogeneity and high variability in the performance of schizophrenic patients,6–8 or whether, as we have recently suggested, dysfunction in a fundamental cognitive process could result in the diversity of symptoms observed in schizophrenic patients.9–11
In addition to lower average performance on cognitive tasks, groups of schizophrenic individuals also typically have much greater variability in comparison to control groups. This increased variability could have several causes. Increased variability would result if each patient's performance was inconsistent across tasks. For example, a patient might perform poorly on a task when attending to a hallucinatory voice, but perform well on a similar task when not hallucinating. Alternatively, increased variability would result if patients with differing types of symptoms had different cognitive deficits. Schizophrenic individuals manifest diverse clinical symptoms, ranging from disorders in the perception and interpretation of events in the world (i.e., hallucinations and delusions), to disorders in thought and language (i.e., derailment, tangentiality, incoherence), to abnormalities in emotional expression, social interaction, and volition (i.e., negative symptoms). If clinical symptoms are related to abnormalities in brain systems that also cause disorders of cognition, then high variability in cognitive performance would be expected in groups of clinically heterogeneous patients with schizophrenia. That is, different cognitive abnormalities would be associated with the different symptoms expressed by individual patients.
One approach to assessing the heterogeneous symptoms of schizophrenia involves correlational techniques. Our group at the University of Iowa has performed a number of factor analytic studies on the Scale for the Assessment of Negative Symptoms (SANS)12 and the Scale for the Assessment of Positive Symptoms (SAPS).13 These studies indicate that negative symptoms form a cohesive factor. Positive symptoms, however, load on several factors. The most consistent solution for positive symptoms produces two factors, one reflecting florid psychotic symptoms (i.e., hallucinations and delusions) and the other reflecting disorganized language and behavior and inappropriate affect.14–17 A number of studies from other groups have also found a three-dimensional model of symptomatology (see review17).
The underlying assumption of the factor analytic approach is that symptoms that frequently co-occur may share a common mechanism. If this assumption is correct, then showing that there are characteristic patterns of cognitive dysfunction associated with each dimension can validate symptom dimensions identified by factor analysis. Although the factor analytic evidence is surprisingly consistent in supporting a three-dimensional model of symptomatology, relatively few studies have attempted to validate this factor structure by the use of neurobiological or neuropsychological indices.
Bilder et al.18 assessed chronic schizophrenic patients with symptom rating scales and a battery of neuropsychological tests. They found that a symptom cluster reflecting disorganization was most strongly associated with cognitive dysfunction and that a negative symptom cluster showed a less strong association with poor cognitive performance. Liddle19,20 found that a symptom dimension reflecting “psychomotor poverty” was associated with poor performance on tests of conceptual thinking, object naming, and long-term memory. Symptoms reflecting disorganization were associated with poor performance on tests of concentration, immediate recall, and list learning. In an independent sample of patients, Liddle and Morris21 found the negative symptom dimension to be associated with a slowing of mental activity, whereas the disorganization dimension was associated with an inability to inhibit inappropriate responses.
Frith et al.22 used stepwise multiple regression to examine the relationship between cognitive measures and symptoms in a large sample (N=283) of schizophrenic patients. They found no relationship between hallucinations and any cognitive measure, and delusions were related only to a decline in IQ. Negative features of schizophrenia (flattening of affect, poverty of speech, and motor retardation) were strongly associated with cognitive impairment. Two positive symptoms reflecting disorganized behavior (incoherence of speech and incongruity of affect) were also associated with cognitive impairment, independently of the effects of the negative symptoms. Cuesta and Peralta23 found only weak associations between cognitive dysfunction and the three symptom dimensions but did find disorganized and negative symptoms to be more strongly associated with cognitive disturbance than were positive symptoms.
Although there have been negative findings,24,25 the majority of studies suggest that cognitive dysfunction is associated with both the negative and the disorganized symptom dimensions of schizophrenia, but that cognitive dysfunction has little or no relationship with the psychotic dimension. The differences in the pattern of association between negative and disorganized symptom dimensions and neuropsychological function reported in these studies preclude more specific hypotheses. The present study assesses the relationship between cognitive function and negative, disorganized, and psychotic symptom dimensions in a large sample (N=134) of well-characterized patients with schizophrenia defined by DSM-IV criteria. Refinement of our understanding of the relationship between cognitive dysfunction and the symptoms of schizophrenia is important for several reasons. Evidence that specific symptom dimensions are associated with different types of cognitive dysfunction could be an important first step in identifying schizophrenic syndromes. Additionally, further knowledge concerning the relationship between cognitive function and symptoms could help answer the question of whether schizophrenia causes generalized cognitive impairment.
METHODS
Patients
The schizophrenic subjects were 90 male and 44 female patients who had a primary DSM-IV diagnosis of schizophrenia, drawn from a group of schizophrenia spectrum patients who were consecutive admissions to the Mental Health Clinical Research Center (MHCRC) at the University of Iowa. Diagnostic subtypes were as follows: 17 disorganized, 61 paranoid, 6 residual, 49 undifferentiated, and 1 catatonic. Thirty-eight of the patients were in their first hospitalization and were neuroleptic-naive at the time of admission. Patients were excluded if their primary diagnosis was not schizophrenia. Patients were also excluded from the present study if they had a history of neurological or medical disorder that would affect neuropsychological function (i.e., seizures, head trauma, stroke, brain tumor, meningitis) or if they had a recent history of abusing alcohol or psychoactive drugs. All subjects gave informed consent to participate in the study.
Diagnosis
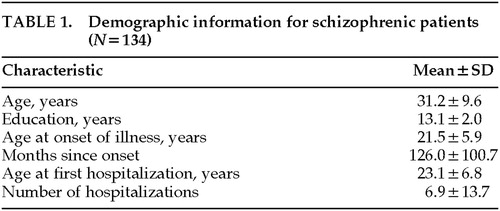
Diagnosis was by consensus of two MHCRC research psychiatrists and was based on both cross-sectional and longitudinal information as reflected in the Comprehensive Assessment of Symptoms and History (CASH), a structured interview and recording instrument.26 The CASH was completed by MHCRC nurses and physicians at the time of each patient's discharge from the hospital and was based on information collected during the entire course of hospitalization. Demographic information is given in Table 1.
Control Subjects
Control subjects were normal volunteers recruited by newspaper advertisement from the local community. Because the patient group may have had a decline in cognitive function, an effort was made to match patient and control groups as closely as possible on parental socioeconomic status. Thus, the control data provide an estimate of the level of cognitive function that would have been expected to be achieved by individuals with the same socioeconomic background as the patient group. Parental educational level (mean±SD) was 13.2±2.8 years and 13.2±years for patients and control subjects, respectively, and there was no significant between-group difference on this variable.
Phenomenological Assessment
The SANS/SAPS symptom ratings used in the present study were drawn from the CASH. The reliability of the SANS/SAPS has been found to be good to excellent for most items.27–29 Information came from research nurses who saw the patients daily and completed weekly SANS/SAPS ratings, from the primary physician during the hospital stay, and from research assistants who interviewed the patients. The SANS/SAPS ratings reflected the most severe symptoms exhibited by the patients during their hospitalization, which, for all of the patients who were not neuroleptic-naive, included a 3-week drug wash. The drug wash enabled us to rate positive symptoms that might otherwise have been suppressed by neuroleptic medication, and to observe negative symptoms independent of drug side effects. Ratings that captured the most severe symptoms exhibited by a patient were used in the correlational analysis because we assume that psychopathological symptoms have a persistent neurophysiological basis (see Discussion).
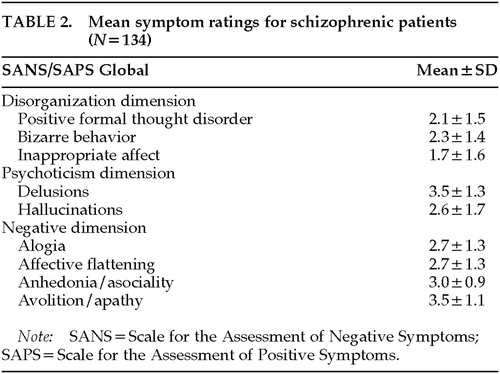
On the basis of previous factor analyses performed by our group, we computed three summary symptom measures. The summary measures reflected the dimensions of negative symptoms, disorganization, and psychoticism. Global ratings were standardized to have the same mean and standard deviation prior to computing each summary measure so that each global score contributed equally to its measure. A negative symptom score was calculated for each subject as the mean of his or her global ratings from the SANS/SAPS for anhedonia/asociality, avolition/apathy, alogia, and affective flattening. The global rating for attention was not included in the negative symptom summary measure because previous studies have shown that it is factorially complex, loading approximately equally on the negative and disorganized dimensions. A disorganized symptom summary score was composed of the mean for each subject of global ratings from the SANS/SAPS for inappropriate affect, positive formal thought disorder, and bizarre behavior. A psychotic symptom summary score was composed of the mean for each subject of global ratings from the SANS/SAPS for hallucinations and delusions. Table 2 lists the mean values for the SANS/SAPS global symptom ratings that made up each dimension.
Neuropsychological Battery
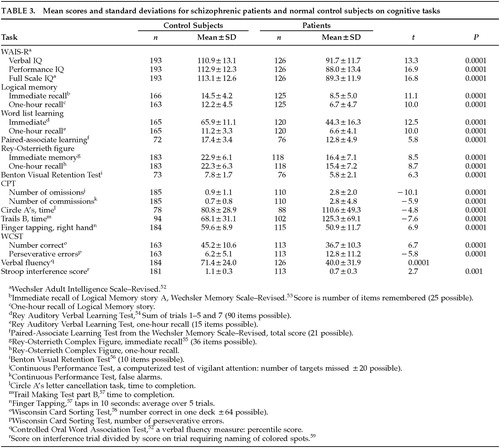
During their hospitalization, all patients received a neuropsychological battery consisting of a complete Wechsler Adult Intelligence Scale–Revised (WAIS-R) IQ test and measures of attention, verbal and nonverbal memory, verbal fluency, and executive function. For the patients in their first hospitalization, testing took place prior to administration of neuroleptic medication. For the remaining patients, testing took place at various times throughout the 3-week drug wash. Thus, cognitive assessment and rating of symptoms occurred over the same time period. Testing took place in a quiet room in sessions of about an hour each, at times when the patient was most cooperative and alert. Specific tests and the scores used in the analyses are described in Table 3. Each test was scored in the standard fashion. The battery was administered by neuropsychologists or experienced research assistants and typically took 3 to 4 hours to administer. Experienced research assistants also tested control subjects, typically during two or three scheduled sessions.
For a variety of reasons, not all control subjects and patients have scores for all of the measures listed in Table 3. The reasons include a change in the tests included in the battery, missed appointments by control subjects, and early discharge from the hospital or refusal to continue by a few patients. For both patients and control subjects, the WAIS-R was considered to be the highest priority if a complete battery could not be administered, and the numbers of both patients and control subjects are consequently highest for this measure.
RESULTS
Neuropsychological Function
As can be seen in Table 3, the mean performance level of the patients on the measures in the neuropsychological battery showed the pattern of generalized impairment that is typical of group studies of patients with schizophrenia. Results of t-tests showed that the schizophrenic patients performed more poorly than the control subjects on every measure in the battery. There is no correction for the multiple statistical tests listed in the table, but all of the t-tests were significant at the 0.001 level or above.
Correlations Between Cognitive Performance and Symptom Dimensions
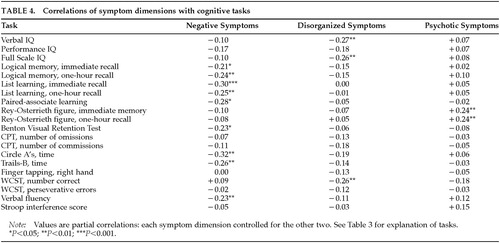
The results of primary interest for the present article involve the relationships between symptom dimensions and cognitive function that are reported in Table 4. Although the three symptom dimensions segregate as independent dimensions in factor analytic studies, they co-occur in schizophrenic patients. In the present sample, the correlation between negative and disorganized symptoms was 0.25 (P<0.001), the correlation between negative and psychotic symptoms was 0.28 (P<0.001), and the correlation between psychotic and disorganized symptoms was 0.33 (P<0.001). We used partial correlations in order to independently assess the relationship between each of the symptom dimensions and cognitive function. For example, the r-values for correlations between the negative dimension and cognitive task performance reported in Table 4 are partial correlations that control for disorganized and psychotic symptoms. Spearman rank-order correlations were performed because not all of the variables were normally distributed. For the sake of consistency in the correlational analysis, the scores in which higher values indicated poorer performance (e.g., error scores or timed scores) were transformed (multiplied by –1), so that higher values always indicate better performance.
As can be seen in Table 4, many of the tests in the battery showed a significant correlation with the negative symptom summary measure. Significant relationships with negative symptoms were found for memory for a verbally presented story (immediate and delayed recall on the Logical Memory test of the Wechsler Memory Scale, story A); memory for a word list (total items remembered across all learning trials [trials 1 through 5 and trial 7] of the Rey Auditory Verbal Learning Test), and delayed (one-hour) recall of the list; paired-associate learning from the Wechsler Memory Scale (total score); performance on the Benton Visual Retention Test; verbal fluency performance (Controlled Oral Word Association Test); time to complete a letter cancellation task (the Circle A Test); and timed performance on Trails B from the Halstead-Reitan Battery. Although performance IQ (PIQ) was not significantly correlated with negative symptoms, one of the performance subscales, Digit Symbol, did show a significant correlation (r=–0.25, P<0.01).
The disorganized symptom dimension was associated with a different and more constrained set of tasks than was the negative symptom dimension. Disorganized symptoms were significantly correlated with lower verbal IQ (VIQ) and full scale IQ (FSIQ) and with number correct on the Wisconsin Card Sorting Test (WCST). The significant FSIQ correlation is largely a function of the VIQ correlation, since PIQ was not significantly related to the disorganization score. Inspection of the subscales for VIQ showed that the disorganized symptom dimension correlated most highly for Vocabulary (r=–0.22, P<0.01) and Arithmetic (r=–0.20, P<0.05).
The psychotic symptom dimension showed no significant negative correlations with cognitive function, and there was a significant positive relationship with performance on the Rey-Osterrieth Figure, at both immediate and delayed (one-hour) assessment. The positive correlation indicates that higher levels of psychotic symptoms were associated with better performance on this task.
The results reported in Table 4 consist of a large number of analyses (60 partial correlations). We assessed, by using a sign test, whether the observed pattern of correlations might have occurred by chance. If there were no associations between any symptom dimension and neuropsychological performance, half of the correlations would be positive and half negative. We used a sign test assuming independent tests to provide approximate P-values for the patterns of correlations. We found that the majority of correlations of cognitive performance with the psychotic dimension were positive (12 of 20, sign test P=0.058, one-tailed), including significant correlations with immediate and delayed reproduction of the Rey-Osterrieth figure. In comparison, all but one of the correlations of cognitive performance with the negative symptom dimensions were negative (9 of the 20 correlations significant, sign test P<0.001), as were 18 out of 20 correlations with the disorganized dimension (three significant, sign test P<0.001).
DISCUSSION
The results of this study support the hypotheses that the negative and disorganized symptom dimensions are associated with cognitive dysfunction and that the psychotic symptom dimension has little or no association with cognitive impairment. The results also support the hypothesis that the pattern of cognitive deficits associated with negative symptoms is different from that associated with disorganized symptoms, suggesting that these two symptom dimensions could have different neurobiological substrates.
Negative Symptom Dimension
Our results indicate that the negative symptom dimension has a more widespread relationship with cognitive impairment than does the disorganization dimension. We found that the negative symptom summary score correlated with a number of different measures of verbal learning and memory, with nonverbal memory, and with verbal fluency. There was also a significant relationship with three measures that assess the ability to quickly perform manual tasks that are guided by visual information: the Digit Symbol test, Trails B, and a letter cancellation task. Interestingly, finger tapping speed, a motor task that does not require visual guidance, was not significantly correlated with negative symptoms.
Previous studies with smaller numbers of subjects found a more discrete set of cognitive functions to be associated with negative symptoms than does the present study.18–21 Liddle and Morris21 suggested that much of the cognitive dysfunction associated with negative symptoms essentially involves slowness of mental activity. We controlled for the effects of mental or motor slowness in a partial correlation analysis that used a composite score derived from the three significant visual-motor tasks (the Digit Symbol Test, Trails B, and letter cancellation). The significant correlations of the negative symptom score with the other cognitive measures changed very little when the variance associated with visual-motor slowness was partialled. This result indicates that the association we observed between the negative symptom dimension and poor verbal learning and memory, poor verbal fluency, and visual memory was independent of a general slowing of visual-motor function.
The neuropsychological measures that correlate with negative symptoms fall into several different cognitive domains (i.e., verbal and visual memory, verbal fluency, visual-motor sequencing), which could occur if high global scores for negative symptoms had heterogeneous etiologies. We assessed this possibility by examining the unique variance in neuropsychological performance that was associated with each of the four negative global scores from the SANS (i.e., alogia, affective flattening, anhedonia, avolition) that made up the negative composite. Partial correlations were computed for each SANS global score, controlling for the effects of the three other negative global scores. When the variability associated with the other negative global scores was controlled by partial correlation analysis, none of the four global scores was significantly correlated to neuropsychological deficit. This result suggests that negative symptoms, as measured by the avolition, anhedonia/asociality, affective flattening, and alogia global SANS scores, represent a unitary dimension.
The results of the present study indicate that when the variability in cognitive performance that is associated with positive symptoms is controlled, negative symptoms are associated with impairment in a variety of cognitive domains. This finding, taken together with the differing patterns of cognitive dysfunction associated with positive versus negative symptoms, suggests that global cognitive deficits are a core feature of the negative symptom dimension. This can be taken as support for the position that there are primary negative or deficit symptoms that have an etiological relationship to brain dysfunction.30 Supporting this possibility is a study31 that found that deficit patients performed more poorly than nondeficit patients on neuropsychological measures that may assess frontal (Stroop interference score and Trails B) and parietal (Mooney Faces Closure Test) function.
Disorganized Symptom Dimension
Our data indicate that the disorganization dimension is related to a more specific form of cognitive dysfunction than is the negative symptom dimension. As can be seen in Table 4, significant correlations were found between the disorganized symptom dimension and VIQ, FSIQ, and the number of correct responses on the WCST. FSIQ is a composite measure reflecting both the verbal and performance subscales of the WAIS-R. Because PIQ was not significantly correlated with the disorganization factor, the significant correlation of FSIQ largely reflects poor performance on the verbal subscales, with the Vocabulary and Arithmetic subscales being most strongly related to the disorganized dimension. The significant correlation of the disorganized composite score with number of correct responses on the WCST reflects poor problem-solving abilities that may result from a difficulty with verbally mediated processes. As noted by Berman and Weinberger,32 “inner speech” may be an important mental activity for identifying the correct response on the WCST. The overall pattern of results suggests that disorganized symptoms may be related to a specific problem with higher-order verbal processing and verbal reasoning.
Our finding that lower VIQ is significantly correlated with the disorganization dimension could be taken as evidence supporting a link between disorganized symptoms and developmental abnormalities.18 Whereas VIQ in adults is relatively insensitive to brain injury, VIQ in children is a very sensitive measure of developmental progress and educational achievement.33 The association of the disorganization dimension and low VIQ might therefore indicate a neurodevelopmental abnormality that would prevent the achievement of normal verbal intellectual skills. There is, however, evidence arguing against this possibility.
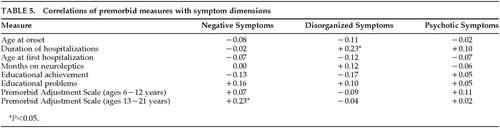
A developmental brain abnormality that preceded the onset of overt schizophrenic symptoms would be likely to cause social difficulties such as withdrawal, a failure to develop normal friendships, poor sociosexual adjustment, or low educational achievement. Table 5 lists zero-order correlations between the three symptom dimensions and variables relating to neurodevelopmental history, such as premorbid adjustment, age at onset, and course of illness. As can be seen, disorganization symptoms were significantly correlated only with duration of hospitalizations. This argues against a developmental hypothesis for the disorganization symptom dimension, and in fact suggests the possibility that disorganized symptoms reflect a neurodegenerative process. The significant correlation between negative symptoms and poor premorbid adjustment during adolescence suggests that negative symptoms may be associated with neurodevelopmental abnormalities.
Psychotic Symptom Dimension
We found no evidence that psychotic symptoms were associated with cognitive dysfunction. The only significant correlations of neuropsychological measures with the psychotic symptom composite were positive, indicating that higher levels of positive symptoms were associated with better immediate and delayed reproduction of the Rey-Osterrieth complex figure. This lack of an association between psychotic symptoms and cognitive dysfunction is consistent with findings from earlier studies that assessed cognitive function in different subtypes of patients with schizophrenia, as well as with the nosology represented in DSM-III-R and DSM-IV.
A number of studies carried out in the 1960s and 1970s found that paranoid patients who had good premorbid adjustment and a short duration of illness showed little or no cognitive impairment.34–36 Because one of the defining characteristics of the paranoid subtype is a high level of delusions, these studies may be interpreted as demonstrating that there is a subgroup of schizophrenic patients in whom there is no relationship between psychotic symptoms and cognitive dysfunction. DSM-III-R and DSM-IV explicitly incorporate this lack of association between psychotic symptoms and cognitive dysfunction into their diagnostic criteria for the paranoid type of schizophrenia. The diagnostic criteria for the paranoid type in DSM-III-R and DSM-IV include prominent delusions and the absence of cognitive dysfunction. The results of the correlational analysis used in the present study are therefore consistent with subtype approaches in showing a lack of association between psychotic symptoms and cognitive dysfunction.
Neuroanatomical Correlates
The pattern of cognitive impairment associated with the three symptom dimensions may offer insight into the neuroanatomical basis of schizophrenic syndromes, although, as discussed below, these data must be interpreted cautiously. Our finding that negative symptoms correlate with poor performance on tests of verbal learning and memory, nonverbal memory, verbal fluency, and visual-motor speed suggests that the negative symptom dimension is associated with the most severe and/or generalized brain abnormality. This finding is consistent with our magnetic resonance imaging (MRI) studies,37–39 which have consistently found negative symptoms to be associated with increased ventricular size, a nonspecific marker of diffuse brain abnormality.
If it is assumed that the left temporal lobe plays an important role in language processing, then our finding that the disorganized dimension is associated with verbal processing deficits is consistent with a report that left temporal lobe MRI abnormalities are correlated with thought disorder in patients with schizophrenia.40 This finding is not consistent, however, with a recent MRI finding from our group that smaller left superior temporal gyral volume was correlated with the psychotic symptom dimension, and specifically with auditory hallucinations.37 A possible explanation for this conflicting finding may be found in current theories of brain organization that indicate that behavior is mediated by distributed networks rather than by isolated brain regions.41
It seems likely that distributed networks that include a variety of cortical and subcortical components mediate both clinical symptoms and cognitive functions in patients with schizophrenia. Components of the distributed network mediating a specific symptom dimension may overlap with components of the networks mediating other symptoms. Supporting this possibility is a study by Friston et al.,42 which used an exploratory canonical correlational analysis of regional cerebral blood flow (rCBF) and symptom data. The analysis indicated that florid and disorganized symptoms together formed a vector that was associated with increased rCBF in left superior temporal gyrus and in other brain regions. If the left superior temporal gyrus is a shared component in the networks mediating psychotic and disorganized symptoms, then subtle abnormalities in this region might be associated with psychotic symptoms in some patients and disorganized symptoms in others.
The nonsignificant correlations of negative symptoms with putative tests of frontal lobe integrity (WCST perseverative errors, Continuous Performance Test [CPT,] Stroop interference score) in the present study are surprising, given the large number of tests that showed significant relationships with negative symptoms. The finding is consistent with MRI findings from our group that showed a lack of association between frontal lobe size and negative symptoms,38,39 but it conflicts with functional brain imaging studies (from Iowa and elsewhere43–45) that have found the negative symptoms of schizophrenia to be associated with lower than normal blood flow in subregions of the frontal lobes (i.e., hypofrontality). One explanation for the apparent discrepancy between our neuropsychological and MRI findings and the findings of functional imaging studies is that possibly neither “frontal lobe tasks” nor frontal lobe size may be sensitive to subtle abnormalities of the frontal lobes that can be detected in blood flow studies. That is, the WCST, Stroop Test, and CPT tap a diversity of cognitive functions, only some of which may be localized to the frontal lobes. Additionally, the frontal lobes are a large and functionally heterogeneous cerebral region whose overall size may have little to do with functionality and blood flow within specific subregions.
There are a number of reasons for being cautious in drawing inferences concerning brain localization from correlations observed between symptom dimensions and cognitive function. Although cognitive tasks may serve a localizing function in patients with structural brain damage, it is not clear that they can play a similar role in schizophrenic patients.46 Inferring the location of a brain lesion from a cognitive task requires finding a specific impairment amidst a group of measures showing relatively normal performance. As can be seen in Table 3, groups of schizophrenic patients perform poorly on almost all cognitive tasks, and the question of whether more specific deficits exist remains controversial. This fact severely limits the possibility of using cognitive tasks as neuropsychological indices of localized brain abnormality in schizophrenia.
An additional reason for caution in attempting to localize abnormalities lies in the nature of the distributed neural networks that are likely to underlie both symptoms and cognitive function. The symptoms of schizophrenia presumably result from dysfunction in one or more components of a functional circuit that includes both cortical and subcortical regions. Finding a significant correlation between a symptom dimension and a cognitive task is evidence that there is overlap between the network underlying the symptoms and the distributed network mediating the task. But localizing the dysfunctional component will require subtle neuroanatomical techniques. Utilizing such a technique, our group has recently found evidence of abnormalities in the thalamus in schizophrenic patients.47 The thalamus is a relatively small subcortical structure with heterogeneous functions that is massively interconnected with all other parts of the brain. Various nuclei of the thalamus are undoubtedly components in the functional circuits that mediate cognition, behavior, and emotion.
We have recently proposed9–11 that schizophrenia is characterized by “cognitive dysmetria,” defined as a disruption of the fluid, coordinated sequences of thought and action that are the hallmark of normal cognition.48 The basis of the “poor coordination” may be a defect in the timing or sequencing component of mental activity. This defect cuts across most of the traditionally defined cognitive systems (e.g., memory, attention) and affects the efficiency and accuracy of their related subprocesses (e.g., memory retrieval, inhibition). Failure of a basic mechanism that synchronizes activity in cognitive subprocesses could lead a schizophrenic individual to incorrectly connect perceptions and associations and to misinterpret both external and internal processes, leading in turn to delusions or hallucinations. Defects in coordinating language processing could lead to “thought disorder.” In addition, the flow of information through the system could become paralyzed, leading to “negative symptoms” such as alogia or affective blunting.
The present study was not designed to explore the dysmetria concept, but rather to assess the unique association of each symptom dimension with cognitive dysfunction. To this end, we used partial correlational techniques, which allowed the correlation of each dimension with cognitive performance to be examined independently of the other two dimensions. This analysis strategy removes any association between cognitive performance and symptoms that may be shared by all three dimensions. The fact that this shared variability was removed is the most likely explanation for the relatively low correlations that we found between symptom dimensions and cognitive function.
Assessment Strategy for Symptoms and Cognitive Function
The present study has both strengths and weaknesses. The large number of schizophrenic patients in the study is a strength, permitting the use of a partial correlational approach that would not otherwise be valid. Using this approach allowed assessment of the unique variance in cognitive task performance that is explained by each symptom dimension. As noted, however, this strategy removes variance shared by the three symptom dimensions.
Another strength of the study is the accuracy of the clinical assessment, which was performed by research personnel who were well trained on the assessment instruments. The clinical ratings were obtained over an extended period of time, giving the raters a large sample of the patients' behavior. Additionally, the clinical ratings were obtained on patients who went through a 3-week medication washout, ensuring that symptoms were not masked or confounded by medication. Research personnel who have extensive experience working with patients with schizophrenia also administered the neuropsychological battery. Patient availability on the MHCRC inpatient unit for an extended period of time allowed testing to take place at times when the patient was cooperative. Testing was stopped when the patient became fatigued or uncooperative. These factors improve the accuracy of the cognitive assessment.
The strategy of rating symptoms when they are at their worst, during a period of hospitalization that includes a drug wash, but assessing cognitive function when the patient is at his or her best during the hospitalization, is undeniably more valid for some symptom dimensions and cognitive tasks than for others. It is based on the assumption that both cognitive dysfunction and clinical symptomatology reflect abnormal neurophysiological processes that are relatively long-standing. It can therefore assess only trait-like relationships between symptoms and cognitive function. The strategy also undoubtedly results in conservative estimates of the relationship between symptoms and cognitive function. Cognitive tasks whose scores are sensitive to subtle changes in cognitive state are less appropriate for this analysis strategy than are tasks that assess more stable cognitive traits. Our failure to find significant correlations of symptoms with putative measures of frontal lobe function (WCST, CPT, and Stroop interference) may have occurred because these measures tap abilities that fluctuate rapidly, being impaired when the patient is ill but recovering when symptoms subside. The strategy used here may also be more valid for negative symptoms, which tend to fluctuate less over time than do psychotic or disorganized symptoms.
The cross-sectional correlational design strategy used in the present study renders it impossible to make causal inferences about the relationship between cognitive dysfunction and symptoms. Additionally, patients were studied at various phases of their illness, and the relationship of symptoms and cognitive performance may be variable over time. These points reflect complex issues that cannot be dealt with in a single study. We have an ongoing longitudinal study that will permit more explicit conclusions about possible changes over time in the relationship between symptoms and cognitive performance. We have published some initial analyses of the data from this ongoing study, which demonstrates that cognitive function in schizophrenic individuals is relatively stable over time.49,50 We have also addressed the issue of cognitive function in first-episode patients and are assessing the effects of chronicity on cognitive function.51
CONCLUSIONS
Using partial correlational analyses to assess the unique variance in neuropsychological test performance that is associated with clinical symptoms in schizophrenic patients, we found evidence that supports a three-dimensional model of symptomatology. Psychotic symptoms did not correlate with cognitive impairment, which indicates that individual variability in severity of hallucinations and delusions is not related to variability in cognitive task performance. A negative symptom composite was associated with poor performance on tests of verbal learning and memory, visual memory, and verbal fluency, and on visual-motor tasks, but not on tasks thought to assess frontal lobe function. This global pattern of impairment was not due to motor slowness, and the pattern suggests that generalized brain dysfunction is a core feature of a primary negative symptom dimension. The correlation of negative symptoms with poor premorbid adjustment during adolescence suggests the possibility that negative symptoms are associated with an abnormal developmental process that affects the final stage of brain maturation and predates the onset of overt symptoms.
In contrast to previous studies, we found in this study that negative symptoms had a stronger relationship to cognitive dysfunction than did disorganized symptoms. Disorganized symptoms were associated with poor performance on verbal intelligence and reasoning tasks, suggesting that more specific brain impairment is associated with this symptom dimension. It may be that the large number of subjects in the present study, along with the use of partial correlational analysis, allowed us to see relationships that were masked by shared variance between negative and disorganized symptoms in previous studies.
 |
 |
 |
 |
 |
1 Braff DL: Information processing and attention dysfunctions in schizophrenia. Schizophr Bull 1993; 19:233–259Crossref, Medline, Google Scholar
2 Kolb B, Whishaw IQ: Performance of schizophrenic patients on tests sensitive to left or right frontal, temporal, or parietal function in neurological patients. J Nerv Ment Dis 1983; 171:435–443Crossref, Medline, Google Scholar
3 Nuechterlein KH, Dawson ME: Information processing and attentional function in the developmental course of schizophrenic disorders. Schizophr Bull 1988; 10:160–203Crossref, Google Scholar
4 Goldberg TE, Weinberger DR, Berman, KF, et al: Further evidence for dementia of the prefrontal type in schizophrenia? A controlled study of teaching the Wisconsin Card Sorting Test. Arch Gen Psychiatry 1987; 44:1008–1014Google Scholar
5 Saykin AJ, Gur RC, Gur RE: Neuropsychological function in schizophrenia, selective impairment in learning and memory. Arch Gen Psychiatry 1991; 48:618–623Crossref, Medline, Google Scholar
6 Blanchard JJ, Neale JM: The neuropsychological signature of schizophrenia: generalized or differential deficit? Am J Psychiatry 1994; 151:40–48Google Scholar
7 Chapman LJ, Chapman JB: The measurement of differential deficit. J Psychiatr Res 1978; 14:303–311Crossref, Medline, Google Scholar
8 Chapman LJ, Chapman JB: Strategies for resolving the heterogeneity of schizophrenics and their relatives using cognitive measures. J Abnorm Psychol 1989; 98:357–366Crossref, Medline, Google Scholar
9 Andreasen NC, O'Leary DS, Cizadlo T, et al: Schizophrenia and cognitive dysmetria: a PET study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci 1996; 93:9985–9990Google Scholar
10 Andreasen NC: Linking mind and brain in the study of mental illnesses: a project for a scientific psychopathology. Science 1997; 275:1586–1593Google Scholar
11 Andreasen NC, Paradiso S, O'Leary DS: “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998; 24:1730–1734Google Scholar
12 Andreasen NC: The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA, The University of Iowa, 1983Google Scholar
13 Andreasen NC: The Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA, The University of Iowa, 1984Google Scholar
14 Andreasen NC, Grove WM: Evaluation of positive and negative symptoms in schizophrenia. Psychiatrie et Psychobiologie 1986; I:108–121Google Scholar
15 Arndt S, Alliger RJ, Andreasen, NC: The positive and negative distinction: failure of the two dimensional model. Br J Psychiatry 1991; 158:317–322Crossref, Medline, Google Scholar
16 Miller DD, Arndt S, Andreasen NC: Alogia, attentional impairment, and inappropriate affect: their status in the dimensions of schizophrenia. Compr Psychiatry 1993; 34:221–226Crossref, Medline, Google Scholar
17 Andreasen NC, Arndt S, Alliger R, et al: Symptoms of schizophrenia: methods, meanings, and mechanisms. Arch Gen Psychiatry 1995; 52:341–351Crossref, Medline, Google Scholar
18 Bilder RM, Mukherjee S, Rieder RO: Symptomatic and neuropsychological components of defect states. Schizophr Bull 1985; 11:409–419Crossref, Medline, Google Scholar
19 Liddle PF: The symptoms of chronic schizophrenia: a re-examination of the positive-negative dichotomy. Br J Psychiatry 1987; 151:145–151Crossref, Medline, Google Scholar
20 Liddle PF: Schizophrenic symptoms, cognitive performance and neurological dysfunction. Psychol Med 1987; 17:49–57Crossref, Medline, Google Scholar
21 Liddle PF, Morris D: Schizophrenic syndromes and frontal lobe performance. Br J Psychiatry 1991; 158:340–345Crossref, Medline, Google Scholar
22 Frith CD, Leary J, Cahill C, et al: Performance on psychological tests: demographic and clinical correlates of these results. Br J Psychology 1991; 159(suppl 13):26–29Google Scholar
23 Cuesta MJ, Peralta V: Cognitive disorders in the positive, negative and disordered syndromes of schizophrenia. Psychiatr Res 1995; 58:227–235Crossref, Medline, Google Scholar
24 Van der Does AJ, Dingemans PM, Linszen DH, et al: Symptom dimensions and cognitive and social function in recent onset schizophrenia. Psychol Med 1993; 23:745–753Crossref, Medline, Google Scholar
25 Morrison-Stewart SL, Williamson PC, Corning WC, et al: Frontal and non–frontal lobe neuropsychological test performance and clinical symptomatology in schizophrenia. Psychol Med 1992; 22:353–359Crossref, Medline, Google Scholar
26 Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing psychopathology and diagnosis. Arch Gen Psychiatry 1992; 49:615–623Crossref, Medline, Google Scholar
27 Andreasen NC: The American concept of schizophrenia. Schizophr Bull 1989; 15:519–531Crossref, Medline, Google Scholar
28 Andreasen NC, Flaum M: Schizophrenia: the characteristic symptoms. Schizophr Bull 1991; 17:25–49Crossref, Google Scholar
29 Andreasen NC, Flaum M, Arndt S, et al: Positive and negative symptoms: assessment and validity, in Negative Versus Positive Schizophrenia, edited by Marneros A, Andreasen NC, Tsuang MT. Berlin and Heidelberg, Springer-Verlag, 1991, pp 28–51Google Scholar
30 Carpenter WT: Psychopathology of common sense. Biol Psychiatry 1991; 29:735–737Crossref, Medline, Google Scholar
31 Buchanan RW, Strauss ME, Kirkpatrick B, et al: Neuropsychological impairments in deficit vs nondeficit forms of schizophrenia. Arch Gen Psychiatry 1994; 51:804–811Crossref, Medline, Google Scholar
32 Berman KF, Weinberger DR: Lateralization of cortical function during cognitive tasks: regional cerebral blood flow studies of normal subjects and patients with schizophrenia. J Neurol Neurosurg Psychiatry 1991; 53:150–160Crossref, Google Scholar
33 Boll TJ, Barth JT: Neuropsychology of brain damage in children, in Handbook of Clinical Neuropsychology, edited by Filskof SB, Boll TJ. New York, Wiley, 1981, pp 418–452Google Scholar
34 Cromwell RL: Assessment of schizophrenia. Annu Rev Psychol 1975; 26:593–619Crossref, Medline, Google Scholar
35 Silverman J: The problem of attention in research and theory in schizophrenia. Psychol Rev 1964; 71:352–379Crossref, Medline, Google Scholar
36 Venables PH: Input Dysfunction in Schizophrenia (Progress in Experimental Personality Research), edited by Maher BA. New York, Academic Press, 1964, pp 1–42Google Scholar
37 Flaum M, O'Leary DS, Swayze VW, et al: Symptom dimensions and brain morphology in schizophrenia: a magnetic resonance study. J Psychiatr Res 1995; 4:261–276Crossref, Google Scholar
38 Andreasen NC, Nasrallah HA, Dunn VD, et al: Structural abnormalities in the frontal system in schizophrenia. Arch Gen Psychiatry 1986; 43:136–144Crossref, Medline, Google Scholar
39 Andreasen NC, Ehrhardt JC, Swayze VW, et al: Magnetic resonance imaging of the brain in schizophrenia. Arch Gen Psychiatry 1990; 47:35–44Crossref, Medline, Google Scholar
40 Shenton ME, Kikinis R, Jolesz FA, et al: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
41 Goldman-Rakic PS: Changing concepts of cortical connectivity: parallel distributed networks, in Neurobiology of Neocortex, edited by Rakic P, Singer W. New York, Wiley, 1989, pp 177–202Google Scholar
42 Friston KJ, Liddle PF, Frith CD, et al: The left medial temporal region and schizophrenia: a PET study. Brain 1992; 115:367–382Crossref, Medline, Google Scholar
43 Andreasen NC, Rezai K, Alliger R, et al: Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia: assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49:943–958Crossref, Medline, Google Scholar
44 Liddle PF, Friston KJ, Frith CD, et al: Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry 1992; 60:179–186Crossref, Google Scholar
45 Kaplan RD, Szechtman H, Franco S, et al: Three clinical syndromes of schizophrenia in untreated subjects: relation to brain glucose activity measured by positron emission tomography (PET). Schizophr Res 1993; 11:47–54Crossref, Medline, Google Scholar
46 David AS: Frontal lobology: psychiatry's new pseudoscience. Br J Psychiatry 1992; 161:244–248Crossref, Medline, Google Scholar
47 Andreasen NC, Arndt S, Swayze VW, et al: Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 1994; 266:294–298Crossref, Medline, Google Scholar
48 Schmahmann JD: An emerging concept: the cerebellar contribution to higher function. Arch Neurol 1991; 48:1178–1187Google Scholar
49 Nopoulos P, Flashman L, Flaum M, et al: Stability of cognitive functioning early in the course of schizophrenia. Schizophr Res 1994; 14:29–37Crossref, Medline, Google Scholar
50 Gold S, Arndt S, Nopoulos P, et al: Longitudinal study of cognitive function in first episode and recent-onset schizophrenia. Am J Psychiatry 1999; 156:1342–1348Google Scholar
51 Mohamed S, Paulsen J, O'Leary DS, et al: Generalized cognitive deficits in schizophrenia. Arch Gen Psychiatry 1999; 56:749–775Crossref, Medline, Google Scholar
52 Wechsler D: Wechsler Adult Intelligence Scale–Revised. San Antonio, TX, Harcourt Brace, 1982Google Scholar
53 Wechsler D: Wechsler Memory Scale–Revised. New York, The Psychological Corporation, 1987Google Scholar
54 Lezak MD: Neuropsychological Assessment, 2nd edition. New York, Oxford University Press, 1983Google Scholar
55 Osterrieth PA: Le test de copie d'une figure complex: contribution a l'etude de la perception et de la memoire. Archives de Psychologie 1944; 30:286–356Google Scholar
56 Spreen O, Benton AL: Neurosensory Center Comprehensive Examination for Aphasia (revised edition). Victoria, BC, University of Victoria Neuropsychology Laboratory, 1977Google Scholar
57 Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ, Neuropsychology Press, 1985Google Scholar
58 Heaton RK: Wisconsin Card Sorting Test Manual. Odessa, FL, Psychological Assessment Resources, 1981Google Scholar
59 Golden JC: Stroop Color and Word Test. Chicago, Stoelting, 1978Google Scholar



