A Review of the Cognitive and Behavioral Symptoms in Dementia With Lewy Bodies
Abstract
Dementia with Lewy bodies is a relatively common cause of dementia. Much has been learned about this disorder, yet much remains to be elucidated, especially in regard to early clinical diagnosis. To clarify the future research agenda in this area, the authors critically appraise the literature on cognitive and behavioral changes in DLB and provide a brief overview of the history of DLB, the main pathological changes, and the findings related to extrapyramidal symptoms and treatment issues. Twenty-one studies on cognition and 47 on behavioral changes in DLB are reviewed. Impairments of working memory and visuospatial functions, visual hallucinations, and depression (or symptoms of depression such as apathy and anxiety) have been identified as early indicators of DLB. However, longitudinal and cross-sectional data are lacking, particularly for different aspects of working memory, visual perception, and nonpsychotic behavioral symptoms.
According to the neuropathological findings of McKeith et al.,1 gathered in a U.K. geriatric department between 1982 and 1987, dementia with Lewy bodies (DLB) is the second most common cause of dementia following Alzheimer's disease (AD), with a prevalence of 19% compared with 52% for AD. Other data gathered in 284 autopsied cases, in a Norwegian general hospital without a geriatric department, suggest that Lewy body pathology is the third most common cause of dementia (7.7%), after AD changes (71.4%) and cerebrovascular lesions (33.8%).2 Almost 96% of subjects with Lewy bodies also had AD changes. Clinically, the prevalence of dementia with Lewy bodies varies depending on the clinical criteria that are applied. Shergill et al.3 reported that the prevalence of DLB among 114 patients diagnosed with dementia (ICD-10) was 26.3% according to the McKeith et al.4 criteria for Senile Dementia of Lewy Body Type, 7% according to Byrne “probable” criteria, and 16.6% according to Byrne “possible” criteria.5
Although prevalence estimates clearly vary, it is also clear that DLB is relatively common. Much has been learned about this disorder, yet much remains to be elucidated, especially with regard to the cognitive and behavioral symptoms of this disease. A proper and early detection of the cognitive and behavioral symptoms is crucial for the differential diagnosis of dementia with Lewy bodies, because these symptoms frequently are the principal complaints at the initial consultation. Therefore, our goals in this review are 1) to provide a critically appraised synopsis of the literature with a focus on the cognitive and behavioral changes of DLB and 2) to target critical areas of needed research. To address these goals, we review the history of DLB, summarize the pathological changes, briefly review findings related to extrapyramidal symptoms and treatment issues regarding behavioral and cognitive symptoms in DLB, and then provide an extensive review of the literature relative to cognitive and behavioral changes in DLB.
Clinically, a better understanding of the cognitive and behavioral symptoms will help to generate an early diagnosis and hence the early planning of an appropriate treatment. In addition, a better understanding of the cognitive and behavioral symptoms of DLB will contribute to the development of a mechanistic model of the disease. Such a model is at this moment difficult to establish because a lot of neuropsychological and imaging data are still lacking.
METHODS OF THE LITERATURE SEARCH
MEDLINE and Psychological Abstracts were searched by using the following key words: 1) “Lewy bodies”; 2) “cognitive ability (explode)” AND “dementia (explode),” “cognitive ability (explode)” AND “Alzheimer's disease,” “cognitive ability (explode)” AND “prediction of dementia”; 3) “dementia (explode)” AND “screening (explode),” 4) “Lewy bodies” AND “psychotic disorders (explode)” OR “delusions” OR “schizophrenia,” and “dementia and psychosis”; and 5) “Lewy bodies” AND “depression (text words),” “Lewy bodies” AND “anxiety (text words),” “Lewy bodies” AND “apathy (text words),” and “Lewy bodies” AND “agitation (text words).” The search included literature that has been published within the past 5 to 8 years. A manual search was also conducted on English and French journal articles to obtain references to other publications. Case reports and retrospective studies that did not mention the presence of cortical Lewy bodies were excluded. For the review of cognitive impairments, only papers with quantified data were retained.
HISTORICAL OVERVIEW
Lewy bodies (LB) are intracytoplasmic, spherical, eosinophilic neuronal inclusions that were originally identified in subcortical nuclei as one of the cardinal characteristics of Parkinson's disease (PD).6–8 Following the publication of several case reports of Japanese and Austrian subjects with dementia presenting Lewy bodies in brainstem nuclei as well as in cortical areas (Table 1, section A),4,5,9 Kosaka10 described the appearance of cortical versus brainstem Lewy bodies. This detailed description had been aimed for researchers using various hematoxylin and eosin staining preparations in combination with other techniques such as Klüver-Barrera, Nissl, Mallory azan, PTAH, Bodian, pyridine silver, bromphenol blue, CTR, Millon, Sakaguchi, Sudan III, Sudan black B, periodic acid-Schiff, Best's carmine, Alcian blue, and Congo red. The development, by Kuzuhara et al.,11 of the monoclonal anti-ubiquitin immunostaining method, a more sensitive technique to detect cortical Lewy bodies than the conventional staining methods, has prompted studies in Europe and America in patients presenting an atypical dementia and cortical Lewy bodies at autopsy. In the 1980s, retrospective studies and case reports showed that some of the atypical patients initially diagnosed as suffering from Alzheimer's disease, Parkinson's disease, and/or multi-infarct dementia12,13 actually had cortical Lewy bodies.1,14–56
Kosaka and colleagues12,57 classified diseases in which Lewy bodies are found into three groups: 1) group A, diffuse type, also called Lewy Body Disease Variant or LBV; 2) group B, transitional type; and 3) group C, “brainstem” type, related to Parkinson's disease. The diffuse type was also classified into two subgroups: 1) the “common form” group in which senile plaques and neurofibrillary tangles as well as Lewy bodies are found in the cortex in sufficient number to meet the pathologic diagnostic criteria for Alzheimer's disease and 2) the “pure form” group in which cortical Lewy bodies are found in sufficient number but the number of senile plaques and neurofibrillary tangles in the cortex does not meet the criteria for Alzheimer's disease per Khatchaturian.58 Recently, the Consortium on Dementia with Lewy Bodies (CDLB) recommended Dementia With Lewy Bodies (DLB) as a generic term for all of these cases, acknowledging the presence of LB without specifying their relative importance in symptom formation with respect to other degenerative or vascular pathology that might be simultaneously present.9
The CDLB defined the pathological features associated with DLB as follows: 1) essential for the diagnosis of DLB: Lewy bodies; 2) associated but not essential: Lewy- related neurites; plaques (all morphologic types); neurofibrillary tangles; regional neuronal loss especially in brainstem (substantia nigra and locus ceruleus) and nucleus basalis of Meynert; microvacuolation (spongiform change) and synapse loss; neurochemical abnormalities and neurotransmitter deficits.9 The Consortium also recommended the examination of three neocortical regions (the frontal, temporal, and parietal cortices); two limbic cortical regions (the anterior cingulate and transentorhinal cortices); and brainstem regions (substantia nigra, locus ceruleus, and dorsal nucleus of vagus) to establish the definite neuropathological diagnosis.9 Following a study with 20 cases having a pathological diagnosis of Alzheimer's disease and/or Parkinson's disease and 8 control cases, Harding and Halliday59 have proposed the following changes to the CDLB neuropathologic diagnostic procedure: 1) elimination of the parietal and frontal association regions from the analysis, 2) elimination of cortical layers I and II from the sampling, and 3) exclusion of cases without brainstem Lewy bodies.
Clinical criteria were developed in the 1990s following the case reports and the first retrospective studies (see Table 1).4,5,9 The first clinical criteria5 were inspired by the school of Kosaka et al.,12 where DLB is viewed as closely related to idiopathic Parkinson's disease. According to Byrne et al.,5 extrapyramidal symptoms (EPS), together with a dementia per DSM-III-R, are necessary for a diagnosis of dementia with Lewy bodies (see section C of Byrne's criteria, Table 1). To meet criteria for probable dementia, a subject must have dementia at onset or later in classical Parkinson's disease or have EPS at onset of dementia. To meet criteria for possible dementia, a subject must have EPS appearing later in the dementing process. The time of onset and the number of EPS make the difference between a diagnosis of possible and one of probable DLB, according to Byrne et al.5 The only indication regarding cognition refers to prominent attention deficits and acute confusional states. Byrne et al.5 did not mention any behavioral or psychotic symptoms.
A year later, criteria for Senile Dementia of Lewy Body Type (SDLT) were published.4 McKeith et al.4 based their clinical description of SDLT primarily on the retrospective data collected in their geriatric psychiatry unit in Newcastle.1,4 More of a focus on the cognitive changes of DLB was evident. The cognitive impairment, affecting memory and higher cognitive functions, was seen to fluctuate. These cognitive fluctuations were felt to be the hallmark of dementia with Lewy bodies. EPS were considered as a possible feature, but they were not felt to be requisite anymore. McKeith et al.4 have introduced to the diagnostic criteria psychiatric features such as hallucinations (auditory or visual) accompanied by paranoid delusions, neuroleptic sensitivity syndrome, repeated unexplained falls, and clouding or loss of consciousness.4 For a subject to meet criteria for SDLT, cognitive fluctuations, occurring with either hallucinations and paranoid delusions, mild EPS, neuroleptic sensitivity, repeated falls, or transient clouding of consciousness, are required. These criteria have been found to have a high diagnostic specificity of 90.0% to 97.0%, with a mean sensitivity of 74%. Sensitivity was greater in the more experienced clinicians (90%) than in the least experienced clinician (55%).48 Interrater reliability has varied between the most experienced raters (94%; kappa=0.87) and the least experienced rater (78%; kappa=0.50).48
In 1996, another set of criteria was proposed by the CDLB, being an update, an integration, and a refinement of the previous criteria.9 The notions of gradual impairment and of the “possible” and “probable” diagnoses were reintroduced, and a progressive cognitive decline became the central feature. Together with the cognitive decline, fluctuations in attention and alertness, recurrent visual hallucinations, and/or EPS (no more than 1 year before the onset of cognitive decline) should be present. The difference between a possible and a probable diagnosis lies in the number of core features exhibited (see Table 1). The CDLB criteria have also added supportive features to the diagnosis of DLB: repeated falls; syncope; transient loss of consciousness; neuroleptic sensitivity syndrome; systemized delusions (usually paranoid); and hallucinations in other modalities.
Using retrospective chart review, done by 5 neurologists, and a blinded pathologic evaluation, Mega et al.60 have studied reliability and validity of the clinical criteria of the CDLB. They found the sensitivity/specificity ratio of the CDLB's probable DLB clinical criteria was 75%/79%. Reformulated clinical criteria that required the presence of EPS significantly predicted those patients with many LBs versus those with few or no LBs and increased clinical specificity to 100%.60 Interrater reliability results for each of the CDLB possible features were used to extract the best-operationalized combination of symptoms. These include (from the most likely to the least likely): hallucinations, cogwheeling, rigidity, bradykinesia, neuroleptic sensitivity, and evidence of fluctuations in cognition. Fluctuation in cognition has been the most difficult feature to identify and agree on.60 Because the typical AD patient loses about 3 points per year61,62 on the Mini-Mental State Examination (MMSE) total score, Mega et al.60 recommended that fluctuations of 5 points or more on the MMSE total score over 3 administrations in a 6-month period be considered significant “fluctuations” for DLB.
PATHOLOGICAL CHANGES IN DLB
Cortical Lewy bodies are found in small and middle neurons, in deep cortical layers of every lobe, with a predilection for the anterior frontal and temporal areas,22,33 the cingulate area,12,33,63 and the insula.12,22,33 In the subcortical structures, Lewy bodies are numerous in the substantia nigra and can also be observed in the nucleus basalis of Meynert, in the locus ceruleus, in the nucleus raphe dorsalis, and in the amygdaloid complex.12,17,22,33,34 In the amygdala, Lewy bodies can be found in conjunction (in the same neuron) with neurofibrillary tangles (NFT) in both DLB and AD.64
Neuritic degeneration in the CA2/3 region of the hippocampus, not seen in AD or in normal aging, is correlated with the presence of cortical Lewy bodies.65 More significant neuronal losses than in AD are described in the substantia nigra, the nucleus basalis of Meynert,34,47,66 and the frontal lobe of subjects with LBV (i.e., DLB).34
Subjects with DLB also present neurochemical dysfunction. Perry et al.67–69 described reductions in the cortical cholinergic enzyme choline acetyltransferase (ChAT) that is more severe in individuals with hallucinations (80%–85%) than in those without (50%–55%).67–68 These ChAT reductions were more extensive in neocortical as opposed to archicortical regions66,69,70 and were more marked in the parietal and temporal cortex67 as well as in the hippocampus and entorhinal cortex in patients with DLB69,71 than in patients with AD.71 Frontal depletion of ChAT was also observed.69–71 As a consequence of these ChAT reductions, there is a lack of cortical acetylcholine in DLB brains. However, an ACh compensation mechanism seems to be present. Unlike the pathological process in AD, in DLB the number of cortical postsynaptic muscarinic receptors (particularly of the low-affinity subtype L) is significantly increased.68,69 Simultaneously to the depletion of cortical ACh, a reduction of dopamine (DA) levels, related to the substantia nigra neuronal loss, has been described in the basal ganglia of subjects with DLB. An approximate loss of 40% to 60% of dopamine in DLB brains, compared with an 80% loss in Parkinson's disease brains, was reported,68–72 as well as a decrement in the basal ganglia levels of homovanillic acid.70 Cell loss in the ventral tegmental area, observed in one case of dementia with cortical LB, suggests abnormalities in the DA mesocorticolimbic system.73
Some recent PET data collected in two groups of mildly to moderately demented subjects meeting criteria for probable DLB (CDLB criteria)9 and probable AD (National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association [NINCDS-ADRDA] criteria)74 showed a more severe glucose hypometabolism in DLB compared with AD in the cerebellar hemispheres and the temporal-parietal-occipital association cortices,75 and especially in the medial and lateral occipital lobes.55 A more severe hypometabolism in the medial temporal and cingulate areas has otherwise been found in AD when compared with DLB.75
RELATIONSHIP TO OTHER DEMENTIAS
The nature of dementia with Lewy bodies is currently a controversial matter. Yoshimura22 and Kosaka et al.12 have suggested that diffuse Lewy body disease or dementia with Lewy bodies might be an extended form of idiopathic Parkinson's disease. Their description of the various forms of the disorder were based on the observation that their demented patients all had clinical signs of parkinsonism and pathological evidence of Lewy bodies in the brainstem. Some of these patients had cortical LB (cLB), and some were devoid of them.12,22 Although it is well recognized that all demented patients with cortical LB also present brainstem LB, cLB have however been reported in demented patients with no clinical signs of parkinsonism but presenting some pathological AD changes.9,16,24 Therefore, other authors hypothesize that DLB is the coexistence of AD and PD.76,77
Brown et al.76,77 argue that LB surimpose on gradually existing AD changes in the brain, starting in the brainstem and then invading the cortex. They demonstrated that cLB were more numerous in 22 AD subjects with concomitant substantia nigra (SN) LB than in 6 pure AD (without SN LB) and 8 pure PD subjects with SN LB.76 All patients with pure AD and AD/PD were clinically demented, whereas only 75% of patients with pure PD (with SN LB) were demented.76 Brown et al.77 also found that synaptophysin concentrations, related to synaptic density, were significantly lower in 14 DLB and 31 AD subjects when compared with 10 healthy control subjects. DLB and AD patients showed a comparable loss of synapses in the frontal, temporal, and parietal lobes.76
Neurofibrillary tangles are a fundamental neuropathological feature of Alzheimer's disease.58,74 However, Brown and co-workers76,77 showed a significant negative correlation between neocortical LB and NFT density in the brain of patients with AD/PD or LBV. The scarcity of NFT in DLB brains has been reported by other authors65,71,78 and was characterized, by Dickson et al.,65 as a hallmark of a disease distinct from Alzheimer's disease. According to Dickson et al.,65 this distinct disease, DLB, can occur independently or in coexistence with pathological aging or AD. In support of their argument, they mention other neuropathological changes that take place in DLB, such as the neuritic degeneration in the CA2/3 region of the hippocampus, which does not occur in AD.65 More recently, other authors have reported findings supportive of the hypothesis of Dickson et al.65 They found a positive correlation between cLB and cognitive impairment, whereas NFT and synapse density did not correlate with cognitive status71,78 and the number of senile plaques correlated less significantly than cLB with the MMSE score.71
ANTEMORTEM PRESENTATION: EXTRAPYRAMIDAL SYMPTOMS
Until recently, results regarding the relationship of EPS and hallucinations to severity of dementia and rate of cognitive decline, as measured with brief assessments in cross-sectional and longitudinal studies, have been controversial (see review by Ellis et al.79 on EPS in Alzheimer's disease). Methodological problems might explain this controversy. There has been confusion between neuroleptic-induced and noninduced EPS, a lack of consideration for concomitant behavioral disturbances and institutionalization, and an absence of matching of the groups with and without EPS on baseline dementia severity.79
One group of researchers80–82 has strictly and retrospectively studied the presentation of EPS in autopsied cases of demented (per DSM-III-R) subjects with DLB and PD. They have identified some EPS that may distinguish between idiopathic Parkinson's disease and dementia with Lewy bodies. They found, in 31 DLB and 34 PD subjects, that the occurrence of any one of four clinical features—myoclonus, absence of resting tremor, no response to levodopa, or no perceived need to treat with levodopa—was 10 times more likely in DLB than in PD (odds ratio=10.29, 95% confidence interval=2.58–41.11).82 The likelihood of having DLB, given any one of these four features, was 85.7% (positive predictive value). Most patients with DLB had either bradykinesia (89.5%) or rigidity (92.8%).82 Gnanalingham et al.83 compared 16 patients with DLB and 15 patients with PD. The mean duration of illness for the PD group was 9.2±2 years, whereas the mean duration of illness for the DLB group was 6.3±1.1 years. Using the Unified Parkinson's Disease Rating Scale, they have shown that patients with DLB presented more rigidity, but less left/ right asymmetry, than patients with PD.83 There was no significant difference between the two groups on the other measures of parkinsonism.
DEMENTIA WITH LEWY BODIES AND TREATMENT ISSUES
The early clinical diagnosis of DLB is crucial, given that 81% of patients with DLB treated with classical neuroleptics such as haloperidol experience a neuroleptic sensitivity syndrome, compared with 29% in AD.1 In order to avoid or lessen the risk of neuroleptic sensitivity syndrome in patients with DLB, treatment strategies have recently been focused on the use of atypical neuroleptics such as clozapine and risperidone. There are no randomized placebo-controlled trials of atypical neuroleptics for the treatment of behavioral disturbances in dementia, and the anecdotal results have been controversial. Some authors reported a favorable response in patients with DLB treated with clozapine84 and risperidone,35,85 while others86–88 described a neuroleptic sensitivity syndrome mainly characterized by the onset or exacerbation of EPS. However, a new atypical neuroleptic, olanzapine, which binds more on serotonergic receptors and less on dopaminomimetic receptors than the other atypical neuroleptics, could be a good treatment choice, given some promising preliminary data on 1 patient89 and on 5 of 8 patients90 with probable DLB per McKeith et al.9 criteria.
Regarding trials of cognition-enhancing drugs, some retrospective data suggest that patients with DLB respond better to treatment with the cholinesterase inhibitor tacrine than do patients with AD.91 A trial of another anti-acetylcholinesterase compound, donepezil, resulted in improvement in hallucinations, and sometimes in cognition and overall function, in 9 patients meeting CDLB clinical criteria for DLB.92
COGNITIVE SYMPTOMS
Retrospective Studies With Autopsy-Proven DLB
Six retrospective studies, with cross-sectional data, have compared small groups of subjects (mean number of DLB subjects per group=12.51, range 5–23; mean number of AD subjects per group=11.51, range 5–23) using different matching procedures: the Information-Memory-Concentration subtest score of the Blessed scale;93 the MMSE94 or Mattis Dementia Rating scale (MDRS)95 total score, alone or in combination with age, gender, and education; the duration of the disease; and the number of months between last testing and death (see Table 2).34,47,51,52,71,96 These subjects were mildly51,96 to moderately47,52 demented. In two studies, the subjects with Lewy Body Variant (or DLB) and AD were severely demented,34,71 whereas the 5 subjects of Samuel et al.,71 with pure DLB, were moderately demented. Only one study included a healthy control group (of 5 subjects).71
Table 2 (tests described in Table 4) indicates that mildly and moderately demented patients with DLB perform more poorly than patients with AD on tests of visual tracking (Trail Making Test A) and visual attention shifting (Trail Making Test B)51,52 that rely on working memory (WM).97,98 Working memory is the system necessary for holding and manipulating information while performing various tasks including learning, reasoning, and comprehending. The central executive system (CES) is the supra component of this model. It is assumed to be an attention controller and to have, among other roles, that of coordinating and distributing attention resources between the two slave subsystems of WM. The visuospatial sketchpad is the slave subsystem that maintains information under a visuospatial code. The other slave subsystem, the articulatory loop, maintains phonologically encoded material in primary memory through short-term storage and translates, when applicable, visually presented material into verbal material.97,98 Regarding verbal attention (articulatory loop) capacities, two studies with mildly demented patients have reported that patients with DLB and AD were equally impaired in performance on the Digit Span subtest of the Wechsler Adult Intelligence Scale–revised (WAIS)51 and on the Attention subtest of the MDRS.96 Another study, with moderately demented subjects, described a poorer performance of patients with DLB when compared with AD subjects on the Digit Span task.47
In respect to verbal and motor initiation capacities (Table 2), mildly and moderately demented patients with DLB are more impaired than patients with AD.47,52,96 The most sensitive tests in this regard have been the lexical fluency tasks47,52 and the Initiation/Perseveration subtest of the MDRS.96 One study51 reported that subjects with DLB and AD perform equally poorly on the lexical fluency tasks. However, this study had a very small number of subjects per group, and they were matched on the MDRS scores.51 The MDRS includes tasks of verbal fluency and tests of other frontal functions as well, thus potentially masking differences that might otherwise have been found. Performance in tasks of semantic fluency and knowledge (naming) were equally impaired in mildly, moderately, and severely demented DLB and AD patients.34,47,51,52
Regarding executive functions such as abstraction capacity (Table 2), the performance of mildly demented subjects with DLB and AD is equally poor on the Conceptualization subtest of the MDRS and the Similarities subtest of the WAIS.51,96 The performance of moderately demented patients with DLB is more impaired than that of patients with AD on the Similarities subtest of the WAIS.47 The two studies assessing moderately demented subjects reported different results on the Arithmetic subtest of the WAIS. According to Galasko et al.,52 patients with DLB have a poorer performance than patients with AD on the Arithmetic subtest, whereas according to Hansen et al.,47 these two groups perform equally poorly on the Arithmetic subtest. The subjects did not differ enough between the two studies in regard to demographics (education, age) and EPS features to account for the different results. These two studies47,52 both included only a small number of subjects in each group (see Table 2). They both performed several analyses on several variables, sometimes with nonparametric statistics (Mann-Whitney U-test), but often with parametric statistics like analysis of variance and Student's t-test, without any correction for the number of analyzed variables. Thus, some results could have been inappropriately identified as significant, or significant results might have been missed, because of the lack of statistical power.
Regarding visuospatial functions, Table 2 shows that mildly and moderately demented patients with DLB perform more poorly than patients with AD on visuospatial praxis tests such as the Block Design subtest of the WAIS and drawing tasks such as the Clock.51,52 Mildly and moderately demented patients with DLB have been found to be more severely impaired on the copy part of drawing tasks (Clock-copy and the Construction subtest of the MDRS), as opposed to the free drawing part, when compared with AD subjects.51,52 However, some authors have described no difference between the two groups, in mildly and severely demented subjects, regarding visuospatial praxis capacities (drawing in two and three dimensions) and the copy part of these tasks (Cambridge Cognitive Examination [CAMCOG]-visuospatial praxis and the Construction subtest of the MDRS).34,96 Connor et al.96 and Salmon et al.51 obtained different results on the Construction subtest of the MDRS (copy of two-dimensional designs). Both studies assessed mildly demented subjects. The DLB subjects of Connor et al.96 had neuropathological changes associated with AD as well as cortical Lewy bodies, whereas the DLB subjects of Salmon et al. had only cortical Lewy bodies. Given also that subjects of Connor et al. had slightly inferior total scores on the MDRS to the subjects of Salmon et al., one would have expected to find more severe deficits in Connor and colleagues' DLB patients. However, it was not the case. The two patient groups of Connor et al. performed equally badly, whereas the DLB patients of Salmon et al. had a poorer performance than the AD patients. The samples of Connor et al.96 (where the two groups performed equally) were larger than the ones of Salmon et al.51 (where the DLB patients performed worse), and therefore the study of Connor et al.96 had better power than the one of Salmon et al.51
Table 2 (last section) shows that most of the studies found performance on episodic and semantic memory tests to be equally impaired in subjects with DLB and AD with mild,51,96 moderate,47,52 and severe dementia.34,71 The data have been collected with the Buschke Selective Reminding Test (SRT),47,52 the California Verbal Learning Test (CVLT; episodic memory tests with free and cued recall paradigms),51 the Visual Reproduction of the WMS (visual episodic memory test),47,52 the Vocabulary subtest of the WAIS,47 the Number Information Test (NIN; semantic memory tests),52 the CAMCOG Memory subtest,34 and the Information-Memory- Concentration subtest (IMC; attention, semantic and autobiographical memory) of the Blessed Scale.71 However, some caution is in order regarding these findings. The results of Hansen et al.47 might have been prone to masking effects, since the two groups of patients were matched for overall severity of dementia with a measure of memory (IMC of the Blessed Scale). Statistical analyses have not been applied to the data of Salmon et al.51 collected with the CVLT, because the studied groups were too small (n=5 subjects per group). Finally, in the study of Förstl et al.,34 dementia as measured with the MMSE, was more severe in patients with AD (MMSE score=7.5±6.1) than in patients with DLB (9.8±6.7). Only one study, with mildly demented patients, has described better performance in subjects with DLB than in subjects with AD on the memory subscale of the MDRS.96 Although this subscale gives some information on short-term free recall and forced-choice recognition capacities, it does not assess short- and long-term cued recall or long-term free recall and recognition capacities. These paradigms are crucial to differentiate between subcortical and cortical types of dementia, especially in the mild stage of dementia.99,100 Unfortunately, Connor et al.96 did not use other measures of memory.
Studies With Antemortem Clinical Criteria
Eight studies have compared relatively small groups of subjects (mean number of DLB subjects per group=12.6, range 7–26; mean number of AD subjects per group=18.5, range 10–52), using the following matching procedures: the MMSE total score, the Clinical Dementia Rating Scale (CDR),101 or the CAMCOG global score,102 in combination with age, gender, education, the duration of the disease, or the premorbid IQ.56,83,103–107 These subjects were mildly103–107 and moderately56,83,108,109 demented. No severely demented subjects have been assessed. Criteria for Senile Dementia of Lewy Body Type4 have been used to enroll patients with DLB in 7 of 8 studies83,103–108 (see Tables 1 and 3). Gnanalingham et al.83,108 also used the criteria of Byrne et al.5 (see Table 1)4,5,9 for Cortical Lewy Bodies. Only Shimomura et al.56 used the criteria for probable DLB per McKeith et al.9 The NINCDS-ADRDA criteria for probable Alzheimer's disease have been used in order to include patients with AD in 8 of 8 studies.56,83,103–108 Five studies also included a healthy control group, matched with the patients by age106 and premorbid IQ,103–105 and/or by education.83,108
Table 3 (tests described in Table 4) shows that mildly103–106 and moderately56 demented patients with DLB are more impaired than patients with AD on some tests of visuospatial working memory56,103–106 and are impaired to the same extent as AD patients on other tests of visual memory and attention. Galloway et al.103 showed that mildly demented patients with DLB perform significantly worse than subjects with AD on the conditional pattern-location paired-associate learning task (spatial working memory). On a task with similar demands, the Digit Symbol subtest of the WAIS, moderately demented subjects with probable DLB performed more poorly than patients with AD.56 However, the impairments of DLB and AD subjects on a visual recognition memory test (abstract designs) did not differ.103 Sahgal et al.104,105 have assessed visual attention, learning, and memory capacities of DLB and AD patients. They have reported worse deficits for patients with DLB on a delayed-matching-to-sample task104 and on a visual search matching-to-sample task,105 both relying on spatial working memory. Patients with DLB and AD performed equally badly on a semantic learning task, the Kendrick Object Learning Task (KOLT).105 Sahgal et al.106 have reported worse deficits in mildly demented DLB subjects compared with AD subjects on the spatial working memory task testing visuospatial planning abilities, one of the attributes of the central executive system in the working memory model.97,98,110 This spatial working memory task has been previously described as very sensitive to frontal lobe impairment111,112 as well as Parkinson's disease,113–115 even in mild stages of PD,114,115 and to frontal lobe impairment in patients with amygdalo-hippocampectomy.112 However, the two patient groups of Sahgal et al.106 performed equally poorly on the Corsi's block test, a visuospatial span task assessing the visuospatial sketchpad of working memory.106
Regarding verbal attention and memory capacities (Table 3, section 2), the results of three studies suggest that mildly and moderately demented patients with DLB are as impaired as patients with AD on some verbal attention and memory tests, but less impaired than AD subjects on some tasks of episodic memory. Mildly and moderately demented subjects with DLB and AD have been equally impaired on the Attention/Calculation subtest (counting backwards and serial subtractions) of the CAMCOG107 and the Digit Span subtest of the WAIS.83 Mildly demented subjects with DLB have been as impaired as AD subjects on tests of semantic and autobiographical memory such as the Vocabulary subtest of the WAIS105 and the Orientation, Remote Memory (semantic memory), and Current Information (public episodic memory) subtests of the CAMCOG.107 Mildly and moderately demented patients with AD have been more impaired than DLB patients on tests of verbal long-term free recall such as the CAMCOG delayed recall107 and the word recall of the ADAS-COG.56
Regarding visuospatial praxis (Table 3, last section), four studies have reported that patients with DLB are more impaired than patients with AD on various visuospatial tasks. Mildly demented patients with DLB have demonstrated poorer performance than patients with AD on the visuospatial praxis subtest (two- and three- dimensional drawing) of the CAMCOG.107 Moderately demented patients with DLB have performed more poorly than both AD and PD patients on the Copy part of the Clock Test.83,108 The two studies of Gnanalingham et al.83,108 and a third study109 (not included in Table 3 because it involved only one subject with DLB, compared to 64 subjects with AD) investigated the differential performance between the Copy (C) and Free Drawing (D) parts of the Clock Test. They found that patients with DLB also performed equally poorly on the Copy (C) and the Free Drawing (D) parts of the Clock Test, whereas patients with AD and PD performed more poorly on the D part than on the C part of the test.83,108,109 However, the three patient groups in the study of Gnanalingham et al.83 were not matched for severity of dementia. Patients with DLB and AD were moderately demented, whereas patients with PD, in the mild stage of dementia, were significantly less cognitively impaired, as measured with the MMSE. Gnanalingham et al.108 have found a low sensitivity but a high predictive value for DLB with individuals presenting higher scores on the D part than on the C part. This finding has, however, been based on only 3 cases with DLB.108,109 Moderately demented patients with DLB have also performed more poorly than patients with AD on the Object Assembly and Block Design subtests of the WAIS.56 Regarding visual perception, two studies have shown no difference between mildly demented subjects with DLB and AD104,107 on tasks depending on the functioning of the occipital and parietal lobes (object, design, and face recognition tasks).
Regarding executive functions (Table 3, section 2), the only difference between patients with DLB and AD has been found in moderately demented patients, on tasks of visuospatial conceptualization and visuospatial judgment. The capacities of visuospatial conceptualization and judgment, as measured by the Raven Progressive Matrices and the Picture Arrangement subtest of the WAIS, have been more disrupted in moderately demented patients with DLB compared to patients with AD.56 However, mildly and moderately demented patients with DLB and AD performed equally badly on two tasks of mental flexibility, a test of visual set-shifting,105 and the Nelson Card Sorting Task.83 Nevertheless, subjects with DLB and AD were more impaired than PD and control subjects on the Nelson test.83
The capacities of verbal conceptualization and judgment, as measured by the Abstract Reasoning subtest of the CAMCOG107 and the Comprehension subtest of the WAIS-R,105 have not differed between mildly demented subjects with DLB and AD.105,107 Other executive functions, such as the motor (motor sequencing task) and verbal (lexical and semantic fluency tasks) initiation capacities, have been equally impaired in mildly107 and moderately83 demented patients with DLB and AD.
Longitudinal Studies
Four of the six longitudinal studies assessed demented patients at the baseline evaluation by using a short cognitive test, the MMSE.94 Another study used the short assessment CAMCOG, from the CAMDEX,102 which is an elaborated version of the MMSE. Only one longitudinal study followed patients with an end diagnosis of DLB confirmed at autopsy.116 Of the remaining five studies, only one117 recruited patients with DLB by using criteria for DLB or SDLT.4 Four studies enrolled patients with the diagnosis of AD with EPS and/or hallucinations.118–121
Twenty-four of the 81 AD patients followed by Miller et al.118 had non–neuroleptic-induced extrapyramidal symptoms. Patients with EPS deteriorated 67% faster on the MMSE (4.5 points per year) than patients without EPS (2.7 points per year). All of the subjects were matched at baseline for age and MMSE total score (mean scores of 18 and 20).118 Chui et al.120 reported that 85 of 135 patients reached the individual end point of a 6- point decline on the MMSE. Among these 85 patients, 46 were mildly demented and 39 were moderately demented at baseline. Significant predictors of cognitive decline were hallucinations and agitation in the mild dementia group, and the presence of non–neuroleptic- induced EPS at baseline in the moderate group.120 In another study, the EEG at baseline, but not the presence of hallucinations, was the best predictor of more rapid cognitive decline in moderately demented patients, as measured by the MMSE.121 Nevertheless, hallucinations predicted a more rapid functional decline.121
Only one longitudinal study has given more information about the cognitive deterioration of subjects with AD and EPS compared with 1) subjects with AD lacking EPS and 2) subjects with PD and dementia.119 At baseline, no significant difference on the formal neuropsychological testing was found between the three groups, with the exception that patients with AD (with and without EPS) had more false positive targets on the recognition paradigm of the word learning task compared with PD patients. After a year of follow-up, the difference between the AD and PD patients regarding the false positive targets remained the only significant distinction between AD (with and without EPS) and PD. Nevertheless, 14 subjects with AD+EPS had greater deficits on tests of verbal comprehension, automatic speech, semantic fluency, and praxis than 16 AD subjects without EPS.119 This study presents a methodological problem of importance. The two groups of patients with AD were not properly matched at baseline, the subjects with AD+EPS being significantly more impaired at baseline than the group without EPS as measured on the Brief Cognitive Rating Scale of Reisberg et al.122 The AD+EPS patients also tended to be more impaired at baseline than the group without EPS as measured on the Clinical Dementia Rating Scale (which showed that 57.14% of subjects with AD+EPS, versus 31.25% of AD subjects without EPS, were moderately demented).
Ballard et al.117 followed, for one year, subjects with Alzheimer's disease, vascular dementia (VaD), and dementia with Lewy bodies, using, respectively, the NINCDS-ADRDA criteria for probable and possible AD,74 the Hachinski scale for vascular dementia,123 and the criteria for SDLT.4 The total CAMCOG scores of the 116 moderately demented subjects with AD, VaD, and SDLT did not differ significantly at baseline, having respective means of 42.68, 44.50, and 47.67 (total possible score on the CAMCOG=106). Nevertheless, patients with DLB had a better performance than patients with AD on the recent memory subtest and a better performance than patients with VaD on the visual memory subtest. After one year, approximately 70% of the patients were reassessed. The 7 patients with DLB tended to deteriorate more rapidly on only the CAMCOG total score versus the 53 AD subjects and the 14 VaD subjects (mean deterioration=27±19.77, 13.21±12.61, and 13.29±13.48 points, respectively). However, a logistic regression analysis showed that patients with DLB deteriorated significantly more than patients with AD and VaD on the verbal (semantic) fluency subtest, and also more than patients with VaD on the Remote Memory subtest of the CAMCOG.117 There was no significant difference among the three groups of patients on the other subtests of the CAMCOG: Orientation, Comprehension, Expression, Praxis, Recent Memory, Visual Memory, Attention and Calculation, Perception (Agnosia), and Abstract Thinking.117
More recently, Olichney et al.116 reviewed the autopsied cases of 40 patients with Lewy body variant and 148 patients with Alzheimer's disease. The two groups of moderately demented patients were matched according to their age, education, and MMSE scores (mean MMSE scores: LBV, 18.2; AD, 17.8) at baseline. They found that LBV subjects had a rate of cognitive decline significantly more rapid and severe than the rate for AD subjects as measured by MMSE scores. The average rate of decline was –5.8±4.5 points per year in LBV compared with –4.1±3.0 points per year in AD (t-test, P<0.01). The LBV group declined a similar amount on the MMSE over a significantly shorter time interval than did the AD group (1.9 vs. 2.7 years; P<0.005). The LBV patients presented significantly more parkinsonian signs at entry compared with AD patients (30% vs. 14%; P=0.02). The patients with LBV had a shorter survival time from the onset of cognitive symptoms and a shorter mean survival after baseline.116
Summary and Discussion Related to the Cognitive Changes of DLB
In this review, 9 studies have assessed mildly demented patients,51,96,103–107,118,120 11 studies have assessed moderately demented patients,47,52,56,83,108,109,116,117,119–121 and 2 studies have assessed severely demented patients.34,71 More than one-third (36.36%) of studies with moderately demented patients used comprehensive neuropsychological batteries, compared with 11.11% of the studies with mildly demented patients. The other studies used short assessments such as the MMSE,34 the CAMCOG,102 or the MDRS.95 Nearly one-half of the studies (44.44%) with mildly demented patients specifically focused their cognitive assessment on tasks of spatial working memory. The 2 studies with severely demented patients used only short assessments. As a result, more cross-sectional and longitudinal information, regarding different aspects of cognition, is available in moderately demented subjects with DLB and AD than in mildly and severely demented subjects.
All of the longitudinal studies demonstrated a significantly faster cognitive or functional decline in patients with DLB116,117 or with AD plus EPS (or hallucinations/agitation)118–121 over a period of 1,116–119,121 2,120 3,119 or 8 years,121 when compared with patients with AD. The MMSE has been sensitive enough to capture the more severe deterioration over time of subjects with DLB compared with subjects with AD. The MMSE is also sensitive to fluctuations in cognition over a 6-month period of time.60 However, specific data regarding the cognitive profile of deterioration over time are lacking. The only specific longitudinal data available are coming from only two studies. Their findings will be summarized throughout this section.
This review has shown that the most consistently found cognitive deficit in mildly and moderately demented patients with DLB, compared with AD patients, is the impairment of spatial working memory that relies on the central executive system (CES) of working memory. Clinically, this impairment can be tested by using the computerized spatial working memory of Galloway, Sahgal, and co-workers103–106 and also by using the Trail Making Test part B. This deficit has been reported in 5 antemortem and 2 retrospective studies. Spatial working memory tasks have been shown to be sensitive in mild and moderate Parkinson's disease without dementia113,114 and in patients with amygdalo-hippocampectomy,112 and to be dose-dependent on clozapine,124 which acts as an adrenoceptor agonist. Because the pathological processes of DLB involve a dysfunction of the aminergic systems, as well as neuronal loss or abnormalities in the amygdala and hippocampus (see section above on pathological changes), comparisons between patients with DLB, AD, and PD on spatial working memory tasks will be necessary in the future.
An examination of the central executive system of working memory using the dual-task paradigm has not yet been done in subjects with DLB. The dual-task paradigm, one of the most widely recognized measures of the CES,110,125–129 has been devised to measure the capacity of the CES to distribute attentional resources between a visual tracking task (visuospatial sketchpad) and a verbal rehearsing task (articulatory loop and phonological buffer). Subjects with AD, even in mild stages of the disease, have deficits on the dual task110,125,127 and deteriorate on it,125 whereas the single-task performance is maintained over time (over a period of 6 to 12 months). The dual task has been shown to be very sensitive in frontal patients with a dysexecutive syndrome, but not in frontal patients without a dysexecutive syndrome.128,129 It has been hypothesized to rely on the cingulate and orbital frontal areas130,131 and to depend at least on the integrity of the DA nigrostriatal and mesocorticolimbic systems,126,132,133 and also, possibly, on the integrity of frontal cholinergic systems134 in nondemented patients with PD. Given the pathological process involved in DLB (LB in anterior frontal and cingulate areas, neuronal loss in frontal lobes), the assessment of DLB subjects with the dual-task paradigm seems highly appropriate.
The impairment of the visuospatial sketchpad, a subsystem of working memory, in mildly demented subjects with DLB needs further study. A retrospective study has found a more severe deficit (in DLB) on the TMT-A,51 and an antemortem study has reported no difference between the performance of DLB and AD on Corsi's blocks test.106 Two retrospective studies51,96 and one antemortem study83 have found that mildly demented patients with DLB were as impaired as patients with AD on tests of the articulatory loop of working memory (verbal attention). The impairment of verbal attention in moderately demented patients with DLB and AD needs to be investigated because two studies in these populations have obtained different results for verbal attention: one retrospective study described a poorer performance of DLB compared with AD patients,47 whereas an antemortem study found no difference between the performance of the two patient groups.83 In the present state of knowledge, the clinical assessment of the visuospatial sketchpad and the articulatory loop of working memory, using simple span tasks such as Corsi's blocks and the Digit Span task, would add nothing of real value to the process of differential diagnosis in the mild stage of dementia.
The second most important field where more severe deficits have been reported in subjects with DLB compared with AD has been the area of visuospatial functions. Two antemortem studies and one retrospective study with moderately demented patients, and one retrospective study with mildly demented patients, found more severe impairment in DLB than in AD on the copy part of the Clock Test. Two retrospective studies assessing mildly demented patients had contradictory results: one described poorer performance of subjects with DLB,51 whereas the other reported no difference between DLB and AD96 on the Construction subtest of the MDRS. The free drawing part of the Clock Test has been used in two antemortem studies with mildly and moderately demented subjects with DLB and AD, and no difference between the two groups of patients has been found. Moderately demented patients with DLB were found to be more impaired than patients with AD in one retrospective and one antemortem study, using the Block Design subtest52,56 and the Object Assembly subtest56 of the WAIS.
Moderately demented patients with AD and EPS (possible DLB) have also deteriorated more rapidly on tests of visuospatial and ideomotor praxis.119 Mildly demented subjects with DLB have been reported to be more impaired than subjects with AD in a study using the Block Design subtest of the WAIS. A number of studies have used the Block Design and/or the Object Assembly subtest of the WAIS to compare the visuospatial capacities of patients with DLB and AD. However, the WAIS was not designed to be used with a neurological population.135,136 In the standard administration and scoring of the Block Design and Object Assembly subtests, time to accomplish the task is as important as the correctness of the execution. The use of these tests with patients having EPS puts examiners at risk of measuring slowing that is actually due to rigidity and bradykinesia found in DLB.80–83 Therefore, one would not know to what extent higher functions (depending on associative right parietal and bilateral occipital and frontal areas), such as mental rotation, planning, visuospatial organization, synthesis and perception, are involved in the impairment. On the praxis subtest of the CAMCOG, one antemortem study with mildly demented patients has found a more severe impairment in DLB compared with AD, whereas one retrospective study with severely demented patients with DLB and AD found no difference between the two patient groups. Despite the above- mentioned methodological problems, the use of some construction tasks without time limits, such as the Clock Test (especially the copy part), could be of some clinical diagnostic value.
Visual perception has been assessed in only 2 antemortem studies with mildly demented subjects; in each, the two patient groups were equally impaired on tasks of matching designs and object and facial recognition. Clinically, it seems likely that classic tasks of visual recognition (objects and faces) will not allow an early differential diagnosis between DLB and AD. The evolution, over time, of visual perception impairment in DLB remains unknown, as well as the involvement of visual perception impairment in the construction praxis deficits in moderately demented DLB patients. To clarify the visual perception issue, the performance of patients with DLB and AD should be studied, in longitudinal designs, on tasks of “pure” visuospatial perception such as the Benton Judgment Line Orientation Test that measure functions related to the integrity of the right parietal and bilateral occipital areas.
The results related to verbal and motor initiation capacities are controversial. Three retrospective studies have found mildly and moderately demented patients with DLB to be more impaired than patients with AD on tests of lexical fluency or on the Initiation/Perseveration (verbal fluency, motor initiation and sequencing, serial drawing) subtest of the MDRS. However, one retrospective study with mildly demented patients51 and one antemortem study with moderately demented patients83 found that DLB and AD patients performed equally poorly on the lexical fluency tasks and the motor sequencing tasks. The performance on tasks of semantic fluency (category fluency) and knowledge (naming) was equally impaired in mildly, moderately, and severely demented subjects with DLB and AD in four retrospective and two antemortem studies. However, moderately demented patients with DLB deteriorated more than patients with AD (and VaD) on the semantic fluency tasks,117,119 and also more than patients with VaD on the task of remote memory.117,119 One study also found a more severe deterioration over time in speech and language capacities, such as comprehension and production of speech.119 The problems with speech production in fluency and expression tasks can be due to the increased severity of EPS affecting the articulatory process, at the level of motor control, as well as to the deterioration of semantic knowledge and/or semantic access, under cognitive control. Unfortunately, none of the studies assessing verbal fluency and expression have controlled for the motor component of the task in order to isolate the nature of the problem. No study has correlated the EPS of DLB patients with their language performance. Clinically, verbal fluency tasks, and especially lexical fluency tasks such as the FAS, might be useful to detect DLB in the early stages of the disease. Semantic fluency tasks might be useful to follow the cognitive deterioration over time in DLB.
Regarding executive functions, one antemortem study has demonstrated a greater impairment in moderately demented DLB patients, compared with AD patients, on tasks of visuospatial abstraction and judgment. The capacity of verbal abstraction, principally assessed with the Similarities subtest of the WAIS, was found, in two retrospective and two antemortem studies, to be equally impaired in mildly demented patients with DLB and AD. However, moderately demented patients with DLB registered a poorer performance in verbal abstraction in one retrospective study. The mental flexibility capacity has been found to be equally impaired in mildly and moderately demented subjects with DLB and AD. Clinically, tests such as the Progressive Matrices of Raven, testing visuospatial abstraction, could be relevant in a process of differential diagnosis in moderate stages of dementia, patients with DLB being more impaired than patients with AD.
The functioning of episodic, semantic, and autobiographical memory has been found to be equally impaired in mildly demented patients with DLB and AD in two retrospective and two antemortem studies. Four retrospective studies also reported the same degree of impairment in moderately and severely demented patients with DLB and AD. However, one antemortem study with mildly demented patients,107 and another antemortem study with moderately demented patients,56 found that DLB subjects had a better performance than AD subjects in tests of verbal episodic memory (delayed recall paradigm)56,107 and personal semantic memory (name and address, in the CAMCOG).107 Clinically, tests of episodic and semantic memory, such as the California Verbal Learning Test or Visual Reproduction on the Wechsler Memory Scale, will not distinguish patients with DLB from patients with AD because the performance of both populations will be equally impaired on most measures of these tests, with the exception of the free recall paradigm. Subjects with DLB might perform better on this paradigm than would be expected for patients with AD in the mild and moderate stages of dementia.
BEHAVIORAL PROBLEMS
Forty-seven articles have been reviewed for this section (see Table 5 and Table 5a). The majority of these studies are case reports (26/47; 55.32%). Other reviewed papers have been retrospective controlled studies (10/47; 21.28%), prospective cohorts (8/47; 17.02%), and controlled studies (3/47; 6.38%). Most of the studies (28/47; 59.47%) have assessed and/or registered behavioral problems principally by using clinical notes. The other studies have used various instruments such as the Burns Symptoms Checklist137 (BSCL; 2 studies), the Present Behavioral Examination138 (PBE; 2 studies), a variant of the PBE, the Past Behavioral History Interview (PBHI; 1 study), the Cornell Scale for Depression139 (CSD; 2 studies), and the Diagnostic Interview Schedule (DIS; 2 studies). Other instruments have been used in only one study: the Blessed Dementia Rating Scale (BDRS);93 the Hamilton Depression Rating Scale140 (Ham-D); the Columbia University Scale for Psychopathology in Alzheimer's Disease141,142 (CUSPAD); the Neuropsychiatric Inventory143 (NPI); the Geriatric Mental State Schedule144 (GMSS); the DSM-III criteria for psychosis and Alcohol abuse (DSM-psy, A); and the History and Aetiology Schedule42 (HAS). All of these instruments have been used principally to assess for the presence of psychosis. Other behavioral disturbances, such as anxiety, apathy, or aggressive behavior, have for the most part been reported in studies using review of clinical notes.
Psychotic Symptoms at Any Stage of the Disease
Table 5 and 5a show the number of patients with DLB and AD that have presented psychotic symptoms at any stage during their disease. Visual hallucinations have been the most frequently reported psychotic symptom in DLB (54.32%; 270/497 reported cases). Comparatively, delusions have been the most frequent psychotic symptoms in AD (31.12%; 111/356 reported cases). The second through fifth most common psychotic symptoms in patients with DLB have been, respectively: delusions (48.51%; 213/439 reported cases); auditory hallucinations (25.44%; 86/338 reported cases); olfactory hallucinations (6.63%; 13/196 reported cases); and tactile hallucinations (0.66%; 1/152 reported cases). The second through fourth most common psychotic symptoms in patients with AD have been, respectively: visual hallucinations (22.96%; 104/453 reported cases); auditory hallucinations (5.76%; 17/295 studied cases); and olfactory hallucinations (2.86%; 3/105 studied cases). No tactile hallucinations have been reported in 47 studied subjects with AD.
Psychotic symptoms at any stage of the disease in AD have been less systematically studied and registered than psychotic symptoms in DLB (see Table 5 and Table 5a). Nevertheless, many studies have shown a more significant amount of psychosis in DLB than in AD. Hallucinations in general have been found significantly more frequently in DLB than in AD.50,52–54 Visual hallucinations have been significantly more frequent in DLB than AD.1,39,43–46,48 Auditory hallucinations at any stage of the disease have also been found significantly more frequently in DLB than in AD.1,46,48
Results regarding delusions have been more controversial (see Table 5 and Table 5a). Some studies reported significantly more delusions in DLB than in AD.4,39,46,48,53 However, other studies described no significant difference between DLB and AD.42,46,50,52 Although the misidentification syndrome was the most frequently reported delusion in both DLB (32.72%; 89/272 reported cases) and AD (18.79%; 37/199 reported cases),14,16,17,23–27,29–32,34–37,43–46,50–51 the types of delusions presenting at any stage of the disease in patients with DLB and AD have generally differed. Ballard et al.,46 however, found the misidentification syndrome to be significantly more common in DLB, at any stage of the disease, than in AD.
The second through sixth most common delusions at any stage of the disease in DLB were, respectively, paranoid delusions (28.57%; 50/175 reported cases);4,10,12,14–27,29–32,34–37,49–51 phantom boarder (15.04%; 20/133 reported cases);14,16,17,23–27,29–32,34–37,43–45,47,50,51 theft delusions (5.31%; 6/113 reported cases);14,16,17,23–27,29–32,34–37,43–45,47,50,51 other delusions (2.5%; 2/80 reported cases);14,16,17,23,25–27,29–32,34–37,50,51 and abandonment delusion (1.67%; 1/60 reported cases).14,16,17,23,25–27,29–32,35–37,47,50,51 The second through fifth most common delusions at any stage of the disease in AD have been, respectively: theft delusions (13.43%; 9/67 reported cases);47,50 paranoid delusions (12.35%; 20/162 reported cases);4,47,50 phantom boarder (7.46%; 5/67 reported cases); and other delusions (4.48%; 3/67 reported cases).47,50 No abandonment delusion has been described in the 9 AD studied cases.47 Unfortunately, no intergroup statistical analyses have been applied to delusions by the authors of the studies.
Major Depression at Any Stage of the Disease
Four studies found that major depression, at any stage of the disease, was significantly more common in DLB (24.45%; 111/454 reported cases) than in AD (9.32%; 58/622 reported cases).4,39,46,50 However, some studies have found no difference between DLB and AD in regard to major depression.42,52–54
Other Behavioral Disturbances at Any Stage of the Disease in Dementia with Lewy Bodies
Data regarding other behavioral disturbances at any stage of the disease have been gathered among the case report studies because, apart from the study of Ballard et al.,46 no controlled study mentioned these symptoms. Thus, no comparative data on patients with Alzheimer's disease are available, apart from the results of Ballard et al.46 The most frequent nonpsychotic behavioral disturbance that has been reported in DLB is anxiety (38.14%; 58/152 reported cases).24,37,38,46 However, Ballard et al.46 did not find any significant difference between DLB (55/138 cases) and AD patients (51/132 cases) in this symptom. This finding needs to be replicated.
Other symptoms found have been irritability (33.33%; 3/9 reported cases);17,23,24 apathy (22.22%; 8/36 reported cases);17,24–27,38 violent behavior/aggressivity (21.43%; 3/14 reported cases);23,37,38 nocturnal confusion/insomnia (15%; 3/20 reported cases);12,23,24 and restlessness/agitation (10.52%; 4/38 reported cases).12,24–27
Psychiatric Symptoms at Presentation
At the onset of the disease, patients with DLB have presented primarily visual hallucinations (51.29%; 139/271 reported cases).4,23,24,26,27,33,36,38,46,54,67 McShane et al.40 studied 8 patients with AD and 5 patients with DLB. They reported that patients who had severe hallucinations were more likely to have poor eyesight. In the study of McShane et al.,41 among the 24 subjects who had hallucinations at any stage of the disease and have come to autopsy, those with hallucinations of early onset were more likely to show cortical LB than those with hallucinations of late onset. Hallucinations began most commonly in the fourth year of dementia and, once established, tended to be a stable phenomenon, persisting into the year before death in 71% of cases.41 Unfortunately, the instrument used by McShane et al.40,41 to assess psychiatric symptoms, the Present Behavioural Examination (PBE),138 does not specify the type of hallucinations (e.g., visual, auditory, olfactory, tactile), reporting only “hallucinations.” Therefore, one does not know if the results of McShane et al.40,41 refer to visual hallucinations.
At the onset of the disease, patients with DLB have also shown the following: delusions in general (50.25%; 97/193 reported cases),1,25–27,34,46,48,51 misidentification syndrome (44.52%; 65/146),34,46 apathy (40%; 2/5 reported cases),23,32 anxiety (36.6%; 56/153 reported cases),26,27,46 paranoid delusions (33.33%; 22/66 reported cases),4,12,15,26,27,49 irritability (25%; 4/16 reported cases),16,23–25 major depression (20.88%; 57/273 reported cases),4,12,15,23,25–27,30,34,36,38,46,49,54,67 and auditory hallucinations (19.65%; 12/61 reported cases).1,4,23,26,27,30
At presentation, patients with AD have comparatively presented the following: anxiety (32.57%; 43/132 cases),46 delusions (26.14%; 40/153 reported cases),4,34,46,48 misidentification syndrome (15.15%; 20/ 132 reported cases),46 visual hallucinations (13.55%; 34/ 251 reported cases),1,4,46,54 paranoid delusions (8.11%; 3/ 37 reported cases),4 major depression (7.83%; 4/61 reported cases),4,46 and auditory hallucinations (1.72%; 1/ 58 reported cases).1,4
Summary and Discussion Related to the Behavioral Changes in DLB
This review has shown that behavioral disturbances, and particularly psychosis, are much more common in DLB than in AD. This review has also confirmed that the presence of visual hallucinations differentiates between DLB and AD, as stated by the current criteria for DLB.9 At the onset of the disease, subjects with DLB present visual hallucinations, delusions (in general), misidentification syndrome, apathy, anxiety, paranoid delusions, irritability, major depression, and auditory hallucinations. It appears that at the onset of the disease, patients with DLB are more likely to have major depression or depressive symptoms (apathy, anxiety, or irritability) than auditory, olfactory, and tactile hallucinations. This finding suggests that major depression should be included as one of the supportive features of the DLB diagnosis. Information regarding the past psychiatric history of patients is crucial in patients presenting depression, because preliminary results with people being treated for depression suggest that late-onset depression could be an early marker of dementia.145 At any stage of the disease, the most common psychotic symptoms in DLB will be visual hallucinations, delusions, auditory hallucinations, olfactory hallucinations, and tactile hallucinations. The most frequent other behavioral disturbances in DLB will be anxiety, irritability, major depression, apathy, violent behavior, nocturnal confusion/insomnia, and restlessness/agitation.
At the onset of the disease, subjects with Alzheimer's disease are less likely than subjects with DLB to present visual hallucinations. They are more likely to show anxiety, delusions, misidentification syndrome, visual hallucinations, paranoid delusions, major depression, and auditory hallucinations. Later on, or at any stage of AD, the psychiatric symptoms mostly likely to appear will be delusions, visual hallucinations, major depression, auditory hallucinations, and olfactory hallucinations. None of the 47 studies reviewed in this paper has reported tactile hallucinations in AD. Unfortunately, no data have been available, in this review, in regard to other behavioral disturbances likely to be present at any stage of the disease in AD.
Apart from the misidentification syndrome, which appears to be the most common delusion in both DLB and AD, the presentation of delusions is different in the two patient groups. Paranoid delusions in general have been more frequent than other kinds in DLB, whereas theft delusions have been especially common in AD. Patients with DLB have shown abandonment delusions; none of the 47 papers has mentioned that type of delusion in AD. The phantom boarder seems to appear more frequently in DLB than in AD.
One of the biggest limitations of this behavioral review is the lack of objectively gathered quantified data. Most of the data are from case reports and clinical notes, relying on the clinical judgment and observations of individuals. In addition, the first cases to be reported were described repetitively by the authors.10,12,18–21 This may potentially mislead a naïve reader with regard to the prevalence of the symptoms reported. Other limitations lie in data collected by some of the few instruments that have been used. The Burns Symptoms Checklist137,146 inquires exhaustively into various delusions and visual, auditory, and olfactory hallucinations, using a negative quantified scale that scores the frequency, emotion, and insight for each symptom. However, this checklist makes no distinction between overvalued ideas and delusions and has no question related to major depression or other behavioral disturbances. The CUSPAD141,142 and the GMSS144 are both semistructured instruments that must be administered by an experienced clinician or trained lay interviewer to the relatives or caregivers of patients with probable Alzheimer's disease. The semistructured design of these instruments can affect the good interrater reliability of the instruments. In addition, no comparable instrument exists for DLB. The Present Behavioural Examination138 has been designed to assess behavioral abnormalities in dementia such as wandering and agitation. However, this instrument does not make any distinction between the different kinds of hallucinations.40 Finally, the Ham-D,140 although an instrument with good validity and interrater reliability, has been designed for a young adult population and therefore contains some questions inappropriate for geriatric patients. In future research into DLB, as well as in clinical settings, the use of an instrument such as the NPI143 is advisable. It evaluates 12 neuropsychiatric disturbances common in dementia in general and also in DLB: delusions, hallucinations, agitation, dysphoria, anxiety, apathy, irritability, euphoria, disinhibition, aberrant motor behavior, nighttime behavior disturbances, and appetite and eating abnormalities. The NPI is brief, employing screening questions, and it allows measurement of both severity and frequency of behaviors as well as the impact of these behaviors on the caregiver. Cummings et al. have established good content validity, concurrent validity, interrater reliability, and test-retest reliability of this instrument.143
CONCLUSIONS AND FUTURE RESEARCH AGENDA
This review clearly shows that much additional research into DLB is needed. The existing research is limited by small sample sizes, problems with matching of samples, diagnostic issues, and outcome assessment limitations. Nonetheless, the available research does suggest some tentative conclusions, as follows.
The cognitive impairments and behavioral disturbances of DLB reported in this review suggest two anatomic poles: a frontal–anterior temporal/subcortical (anterior) pole and a parietal-temporal-occipital (posterior) pole.
Visual hallucinations and problems with construction praxis might represent the posterior pole. This review has shown that visual hallucinations have been the most frequently reported psychotic symptoms in DLB at onset and at any stage of the disease. Studies with AD147 and DLB40 have found a relationship between visual hallucinations and a decrease of visual acuity40,147 and presence of visual agnosia.147 Therefore, there could be an involvement of visual systems in patients having visual hallucinations. Interestingly, the recent PET data collected in mildly to moderately demented subjects with visual hallucinations have demonstrated a more severe hypometabolism in DLB, compared with AD, in the temporal-parietal-occipital association cortices75 and in the medial and lateral occipital lobes55 involved in the “what” and “where” visual systems. The cognitive findings of the present review, related to the “what” system, have shown that visual perception was similarly impaired in mild DLB and AD (see section above on cognitive symptoms). No data were available in more advanced stages of the disease on tasks of visual perception that test the “what” and “where” systems, such as the Benton Judgment Line Orientation Test. Visual hallucinations could thus be the first symptoms, before the cognitive ones, of an alteration of the “what” system. Several neuropsychological studies have demonstrated a more severe impairment in DLB than in AD in construction praxis, which could involve both the “what” and the “where” systems as well as the frontal and subcortical areas. However, since no motor control measures have been applied in these studies, one does not know to what extent the visual systems are altered in mild and moderate DLB, on the sole basis of the cognitive testing. Unfortunately, no correlative data are available between impairments on visuoconstructive tasks and brain hypometabolism in DLB subjects.
Apart from visual hallucinations, at onset of the disease patients with DLB are more likely to have delusions, major depression (or depressive symptoms of apathy or irritability), and anxiety, rather than auditory, olfactory, and tactile hallucinations. The misidentification syndrome appears to be the most common delusion. These behavioral symptoms, as well as cognitive impairments in working memory, executive functions, and verbal and motor initiation, will be designated as the anterior pole. The anterior pole can be explained by the pathological processes involved in DLB (see, for details, the section on neuropathology). Cortical Lewy bodies are mostly found in anterior frontal, temporal, insula, and cingulate areas, the substantia nigra (dopaminergic ascending pathways origin), the nucleus basalis of Meynert (acetylcholinergic ascending pathways origin), the locus ceruleus (noradrenergic ascending pathways origin), the nucleus raphe dorsalis (serotonergic ascending pathways origin), and the amygdala.
Neuronal losses in DLB occur in the frontal lobes, in the hippocampus, and in the nuclei and areas in which originate the nigrostriatal and the mesocorticolimbic dopaminergic pathways as well as cortical cholinergic pathways. The neuronal losses in the frontal lobes and the disruption of several ascending neurochemical pathways, such as the nigrostriatal and the mesocorticolimbic dopaminergic pathways, as well as the noradrenergic, serotonergic, and acetylcholinergic pathways, ending in the prefrontal areas, might account for the more severe deficits in early DLB, compared with early AD, on tasks of spatial working memory, visuospatial executive function, and verbal and motor initiation. The spatial working memory paradigm has indeed been shown, in some cerebral imagery studies with healthy young control subjects, to depend more on the right than the left Brodmann areas 8, 9,148 46,148,149 and 47,149,150 and on the parietal and occipital cortices.150
The amygdala is also severely altered in DLB, with LB and NFT sometimes localized in the same neuron (see the section on pathology above). Interestingly, animal and human studies have shown that lesions of the amygdala can be related to attention deficits,151 fear, and anxiety,151–153 but not to learning impairments, which are more related to the disruption of the hippocampus.154–155 A more severe impairment of the amygdala in early DLB, compared with a more severe impairment of the hippocampus in early AD, could therefore explain some cognitive and behavioral findings described in the present review. These different alterations could at least partially account for the more severe attention deficits in DLB and the comparable and sometimes more severe impairment of episodic memory or learning capacity in AD. The more severe alteration of the frontal lobes and amygdala in DLB patients, compared with AD patients, can perhaps explain the more frequent paranoid delusions reported in this population. Presence of delusions have been correlated, in previous studies, with hypometabolism in temporal and paralimbic structures156–157 as well as hypometabolism in frontal lobes157–160 in psychotic AD subjects.
In the anterior pole as described above, the study of different functions of working memory may greatly contribute, in the future, to the distinction of DLB and AD. Reciprocally, the study of DLB and AD via their distinct neuropathological processes may help to clarify the physiological and anatomical substrate of working memory. This review has demonstrated that patients with DLB have more severe impairment of spatial working memory than patients with AD. Most of the studies reporting that finding utilized the same task103–106—and therefore measured the same aspect of spatial working memory, and possibly the same anatomical substrate. However, recent cognitive studies mention the various aspects of visuospatial working memory: the passive store and the active imagery operations;161 the separate visual and spatial dimensions following the “what” (ventral visual pathway) and “where” (dorsal visual pathway) visual systems.162 Only two studies51,106 assessed the visuospatial sketchpad of working memory, which could be associated with a more passive visuospatial working memory buffer, and they found no difference between the two patient groups in the mild stages of dementia. There is a need to investigate different aspects of the visuospatial working memory in both DLB and AD subjects. In addition, the status of verbal working memory (articulatory loop) for the DLB and AD patient populations is still unclear because the results are contradictory on the Digit Span task (measuring subvocal rehearsal). The articulatory loop or verbal working memory also has subcomponents, with different biological substrates, that could be assessed with different tests: a subvocal rehearsal system associated with Broca's area;163 a phonological store associated with the left supramarginal gyrus;163 and semantic working memory associated with the left inferior prefrontal cortex.164–166
Finally, one important paradigm of the central executive system of working memory, the dual-task paradigm, has not yet been studied in patients with DLB, and its anatomical substrate is still unclear. This paradigm has been associated in one study with the bilateral dorsolateral prefrontal cortex167 and has been hypothesized by other authors to rely on the cingulate and orbital frontal areas130,131 and to depend at least on the integrity of the dopaminergic nigrostriatal and mesocorticolimbic systems,126,132,133 and on the acetylcholinergic systems in Parkinson's disease.134
The findings regarding the biological substrates of components of working memory are derived from cerebral imagery studies in young healthy subjects, with very small sample sizes. The study of different components of working memory in DLB, AD, and healthy control subjects could thus contribute to the understanding of the cognitive impairment of the two patient populations but would also contribute to the further development of the cognitive model of working memory.
Cross-sectional and, particularly, longitudinal comparisons of cognitive deficits, together with behavioral symptoms presented by patients with dementia with Lewy bodies, Alzheimer's disease, and Parkinson's disease (with and without dementia), will be necessary to uncover the nature of DLB. DLB could be, like AD, heterogeneous, at least at onset of the disease: an anterior pole form with depression or acute anxiety, EPS, and working memory deficits and a posterior pole form with visual hallucinations and construction dyspraxia. The current antemortem diagnostic criteria of the CDLB9 make it easier for investigators, using the gradual diagnosis system of possible and probable DLB,9 to start the observation of DLB at a very early stage. These criteria have a good specificity, despite a poor interrater reliability (see McKeith et al.168 for a review). At onset, DLB with EPS seems clinically different from PD, at least in terms of motor symptoms (see section on EPS above). However, data comparing DLB, PD, and AD are very scarce.
This review has shown that comparisons between AD and DLB are currently the most accessible in literature. However, very few of the reviewed studies have correlated the behavioral disturbances with specific and quantified cognitive impairments, at onset or at any stage of the disease. The cognitive and behavioral characterization of dementia with Lewy bodies will be the first step toward efficient treatment solutions for the disease.
ACKNOWLEDGMENTS
This work has been supported by the Alzheimer Society of Canada (postdoctoral research fellowship #97-15 to the first author) and by the Kunin-Lunenfeld Applied Research Unit of Baycrest (M.S. and R.v.R.). R.v.R. and M.S. are currently involved in research granted by the Alzheimer Society of Canada.
 |
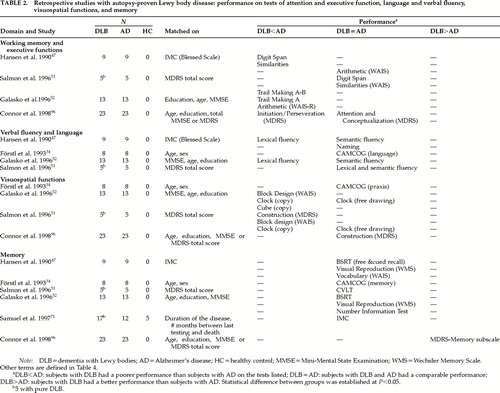 |
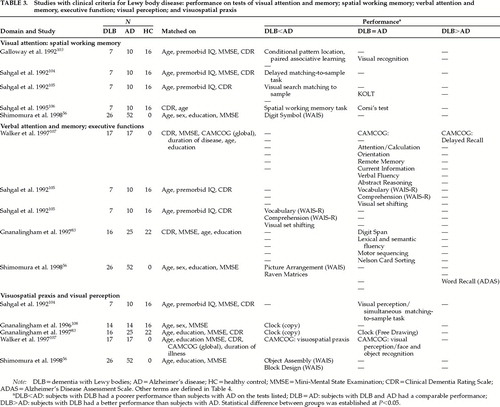 |
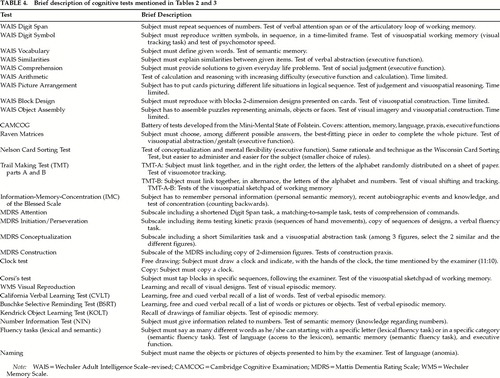 |
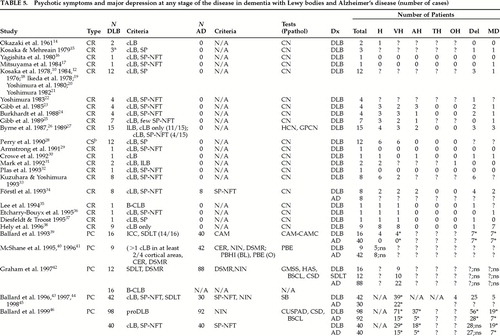 |
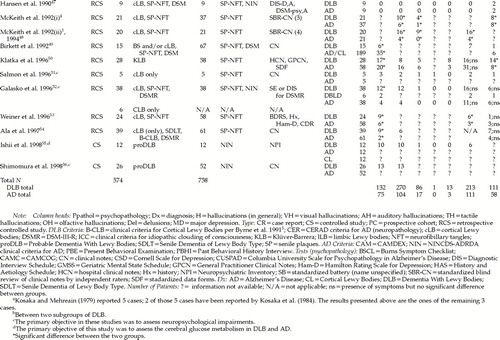 |
1 McKeith IG, Fairbairn A, Perry R, et al: Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ 1992; 305:673–678Crossref, Medline, Google Scholar
2 Lindboe CF, Hansen HB: The frequency of Lewy bodies in a consecutive autopsy series. Clin Neuropathol 1998; 17:204–209Medline, Google Scholar
3 Shergill S, Mullan E, D'ath P, et al: What is the clinical prevalence of Lewy body dementia? Int J Geriatr Psychiatry 1994; 9:907–912Google Scholar
4 McKeith IG, Perry RH, Fairbairn AF, et al: Operational criteria for senile dementia of Lewy body Type (SDLT). Psychol Med 1992; 22:911–922Crossref, Medline, Google Scholar
5 Byrne EJ, Lennox GG, Goodwin-Austen RB, et al: Dementia associated with cortical Lewy bodies: proposed clinical diagnostic criteria. Dementia 1991; 2:283–284Google Scholar
6 Lewy FH: Paralysis agitans, in Handbuch der Neurologie, edited by Lawandowsky M. Berlin, Springer-Verlag, 1912 , pp 920–958Google Scholar
7 Lipkin LE: Cytoplasmic inclusions in ganglion cells associated with Parkinsonian states: a neurocellular change studied in 53 cases and 206 control cases. Am J Pathol 1959; 35:1117–1133Google Scholar
8 Forno LS: Concentric hyalin intraneuronal inclusions of Lewy body type in the brains of elderly persons (50 incidental cases): relationship to parkinsonism. J Am Geriatr Soc 1969; 17:557– 575Crossref, Medline, Google Scholar
9 McKeith IG, Galasko D, Kosaka K, et al: Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB). Neurology 1996; 47:1113–1124Google Scholar
10 Kosaka K: Lewy bodies in cerebral cortex: report of three cases. Acta Neuropathol (Berl) 1978; 42:127–134Crossref, Medline, Google Scholar
11 Kuzuhara S, Mori H, Izumiyama N, et al: Lewy bodies are ubiquitinated: a light and electron microscopic immunocytochemical study. Acta Neuropathol (Berl) 1988; 75:345–353Crossref, Medline, Google Scholar
12 Kosaka K, Yoshimura M, Ikeda K, et al: Diffuse Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree: a new disease? Clin Neuropathol 1984; 3:185–192Google Scholar
13 Perry RH, Irving D, Blessed G, et al: Clinically and neuropathologically distinct form of dementia in the elderly. Lancet 1989; 21:166Crossref, Google Scholar
14 Okazaki H, Lipkin LE, Aronson SM: Diffuse intracytoplasmic ganglionic inclusions (Lewy type) associated with progressive dementia and quadriparesis in flexion. J Neuropathol Exp Neurol 1961; 20:237–244Crossref, Medline, Google Scholar
15 Kosaka K, Mehreain P: Dementia: parkinsonism syndrome with numerous Lewy bodies and senile plaques in cerebral cortex. Archiv für Psychiatrie und Nervenkrankeiten 1979; 226:241– 250Crossref, Medline, Google Scholar
16 Yagashita S, Itoh Y, Amano N, et al: Atypical senile dementia with widespread Lewy type inclusion in the cerebral cortex. Acta Neuropathol 1980; 49:187–191Crossref, Medline, Google Scholar
17 Mitsuyama Y, Fukunga H, Yamashita M: Alzheimer's disease with widespread presence of Lewy bodies. Folia Psychiatria et Neurologica Japonica 1984; 38:82–88Google Scholar
18 Kosaka K, Oyanagi S, Matsushita M, et al: Presenile dementia with Alzheimer-, Pick- and Lewy-body changes. Acta Neuropathol (Berl) 1976; 36:221–233Crossref, Medline, Google Scholar
19 Ikeda K, Hori A, Bode G: Progressive dementia with “diffuse Lewy-type inclusions” in cerebral cortex. Archiv für Psychiatrie und Nervenkrankeiten 1978; 228:243–248Crossref, Google Scholar
20 Yoshimura M, Schimada H, Nagura H, et al: Two autopsy cases of Parkinson's disease with Shy-Drager syndrome (abstract). Transactions of the Society of Pathology in Japan (Japan) 1980; 69:423Google Scholar
21 Yoshimura M: Corticale Veränderungen bei Paralysis agitans [Cortical alterations with paralysis agitans]. Aktiengesellschaft für Problematisch Neuropathologie (Wien) 1982; 7:77–91Google Scholar
22 Yoshimura M: Cortical changes in the parkinsonian brain: a contribution to the delineation of “diffuse Lewy body disease.” J Neurol 1983; 229:17–32Crossref, Medline, Google Scholar
23 Gibb WRG, Esiri MM, Lees AJ: Clinical and pathological features of diffuse cortical Lewy body disease (Lewy body dementia). Brain 1985; 110:1131–1153Google Scholar
24 Burkhardt CR, Filley CM, Kleinschmidt-DeMasters BK, et al: Diffuse Lewy body disease and progressive dementia. Neurology 1988; 38:1520–1528Google Scholar
25 Gibb WF, Luthert PJ, Janota I, et al: Cortical Lewy body dementia: clinical features and classification. J Neurol Neurosurg Psychiatry 1989; 52:185–192Crossref, Medline, Google Scholar
26 Byrne EJ, Lowe J, Goodwin-Austen RB, et al: Dementia and Parkinson's disease associated with diffuse cortical Lewy bodies. Lancet 1987; 8531:501Crossref, Google Scholar
27 Byrne EJ, Lennox G, Lowe J, et al: Diffuse Lewy body disease: clinical features in 15 cases. J Neurol Neurosurg Psychiatry 1989; 52:509–517Crossref, Google Scholar
28 Perry RH, Irving D, Blessed G, et al: Senile dementia of Lewy body type: a clinically and neuropathologically distinct form of Lewy body dementia in the elderly. J Neurol Sci 1990; 95:119– 139Crossref, Medline, Google Scholar
29 Armstrong TP, Hansen LA, Salmon DP, et al: Rapidly progressive dementia in a patient with the Lewy body variant of Alzheimer's disease. Neurology 1991; 41:1178–1180Google Scholar
30 Crowe SF, Peppard RF, Borenstein R, et al: Diffuse Lewy body disease and progressive dementia in a young woman. Aust NZ J Psychiatry 1992; 26:507–511Crossref, Medline, Google Scholar
31 Mark MH, Sage JI, Dickson DW, et al: Levodopa-nonresponsive Lewy body parkinsonism: clinicopathologic study of two cases. Neurology 1992; 42:1323–1327Google Scholar
32 Plas J, Dubrugeaud G, Jeanneau A, et al: Demence a corps de Lewy corticaux diffus [Diffuse cortical Lewy body dementia]. L'Encephale, 1993; XIX:541–545Google Scholar
33 Kuzuhara S, Yoshimura M: Clinical and neuropathological aspects of diffuse Lewy body disease in the elderly. Adv Neurol 1993; 60:464–469Medline, Google Scholar
34 Förstl H, Burns A, Luthert P, et al: The Lewy-body variant of Alzheimer's disease, clinical and pathological findings. Br J Psychiatry 1993; 162:385–392Crossref, Medline, Google Scholar
35 Lee H, Cooney JM, Lawlor BA: Case report: the use of risperidone, an atypical neuroleptic, in Lewy body disease. Int J Geriatr Psychiatry 1994; 9:415–417Crossref, Google Scholar
36 Etcharry-Bouyx F, Gambarelli D, Kassis N, et al: Troubles cognitifs et syndrome parkinsonien: maladie a corps de Lewy diffus? [Cognitive problems and parkinsonian syndrome: diffuse Lewy body disease?] Rev Neurol. (Paris) 1995; 151,6–7:410–412Google Scholar
37 Diesfeldt HFA, Troost D: Delusional misidentification and subsequent dementia: a clinical and neuropathological study. Dementia 1995; 6:94–98Medline, Google Scholar
38 Hely MA, Reid WGJ, Halliday GH, et al: Diffuse Lewy body disease: clinical features in nine cases without coexistent Alzheimer's disease. J Neurol Neurosurg Psychiatry 1996; 60:531– 538Crossref, Medline, Google Scholar
39 Ballard CG, Mohan RNC, Patel A, et al: Idiopathic clouding of consciousness: do the patients have cortical Lewy body disease? Int J Geriatr Psychiatry 1993; 8:571–576Google Scholar
40 McShane R, Gedling K, Reading M, et al: Prospective study of relations between cortical Lewy bodies, poor eyesight, and hallucinations in Alzheimer's disease. J Neurol Neurosurg Psychiatry 1995; 59:185–188Crossref, Medline, Google Scholar
41 McShane R, Keene J, Gedling K, et al: Hallucinations, cortical Lewy body pathology, cognitive function and neuroleptic use in dementia, in Dementia With Lewy Bodies: Clinical, Pathological and Treatment Issues, edited by Perry RH, McKeith IG, Perry EK. New York, Cambridge University Press, 1996, pp 85–98Google Scholar
42 Graham C, Ballard C, Saad K: Variables which distinguish patients fulfilling clinical criteria for dementia with Lewy bodies from those with Alzheimer's disease. Int J Geriatr Psychiatry 1997; 12:314–318Crossref, Medline, Google Scholar
43 Ballard C, Lowery K, Harrison R, et al: Noncognitive symptoms in Lewy Body dementia, in Dementia With Lewy Bodies: Clinical, Pathological and Treatment Issues, edited by Perry RH, McKeith IG, Perry E. New York, Cambridge University Press, 1996, pp 67–83Google Scholar
44 Ballard C, McKeith I, Harrison R, et al: A detailed phenomenological comparison of complex visual hallucinations in dementia with Lewy bodies and Alzheimer's disease. Int Psychogeriatr 1997; 9:381–388Crossref, Medline, Google Scholar
45 Ballard C, McKeith IG: Psychiatric features in diffuse Lewy body disease (letter). Neurology 1998; 50:573Crossref, Medline, Google Scholar
46 Ballard C, Holmes C, McKeith IG, et al: Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer's disease. Am J Psychiatry 1999; 156:1039–1045Google Scholar
47 Hansen L, Salmon D, Galasko D, et al: The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology 1990; 40:1–8Crossref, Medline, Google Scholar
48 McKeith IG, Fairbairn AF, Bothwell RA, et al: An evaluation of the predictive validity and inter-rater reliability of clinical diagnostic criteria for senile dementia of Lewy body type. Neurology 1994; 44:872–877Crossref, Medline, Google Scholar
49 Birkett DP, Desouky A, Han L, et al: Lewy bodies in psychiatric patients. Int J Geriatr Psychiatry 1992; 7:235–240Crossref, Google Scholar
50 Klatka LA, Louis ED, Schiffer RB: Psychiatric features in diffuse Lewy body disease: a clinicopathologic study using Alzheimer's disease and Parkinson's disease comparison groups. Neurology 1996; 47:1148–1152Google Scholar
51 Salmon DP, Galasko D, Hansen LA, et al: Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn 1996; 31:148–165Crossref, Medline, Google Scholar
52 Galasko D, Katzman R, Salmon DP, et al: Clinical and neuropathological findings in Lewy body dementias. Brain Cogn 1996; 31:166–175Crossref, Medline, Google Scholar
53 Weiner MF, Risser RC, Cullum CM, et al: Alzheimer's disease and its Lewy body variant: a clinical analysis of postmortem verified cases. Am J Psychiatry 1996; 153:1269–1273Google Scholar
54 Ala TA, Yang KH, Sung JH, et al: Hallucinations and signs of parkinsonism help distinguish patients with dementia and cortical Lewy bodies from patients with Alzheimer's dementia at presentation: a clinicopathological study. J Neurol Neurosurg Psychiatry 1997; 62:16–21Crossref, Medline, Google Scholar
55 Ishii K, Imamura T, Sasaki M, et al: Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer's disease. Neurology 1998; 51:125–130Crossref, Medline, Google Scholar
56 Shimomura T, Mori E, Yamashita H, et al: Cognitive loss in dementia with Lewy bodies and Alzheimer disease. Arch Neurol 1998; 55:1547–1552Google Scholar
57 Kosaka K: Dementia and neuropathology in Lewy body disease. Adv Neurol 1993; 60:456–463Medline, Google Scholar
58 Khatchaturian Z: Diagnosis of Alzheimer's disease. Arch Neurol 1985; 42:1097–1105Google Scholar
59 Harding AJ, Halliday GM: Simplified neuropathological diagnosis of dementia with Lewy bodies. Neuropathol Appl Neurobiol 1998; 24:195–201Crossref, Medline, Google Scholar
60 Mega MS, Masterman DL, Benson F, et al: Dementia with Lewy bodies: reliability and validity of clinical and pathologic criteria. Neurology 1996; 47:1403–1409Google Scholar
61 Becker JT, Huff EJ, Nebes RD, et al: Neuropsychological function in Alzheimer's disease: pattern of impairment and rates of progression. Arch Neurol 1988; 45:263–268Crossref, Medline, Google Scholar
62 Burns A, Jacoby R, Levy R: Progression of cognitive impairment in Alzheimer's disease. J Am Geriatr Soc 1991; 39:39–45Crossref, Medline, Google Scholar
63 Del-Ser T, Munoz DG, Hachinski V: Temporal pattern of cognitive decline and incontinence is different in Alzheimer's disease and diffuse Lewy body disease, Neurology 1996; 46:682– 286Google Scholar
64 Schmidt ML, Martin JA, Lee VMY, et al: Convergence of Lewy bodies and neurofibrillary tangles in amygdala neurons of Alzheimer's disease and Lewy body disorders. Acta Neuropathol 1996; 91:475–481Crossref, Medline, Google Scholar
65 Dickson DW, Wu E, Crystal HA, et al: Alzheimer's Disease and age-related pathology in diffuse Lewy body disease, in Heterogeneity of Alzheimer's Disease, edited by Boller F, Forette F, Khatchaturian Z, et al. Berlin-Heidelberg, Springer-Verlag, 1992, pp 168–186Google Scholar
66 Crystal HA, Dickson DW, Lizardi JE, et al: Antemortem diagnosis of diffuse Lewy body disease. Neurology 1990; 40:1523– 1528Google Scholar
67 Perry EK, Kerwin J, Perry RH, et al: Visual hallucinations and the cholinergic system in dementia (letter). J Neurol Neurosurg Psychiatry 1990; 53:88Crossref, Medline, Google Scholar
68 Perry EK, Marshall E, Perry RH, et al: Cholinergic and dopaminergic activities in senile dementia of Lewy body type. Alzheimer Dis Assoc Disord 1990; 4:87–95Crossref, Medline, Google Scholar
69 Perry EK, Irving D, Kerwin JM, et al: Cholinergic transmitter and neurotrophic activities in Lewy body dementia: similarity to Parkinson's and distinction from Alzheimer disease. Alzheimer Dis Assoc Disord 1993; 7:69–79Crossref, Medline, Google Scholar
70 Langlais PJ, Thal LJ, Hansen L, et al: Neurotransmitters in basal ganglia and cortex of Alzheimer's disease with and without Lewy bodies. Neurology 1993; 43:1927–1934Google Scholar
71 Samuel W, Alford M, Hofstetter R, et al: Dementia with Lewy bodies versus pure Alzheimer disease: differences in cognition, neuropathology, cholinergic dysfunction, and synapse density. J Neuropathol Exp Neurol 1997; 56:499–508Crossref, Medline, Google Scholar
72 Perry EK, Marshall E, Thompson P, et al: Monoaminergic activities in Lewy body dementia: relation to hallucinations and extrapyramidal features. J Neural Transm 1993; 6:167–177Crossref, Google Scholar
73 Eggerston DE, Sima AAF: Dementia with cerebral Lewy bodies: a mesocortical dopaminergic defect? Arch Neurol 1986; 43:524–527Google Scholar
74 McKhann G, Drachman D, Folstein M, et al: Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
75 Imamura T, Ishii K, Sasaki M, et al: Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer's disease: a comparative study using positron emission tomography. Neurosci Lett 1997; 235:49–52Crossref, Medline, Google Scholar
76 Brown DF, Dababo MA, Bigio EH, et al: Neuropathologic evidence that the Lewy body variant of Alzheimer disease represents coexistence of Alzheimer disease and idiopathic Parkinson disease. J Neuropathol Exp Neurol 1998; 57:39–46Crossref, Medline, Google Scholar
77 Brown DF, Risser RC, Bigio EH, et al: Neocortical synapse density and Braak stage in the Lewy body variant of Alzheimer disease: a comparison with classic Alzheimer disease and normal aging. J Neuropathol Exp Neurol 1998; 57:955–960Crossref, Medline, Google Scholar
78 Mattila PM, Roytta M, Torikka H, et al: Cortical Lewy bodies and Alzheimer-type changes in patients with Parkinson's disease. Acta Neuropathol 1998; 95:576–582Crossref, Medline, Google Scholar
79 Ellis RJ, Caligiuri M, Galasko D, et al: Extrapyramidal motor signs in clinically-diagnosed Alzheimer's disease (review). Alzheimer Dis Assoc Disord 1996; 10:103–114Crossref, Medline, Google Scholar
80 Louis ED, Goldman JE, Powers JM, et al: Parkinsonian features of eight pathologically diagnosed cases of diffuse Lewy body disease. Mov Disord 1995; 10:188–194Crossref, Medline, Google Scholar
81 Louis ED, Fahn S: Pathologically diagnosed diffuse Lewy body disease and Parkinson's disease: do the parkinsonian features differ? Adv Neurol 1996; 69:311–314Google Scholar
82 Louis ED, Klatka LA, Liu Y, et al: Comparison of extrapyramidal features in 31 pathologically confirmed cases of diffuse Lewy body disease and 34 pathologically confirmed cases of Parkinson's disease. Neurology 1997; 48:376–380Crossref, Medline, Google Scholar
83 Gnanalingham KK, Byrne EJ, Thornton A: Motor and cognitive function in Lewy body dementia: comparison with Alzheimer's and Parkinson's diseases. J Neurol Neurosurg Psychiatry 1997; 62:243–252Crossref, Medline, Google Scholar
84 Chacko RC, Hurley RA, Jankovic J: Use in diffuse Lewy body disease. J Neuropsychiatry 1993; 5:206–208Link, Google Scholar
85 Allen RL, Walker Z, J D'ath P, et al: Risperidone for psychotic and behavioural symptoms in Lewy body dementia. Lancet 1995; 346:185Crossref, Medline, Google Scholar
86 McKeith I, Ballard CG, Harrison RWS: Neuroleptic sensitivity to risperidone in Lewy body dementia. Lancet 1995; 346:699Crossref, Medline, Google Scholar
87 Burke WJ, Pfeiffer RF, McComb RD: Neuroleptic sensitivity to clozapine in dementia with Lewy bodies. J Neuropsychiatry Clin Neurosci 1998; 10:227–229Link, Google Scholar
88 Herrmann N, Rivard M-F, Flynn M, et al: Risperidone for the treatment of behavioral disturbances in dementia: a case series. J Neuropsychiatry Clin Neurosci 1998; 10:220–223Link, Google Scholar
89 Conn DK, Simard M: Case report: successful treatment of psychosis with olanzapine in a case of early dementia with Lewy bodies. Int J Geriatr Psychopharmacol 1999; 2:47–49Google Scholar
90 Walker Z, Grace J, Overshot R, et al: Olanzapine in dementia with Lewy bodies: a clinical study. Int J Geriatr Psychiatry 1999; 14:459–466Crossref, Medline, Google Scholar
91 Levy R, Eagger S, Griffiths M, et al: Lewy bodies and response to tacrine in Alzheimer's disease. Lancet 1994; 343:176Crossref, Medline, Google Scholar
92 Shea C, MacKnight C, Rockwood K: Aspects of dementia: donepezil for treatment of dementia with Lewy bodies: a case series of nine patients. Int Psychogeriatr 1998; 10:229–238Crossref, Medline, Google Scholar
93 Blessed G, Tomlinson BE, Roth M: The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 1968; 114:797–811Crossref, Medline, Google Scholar
94 Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
95 Mattis S: Mental Status Examination for organic mental syndrome in the elderly patient, in Geriatric Psychiatry, edited by Bellak T, Karasu TB. New York, Grune and Stratton, 1976, pp 77–121Google Scholar
96 Connor DJ, Salmon DP, Sandy TJ, et al: Cognitive profiles of autopsy-confirmed Lewy body variant vs pure Alzheimer disease. Arch Neurol 1998; 55:994–1000Google Scholar
97 Baddeley AD: Working Memory. Oxford, UK, Oxford University Press, 1986Google Scholar
98 Baddeley AD: Human Memory: Theory and Practice. Hove, Hillsdale, NJ, Lawrence Erlbaum, 1990Google Scholar
99 Pillon B, Dubois B, Ploska A, et al: Severity and specificity of cognitive impairment in Alzheimer's, Huntington's, and Parkinson's diseases and progressive supranuclear palsy. Neurology 1991; 41:634–642Crossref, Medline, Google Scholar
100 Pillon B, Deweer B, Michon A, et al: Are explicit memory disorders of progressive supranuclear palsy related to damage to striatofrontal circuits? Comparison with Alzheimer's, Parkinson's, and Huntington's diseases. Neurology 1994; 44:1264–1270Google Scholar
101 Hughes CP, Berg L, Danziger WL, et al: A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140:566–572Crossref, Medline, Google Scholar
102 Roth M, Tym E, Mountjoy CQ, et al: CAMDEX: a standardised instrument for the diagnoses of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986; 149:698–709Crossref, Medline, Google Scholar
103 Galloway PH, Sahgal A, McKeith IG, et al: Visual pattern recognition memory and learning deficits in senile dementia of Alzheimer and Lewy body types. Dementia 1992; 3:101–107Google Scholar
104 Sahgal A, Galloway PH, McKeith IG, et al: Matching-to-sample deficits in patients with senile dementias of the Alzheimer and Lewy body types. Arch Neurol 1992; 49:1043–1046Google Scholar
105 Sahgal A, Galloway PH, McKeith IG, et al: A comparative study of attentional deficits in senile dementias of Alzheimer and Lewy body types. Dementia 1992; 3:350–354Google Scholar
106 Sahgal A, McKeith IG, Galloway PH, et al: Do differences in visuospatial ability between senile dementias of the Alzheimer and Lewy body types reflect differences solely in mnemonic function? J Clin Exp Neuropsychol 1995; 171:035–043Google Scholar
107 Walker Z, Allen RL, Shergill S, et al: Neuropsychological performance in Lewy body dementia and Alzheimer's disease. Br J Psychiatry 1997; 170:156–158Crossref, Medline, Google Scholar
108 Gnanalingham KK, Byrne EJ, Thornton A: Clock-face drawing to differentiate Lewy body and Alzheimer type dementia syndromes. Lancet 1996; 347:696–697Crossref, Medline, Google Scholar
109 Swanwick GRJ, Coen RF, Maguire CP, et al: Clock-face drawing to differentiate dementia syndrome. Lancet 1996; 347:1115Crossref, Medline, Google Scholar
110 Baddeley AD, Logie R, Bressi S, et al: Dementia and working memory. Q J Exp Psychol A 1986; 38A:603–614Google Scholar
111 Owen AM, Downes JJ, Sahakian BJ, et al: Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 1990; 28:1021–1034Google Scholar
112 Owen AM, Morris RG, Sahakian BJ, et al: Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain 1996; 119:1597–1615Google Scholar
113 Morris RG, Downes JJ, Sahakian BJ, et al: Planning and spatial working memory in Parkinson's disease. J Neurol Neurosurg Psychiatry 1988; 51:757–766Crossref, Medline, Google Scholar
114 Owen AM, James M, Leigh PN, et al: Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain 1992; 115:1727–1751Google Scholar
115 Owen AM, Besinska M, James M, et al: Visuospatial memory deficits at different stages of Parkinson's disease. Neuropsychologia 1993; 31:627–644Crossref, Medline, Google Scholar
116 Olichney JM, Galasko D, Salmon DP, et al: Cognitive decline is faster in Lewy body variant than in Alzheimer's disease. Neurology 1998; 51:351–357Crossref, Medline, Google Scholar
117 Ballard C, Patel A, Oyebode F, et al: Cognitive decline in patients with Alzheimer's disease, vascular dementia and senile dementia of Lewy body type. Age Ageing 1996; 25:209–213Crossref, Medline, Google Scholar
118 Miller TP, Tinklenberg JR, Brooks JO III, et al: Cognitive decline in patients with Alzheimer disease: differences in patients with and without extrapyramidal signs. Alz Dis Assoc Disord 1991; 5:251–256Crossref, Medline, Google Scholar
119 Soininen H, Helkala V, Laulumaa, et al: Cognitive profile of Alzheimer patients with extrapyramidal signs: a longitudinal study. J Neural Transm (P-D Sect) 1992; 4:241–254Crossref, Medline, Google Scholar
120 Chui HC, Lyness SA, Sobel E, et al: Extrapyramidal signs and psychiatric symptoms predict faster cognitive decline in Alzheimer's disease. Arch Neurol 1994; 51:676–681Crossref, Medline, Google Scholar
121 Lopez OL, Brenner RP, Becker JT, et al: EEG spectral abnormalities and psychosis as predictors of cognitive and functional decline in probable Alzheimer's disease. Neurology 1997; 48:1521–1525Google Scholar
122 Reisberg B, Schneck MK, Ferris SH: The Brief Cognitive Rating Scale (BCRS): finding in primary degenerative dementia (PDD). Psychopharmacol Bull 1983; 19:734–739Google Scholar
123 Hachinski VC, Iliff LD, Zilhka E, et al: Cerebral blood flow in dementia. Arch Neurol 1975; 32:632–637Crossref, Medline, Google Scholar
124 Coull JT, Middleton HC, Robbins TW, et al: Contrasting effects of clonidine and diazepam on tests of working memory. Psychopharmacology 1995; 120:311–321Crossref, Medline, Google Scholar
125 Baddeley AD, Bressi S, Della Sala S, et al: The decline of working memory in Alzheimer's disease: a longitudinal study. Brain 1991; 114:2521–2542Google Scholar
126 Panisset M, Simard M, Papagno C, et al: Central executive system deficits in Parkinson's disease, in Proceedings of the International Symposium on Dementia and Parkinson's Disease, edited by Korczin A. Bologna, Monduzzi Editore, 1994, pp 249– 251Google Scholar
127 Greene JDW, Hodges JR, Baddeley AD: Autobiographical memory and executive function in early dementia of Alzheimer type. Neuropsychologia 1995; 33:1647–1670Google Scholar
128 Baddeley AD, Della Sala S: Working memory and executive control. Philos Trans R Lond B Biol Sci 1996; 351B:1397–1404Google Scholar
129 Baddeley AD, Della Sala S, Papagno C, et al: Dual task performance in dysexecutive and nondysexecutive patients with a frontal lesion. Neuropsychology 1997; 11:187–194Crossref, Medline, Google Scholar
130 Moscovitch M: Cognitive resources and dual-task interference effects at retrieval in normal people: the role of the frontal lobes and medial temporal cortex. Neuropsychology 1994; 8:524–534Crossref, Google Scholar
131 Stuss DT, Shallice T, Alexander MP, et al: A multidisciplinary approach to anterior attentional functions. Ann N Y Acad Sci 1995; 769:191–211Crossref, Medline, Google Scholar
132 Malapani C, Pillon B, Dubois B, et al: Impaired simultaneous cognitive task performances in Parkinson's disease: a dopamine-related dysfunction. Neurology 1994; 44:319–326Crossref, Medline, Google Scholar
133 Dubois B, Malapani C, Verin M, et al: Fonctions cognitives et noyaux gris centraux: le modèle de la maladie de Parkinson [Cognitive functions and basal ganglia: the model of Parkinson's disease]. Rev Neurol (Paris) 1994; 150:763–770Medline, Google Scholar
134 Simard M: Étude comparée sur les altérations de la mémoire de travail dans la maladie de Parkinson et l'ataxie de Friedreich: implication des processus de l'administrateur central [Comparative study of alterations of working memory in Parkinson's disease and Friedreich's ataxia: implication of processes of the central executive system]. Ph.D. thesis, Département de Psychologie, Université du Québec à Montréal, Montréal, Québec, Canada, 1997Google Scholar
135 Wechsler D: The Measurement and Appraisal of Adult Intelligence, 4th edition. Baltimore, Williams and Wilkins, 1958Google Scholar
136 Wechsler D: Wechsler Adult Intelligence Scale–Revised, manual. New York, Psychological Corporation, 1981Google Scholar
137 Burns A, Jacoby R, Levy R: Psychiatric phenomena in Alzheimer's disease. Br J Psychiatry 1990; 157:72–76Crossref, Medline, Google Scholar
138 Hope T, Fairburn CG: The Present Behavioural Examination (PBE): the development of an interview to measure current behavioural abnormalities. Psychol Med 1992; 22:223–230Crossref, Medline, Google Scholar
139 Alexopoulos GS, Abrams RC, Young RC, et al: Cornell Scale for Depression in Dementia. Biol Psychiatry 1988; 23:271–284Crossref, Medline, Google Scholar
140 Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
141 Devanand DP, Miller L, Richards M, et al: The Columbia University Scale for Psychopathology in Alzheimer's Disease. Arch Neurol 1992; 49:371–376Crossref, Medline, Google Scholar
142 Devanand DP: Use of the Columbia University Scale to Assess Psychopathology in Alzheimer's Disease. Int Psychogeriatr 1997; 9(suppl 1):137–142Google Scholar
143 Cummings JL, Mega M, Gray K, et al: The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Google Scholar
144 Copeland JRM, Kelleher MJ, Kellet JM, et al: A semi-structured clinical interview for the assessment of diagnosis and mental state in the elderly: the Geriatric Mental State, I: development. Psychol Med 1976; 6:439–449Crossref, Medline, Google Scholar
145 Van Reekum R, Simard M, Clarke D, et al: Late-life depression as a predictor of dementia: cross sectional results from a day hospital for depression database. Am J Geriatr Psychiatry 1999; 7:151–159Medline, Google Scholar
146 Ballard CG, Saad K, Patel A, et al: The prevalence and phenomenology of psychotic symptoms in dementia sufferers. Int J Geriatr Psychiatry 1995; 10:477–485Crossref, Google Scholar
147 Holroyd S, Shelson-Keller A: A study of visual hallucinations in Alzheimer's disease. J Geriatr Psychiatry 1995; 3:198–205Crossref, Google Scholar
148 Petrides M, Alivisatos B, Evans AC, et al: Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci USA 1993; 90:873– 877Crossref, Medline, Google Scholar
149 McCarthy G, Blamire AM, Puce A, et al: Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc Natl Acad Sci USA 1994; 91:8690–8694Google Scholar
150 Jonides J, Smith EE, Koeppe RA, et al: Spatial working memory in humans as revealed by PET. Nature 1993; 363:623–625Crossref, Medline, Google Scholar
151 Davis M: Neurobiology of fear responses: the role of the amygdala (review). J Neuropsychiatry Clin Neurosci 1997; 9:382–402Link, Google Scholar
152 Davis M: Are different parts of the extended amygdala involved in fear versus anxiety? (review). Biol Psychiatry 1998; 44:1239–1247Google Scholar
153 Huston JP, Schildein S, Gerhardt P, et al: Modulation of memory, reinforcement and anxiety parameters by intra-amygdala injection of cholecystokinin-fragments Boc CCK-4 and CCK-8s. Peptides 1998; 19:27–37Crossref, Medline, Google Scholar
154 Squire LR, Zola-Morgan S: The medial temporal lobe memory system. Science 1991; 253:1380–1386Google Scholar
155 Deweer B, Lehéricy S, Pillon B, et al: Memory disorders in probable Alzheimer's disease: the role of hippocampal atrophy as shown with MRI. J Neurol Neurosurg Psychiatry 1995; 58:590– 597Crossref, Medline, Google Scholar
156 Starkstein SE, Vázquez S, Petracca G, et al: A SPECT study of delusions in Alzheimer's disease. Neurology 1994; 44:2055–2059Google Scholar
157 Mentis MJ, Weinstein EA, Horwitz B, et al: Abnormal brain glucose metabolism in the delusional misidentification syndromes: positron emission tomography study in Alzheimer disease. Biol Psychiatry 1995; 38:438–449Crossref, Medline, Google Scholar
158 Binetti G, Padovani A, Magni E, et al: Delusions and dementia: clinical and CT correlates. Acta Neurol Scand 1995; 91:271–275Crossref, Medline, Google Scholar
159 Kotrla KJ, Chacko RC, Harper RG, et al: SPECT findings on psychosis in Alzheimer's disease. Am J Psychiatry 1995; 152:1470–1475Google Scholar
160 Sultzer DL, Mahler ME, Mandelkern MA, et al: The relationship between psychiatric symptoms and regional cortical metabolism in Alzheimer's disease. J Neuropsychiatry Clin Neurosci 1995; 7:476–484Link, Google Scholar
161 Vecchi T, Monticellai ML, Cornoldi C: Visuo-spatial working memory: structures and variables affecting a capacity measure. Neuropsychologia 1995; 33:1549–1564Google Scholar
162 Ruchkin DS, Johnson R Jr, Grafman J, et al: Multiple visuospatial working memory buffers: evidence from spatiotemporal patterns of brain activity. Neuropsychologia 1997; 35:195–209Crossref, Medline, Google Scholar
163 Paulesu E, Frith CD, Frackowiak RSJ: The neural correlates of the verbal component of working memory. Nature 1993; 362:342–345Crossref, Medline, Google Scholar
164 McCarthy G, Blamire AM, Rothman DL, et al: Echo-planar magnetic resonance imaging studies of frontal cortex activation during word generation in humans. Proc Natl Acad Sci USA 1993; 90:4952–4956Google Scholar
165 Cohen JD, Forman SD, Braver TS, et al: Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Hum Brain Mapp 1994; 1:293–304Crossref, Medline, Google Scholar
166 Gabrieli JDE, Poldrack RA, Desmond JE: The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA 1998; 95:906–913Crossref, Medline, Google Scholar
167 D'Esposito M, Detro JA, Alsop DC, et al: The neural basis of the central executive system of working memory. Nature 1995; 379:279–281Crossref, Google Scholar
168 McKeith IG, O'Brien JT, Ballard C: Diagnosing dementia with Lewy Bodies. Lancet 1999; 354:1227–1228Google Scholar



