Functional Imaging as a Window to Dementia: Corticobasal Degeneration
Corticobasal degeneration (CBD) is a rare neurodegenerative dementia that illustrates the use of functional imaging in geriatric psychiatry. There have been numerous publications related to the more common dementias (such as Alzheimer’s disease [AD]) and functional imaging. Fewer reports are available regarding CBD; functional imaging may be cardinal in differentiating this clinical entity from other dementias and/or movement disorders. CBD typically presents with asymmetric parkinsonism, dystonia or focal myoclonus and specific cognitive-behavioral changes.1 These include one or more of the following signs: ideomotor apraxia, cortical sensory loss or alien hand phenomenon,2,3 frontal executive deficits,4,5 and, less often, dementia.6 Although first described in 1968, it was not until the early 1990s that this disorder became a focus of clinical and research interest.7 Investigators have previously referred to CBD as corticodentatonigral degeneration with neuronal achromasia, corticonigral degeneration, corticobasal ganglionic degeneration, or progressive asymmetrical rigidity and apraxia syndrome.7 Some authors have suggested using the term corticobasal syndrome for the constellation of clinical features considered as defining characteristics of corticobasal degeneration, reserving the term CBD for the histopathological disorder.8 CBD is now recognized as part of the spectrum of frontotemporal lobar degeneration.2
The prevalence of this disorder is not clear. CBD is less common than other mid- and late-life basal ganglia disorders. In an autopsy series of 226 elderly demented patients, CBD accounted for 1.3% of cases.9 The mean age at onset is 63 years (SD=7.7), although the disease may occur as young as 28.2,10 The disease has an insidious onset and is steadily progressive, with a mean duration of 7.9 years (SD=2.6).2 The clinical diagnosis of CBD is challenging. This disorder may be markedly underdiagnosed, as one report indicates initial recognition to be as low as 35%.11 CBD may be difficult to differentiate in its early course from Parkinson’s disease (PD) or other parkinsonian disorders, like progressive supranuclear palsy (PSP) or multiple system atrophy (MSA). A lack of initial response to l-dopa therapy, although nonspecific, helps differentiate CBD from PD. Despite recommended diagnostic criteria for probable CBD, a definitive diagnosis requires neuropathological confirmation.3
Neuropsychiatric Features
Depression is the most common neuropsychiatric feature in CBD. The prevalence of depression may be as high as 70%, apathy 40%, and irritability 20%.12 Less frequently described are agitation, obsessive-compulsive symptoms, anxiety, disinhibition, delusions or aberrant motor behavior such as pacing.12,13 The cognitive changes in CBD include a unique combination of focal parietal and frontal-subcortical deficits. Most patients with CBD develop ideomotor apraxia (inability to perform previously learned movements in the absence of weakness or sensory deficits). Frontal deficits may include psychomotor slowing, a dysexecutive syndrome and impaired memory retrieval.2–4 Patients with CBD often have constructional and visuospatial difficulties, acalculia, elements of Gerstmann syndrome and nonfluent aphasia. This may indicate a possible overlap with progressive nonfluent aphasia.14,15 The alien limb phenomenon is a dramatic manifestation of CBD (a “feeling that one limb is foreign or ‘has a will of its own,’ together with observable involuntary motor activity”).16 In addition to CBD, several other disorders have been associated with the alien limb phenomenon: vascular insults (especially in the anterior cerebral artery territory), surgical lesions (corpus callosotomy or thalamotomy), tumors of the corpus callosum, Creutzfeldt-Jakob disease, and AD.16
Patients with CBD may have hemispatial neglect or impaired proprioception, astereognosis, and agraphesthesia in the setting of intact primary sensory modalities, indicating cortical sensory loss.8 Balint’s syndrome—a visuospatial disorder with inability to integrate complex visual scenes (simultanagnosia), inability to accurately direct hand or other movements by visual guidance (optic ataxia), and reduced voluntary eye movements to visual stimuli (oculomotor apraxia)—may also be present.17 Alien limb sign and Balint’s syndrome can co-occur.17
Neuropathological Findings
There are no laboratory markers for CBD. A definitive diagnosis requires neuropathology. It is a “tauopathy,” diagnosed by the presence of intraneuronal tau-immunoreactive inclusions (CBD inclusions) in substantia nigra and cortical layer II.2 Astrocytic plaques and coiled bodies in oligodendroglia are characteristic. Ballooned achromatic cells are present in the involved cortex and subcortical regions.2 There are also cortical neuronal loss, gliosis of the frontoparietal and perirolandic cortices and caudate nuclei, as well as degeneration of the substantia nigra.
Structural Imaging Studies
Findings on magnetic resonance imaging (MRI) studies can support the clinical diagnosis of CBD. MRI typically reveals asymmetric atrophy involving the posterior frontal and parietal regions, corresponding to the clinically notable asymmetry in motor deficits.4,5,18–20 However, a recent volumetric study did not find substantial asymmetry.21 A longitudinal case report has demonstrated the progressive nature of the atrophy on imaging.22 One study has reported a hyperintense appearance of the frontoparietal white matter on diffusion weighted MRI.20 Atrophy of the basal ganglia is present less consistently.19,23 Increased T2 signal intensity in the putamen ipsilateral to the cortical atrophy and decreased T2 signal intensity bilaterally in the putamen have both been reported.5,20 Several studies have reported atrophy of the corpus callosum, predominantly in its mid portion.21,24–26 In one study the degree of callosal atrophy was significantly correlated with measures of cognitive impairment.24 The authors noted that the middle predominance is consistent with degeneration in the posterior frontal and parietal cortices and thus may reflect both the severity and location of neuronal loss.
Functional Imaging Studies
In recent years, investigators have proposed functional imaging studies as diagnostic tests to help differentiate CBD from other pathologic entities. In the last 10 years, functional imaging studies in CBD have included positron emission tomography (PET), single-photon emission computed tomography (SPECT), functional magnetic resonance imaging (fMRI), and magnetic resonance spectroscopy (MRS). Although the CBD diagnoses were rarely pathologically proven, these studies used established diagnostic criteria. Some compared CBD patients with healthy control subjects, while others contrasted them with patients with other parkinsonian or dementing disorders.
In studies utilizing 18F-fluorodeoxyglucose (FDG) PET, regional cerebral metabolic rate (rCMR) was decreased most commonly in areas of frontal and parietal cortex as well as thalamus and basal ganglia (Cover and Figure 1).24,27–34 Although individual patients varied considerably in the exact location and extent of deficits, primary sensorimotor cortex may be particularly affected in CBD.30,32,34 Regional asymmetries were consistently found, with more severe hypometabolism contralateral to the more affected side of the body.24,27–34 However, some studies have reported symmetrical hypometabolism in the presence of asymmetrical clinical deficits or a reversed asymmetry in a few patients.27,29,32
Two studies have compared FDG PET metabolic patterns between patients with PSP, CBD and control subjects.27,32 Both used statistical parametric mapping, an image analysis technique that allows voxel by voxel comparison of groups of images after they are normalized to a standardized space. Images from the CBD groups were mirrored when necessary so that the most affected side was always the same in order to adjust for the characteristic asymmetry, thus allowing group-wise comparisons. Both studies found that metabolic asymmetry was present in most patients with CBD but not in patients with PSP. In one study, comparison between the CBD (n=12) and PSP (n=12) groups revealed a lower metabolism in the inferior parietal lobule, precuneus, and lateral occipital cortex of the more affected hemisphere in the CBD group and a lower metabolism in the anterior cingulate and medial frontal gyri of both hemispheres and the midbrain in the PSP group.27 In the other study, comparison between PSP (n=21) and CBD (n=22) groups indicated more metabolic impairment in sensorimotor, supplementary motor, and parietal cortices in the CBD group and lower metabolism in the midbrain, anterior cingulate, and orbitofrontal regions in the PSP group.32 These studies suggest that FDG PET may be useful in the differential diagnosis due to the more posterior cortical findings in CBD as compared to the more anterior cortical and midbrain findings in PSP.
Regional cerebral blood flow (rCBF) has been measured by SPECT in patients with CBD using several tracers including 99mTc-hexamethylpropyleneamine oxime (HMPAO), 99mTc-ethylene cysteinate dimer, and N-isopropyl-p[123I]-iodoamphetamine. The most common findings have been marked perfusion asymmetry in the posterior frontal and parietal regions, with hypoperfusion contralateral to the most affected side.22,23,35–40 Other commonly affected areas are temporal cortex, basal ganglia, thalamus and pontocerebellar regions.22,36–40 One study reported that CBD patients with dementia had significant reductions of relative rCBF in the inferior prefrontal region of the more affected hemisphere, compared to CBD patients without dementia.40 Another found widespread decreases in absolute rCBF in patients with CBD (n=13), compared to control subjects (n=10), indicating that use of relative measures may not be fully informative in this group.36
Three studies have utilized SPECT to compare rCBF between patients with CBD and other parkinsonian or dementing disorders.37–39 All three measured predetermined regions of interest. Although most regions of interest were placed in roughly similar areas (frontal, parietal, temporal, and occipital cortices, basal ganglia, thalamus, and cerebellum) in both studies comparing patients with CBD and with PSP, the results were quite different. In a study measuring absolute rCBF, values did not differ significantly between PSP (n=12) and CBD (n=12).37 In a study measuring relative rCBF, values were significantly lower in inferior frontal, sensorimotor, and posterior parietal cortices in patients with CBD (n=6) compared to patients with PSP (n=5).39 Technique differences as well as differences in patient samples (e.g., illness duration, clinical presentation, inclusion of cognitive deficits) may be reasons why these findings are not consistent. Both studies found significantly higher asymmetry indices in CBD particularly in posterior frontal and parietal regions, similar to the differences in rCMR described previously. The third study found that rCBF was significantly lower in anterior cingulate cortex, sensorimotor cortex, basal ganglia and thalamus in patients with CBD compared to patients with AD. In contrast, rCBF was significantly lower in posterior parietal cortex in patients with AD compared to patients with CBD.38 Patients with CBD also had significantly higher asymmetry indices in inferior prefrontal and sensorimotor cortices, whereas patients with AD had significantly higher asymmetry indices in lateral and medial prefrontal cortex and posterior parietal cortex.
Several methods have been used to image the dopamine system in CBD. Two studies utilized fluorodopa (FDOPA) PET to assess functional integrity of dopaminergic neurons in the striatum.33,34 Normally, uptake is uniform throughout caudate and putamen bilaterally. Both studies found that uptake was decreased in patients with CBD (total n=10) compared to control subjects (total n=18), particularly contralateral to the more affected side of the body (Figure 2). There was considerable variability in the distribution, with some individuals showing symmetrical decreases.33 Compared to patients with PD (n=15), patients with CBD (n=6) had more uptake (less decrease).33 A third study utilized [123I]-2β-carbomethoxy-3-β-(iodophenyl)tropane ([123I]β-CIT) SPECT, a cocaine derivative with high affinity for dopamine transporters and thus another marker of presynaptic dopaminergic neurons.41 Patients with MSA (n=18), PSP (n=8), PD (n=48), and CBD (n=4) were compared to control subjects (n=14) and each other. Overall β-CIT striatal binding was significantly reduced in all patient groups compared to control subjects, with the CBD group least affected. Asymmetry of striatal β-CIT binding was greatest in patients with CBD, but was found only in 2 of the 4. The authors concluded that β-CIT SPECT was a reliable tool for visualizing presynaptic dopaminergic lesions in patients with MSA, PSP and CBD, however it was inferior to other imaging modalities in differentiating these disorders from PD. Another SPECT tracer that binds to the dopamine transporter is [2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-yl] methyl](2-mercaptoethyl)amino]ethyl]amino]ethanethi olato(3-)-N2,N2′,S2,S2′]oxo-[1R-(exo-exo]-[99mTc]technetium ([99mTc]TRODAT-1). It has been used to compare striatal function in patients with CBD (n=5) and idiopathic PD (n=10) to control subjects (n=10) and each other.42 As was found in previously discussed studies, striatal binding was significantly reduced in both patient groups compared to control subjects. Unlike previous studies, there was no significant difference in striatal binding between the CBD and PD groups and both exhibited asymmetry. Regional analysis revealed that binding was reduced similarly in both caudate and putamen in the patients with CBD, whereas binding was relatively preserved in the caudate and decreased in the putamen in the PD patients. Postsynaptic dopaminergic D2 receptors were measured in an individual with CBD utilizing 123mI-iodobenzamide (IBZM) SPECT.35 Tracer uptake was severely reduced in the basal ganglia contralateral to the symptoms. Overall these studies indicate that the striatal dopaminergic system is impaired in CBD, but probably less severely than in other parkinsonian disorders.
One group has used fMRI to probe cortical function in patients with CBD (total n=8).22,23 Two finger opposition tasks of differing difficulty were used. In the simple task, each finger in order (starting with digit 2, the index finger) was touched to the thumb (digit 1). In the complex task a specified sequence was followed (1–2, 1–4, 1–3, 1–5). Activation of the contralateral sensorimotor, supplementary motor, and parietal cortex and of the ipsilateral prefrontal cortices occurred during the execution of the simple motor task with the unaffected hand. In contrast, decreased activation of the contralateral sensorimotor and parietal cortices and supplementary motor area occurred during performance of the same task with the affected hand (Figure 3). During performance of the complex motor task with the unaffected hand, there was bilateral activation of the sensorimotor and parietal cortices and activation of the contralateral frontal cortex. During performance of the same task with the affected hand, there was bilateral activation of the sensorimotor cortex and supplementary motor area, but only modest bilateral activation of the parietal cortex, particularly contralaterally (Figure 3). These results suggest parietal lobe dysfunction contralateral to the affected hand. The authors comment that the parietal lobe participates in the motor control of movements in the intrapersonal space. There are connections between the inferior parietal lobe and inferior premotor area (which may store elementary motor programs). Thus, parietal lobe dysfunction can disconnect the supplementary motor, premotor, and sensorimotor areas. The authors propose that fMRI can provide evidence of asymmetrical disorganization of the hierarchical cortical motor program, before structural and even SPECT changes become evident.
Magnetic resonance spectroscopy provides a relative measure of particular metabolites, most commonly presented as spectra of the amount of signal produced by each from a volume of interest (voxel) rather than as images. Three studies have utilized proton (1H) MRS to examine patients with CBD and related diseases (PSP, PD, MSA, vascular parkinsonism, primary progressive aphasia, frontotemporal dementia).43–45 With 1H MRS the metabolites of interest are N-acetyl aspartate, choline, and creatine. N-Acetyl aspartate is present almost exclusively within neurons and indicates neuronal/axonal density. Choline is mostly present within membrane constituents and can be elevated both as a result of increased synthesis and destruction. Creatine is present alone and as part of phosphocreatinine, both of which are important for energy metabolism. While this peak is normally quite stable and commonly used as a reference standard, it does change in some conditions. The only region included in all 3 studies was the basal ganglia. Although the voxel placement varied, all reported either decreased N-acetyl aspartate/creatine or decreased N-acetyl aspartate/choline.43–45 Two studies found decreased N-acetyl aspartate/creatine in frontal cortex.44,45 Other areas reported to be affected include the centrum semiovale, parietal cortex, and perisylvian cortex. The factors that differentiated CBD from other entities (e.g., PSP, MSA, frontotemporal dementia) were marked asymmetry and perhaps involvement of parietal cortex.
In summary, the most salient findings in these functional imaging studies are asymmetrical hypoperfusion on SPECT and asymmetrical hypometabolism on PET involving the parietofrontal cortex, basal ganglia and thalamus. These findings suggest that multiple components of neural networks related to both movement execution and production of skilled movements are disturbed in CBD.32 The functional imaging results may confirm a clinical diagnosis of probable CBD and support the diagnosis in patients who do not fulfill sufficient clinical criteria.46 The few reports looking at CBD using fMRI and MRS appear to support the presence of hemispheric asymmetry early in the disease.
Conclusions
In conclusion, CBD is a neurodegenerative dementia with abnormal movements and focal behavioral manifestations. The clinical diagnosis is difficult to make in the absence of pathological findings. Functional imaging studies may be very helpful in demonstrating asymmetrical abnormalities in frontoparietal regions, basal ganglia, and thalamus contralateral to clinical symptoms, particularly in the early stages.46 Future studies are needed to replicate present findings. Comparison with other patient groups, such as PSP and PD, will be particularly important. Although an uncommon form of dementia, CBD exemplifies the future possibilities of functional imaging in the clinical evaluation of dementing illnesses.
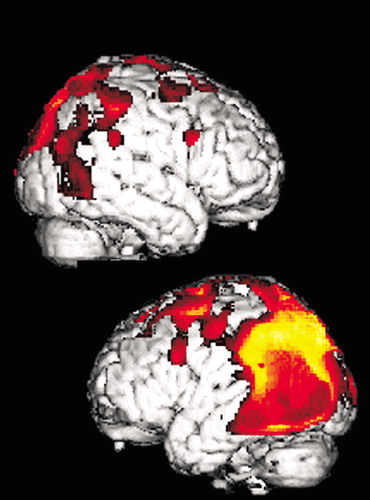
Figure 1. Cover and Statistical parametric map displaying regions of significant hypometabolism superimposed on a three-dimensional reconstruction of magnetic resonance images and selected axial images from the source positron emission tomography 18F-fluorodeoxyglucose data set in a patient with corticobasal degeneration. Note the prominent asymmetrical metabolic decreases in the occipital and parietal lobes (arrows).
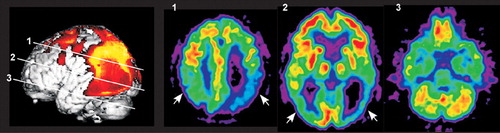
Figure 1. Cover and Statistical parametric map displaying regions of significant hypometabolism superimposed on a three-dimensional reconstruction of magnetic resonance images and selected axial images from the source positron emission tomography 18F-fluorodeoxyglucose data set in a patient with corticobasal degeneration. Note the prominent asymmetrical metabolic decreases in the occipital and parietal lobes (arrows).
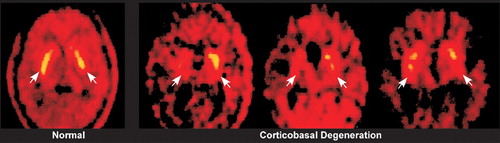
FIGURE 2. [18F]Fluorodopa (FDOPA) positron emission tomography images in a normal subject and three patients with corticobasal degeneration show decreased uptake of FDOPA in the corticobasal degeneration group in the striatum (arrows) as well as the clearly asymmetric pattern (used with permission (34).
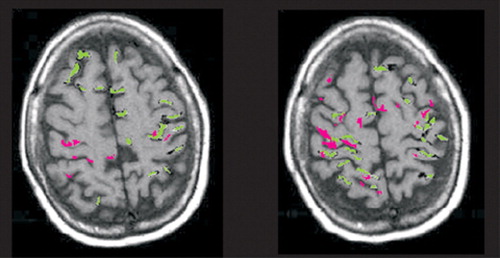
FIGURE 3. Functional magnetic resonance imaging results of finger opposition tasks in a patient with corticobasal degeneration are overlaid onto axial magnetic resonance images. There are clear differences in cortical activation when movement is performed with the impaired (pink) and the unaffected (green) hands. Note the decreased areas and extent of activation with the affected hand with a simple (left) and complex (right) task (adapted with permission (23).
1 Wenning GK, Litvan I, Jankovic J, et al: Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry 1998; 64:184–189Crossref, Medline, Google Scholar
2 Mendez MF, Cummings JL: Dementia. A Clinical Approach, 3rd ed. Philadelphia, Butterworth Heinemann, 2003Google Scholar
3 Cummings JL: The Neuropsychiatry of Alzheimer’s Disease and Related Dementias. London, Martin Dunitz, 2003Google Scholar
4 Soliveri P, Monza D, Paridi D, et al: Cognitive and magnetic resonance imaging aspects of corticobasal degeneration and progressive supranuclear palsy. Neurology 1999; 53:502–507Crossref, Medline, Google Scholar
5 Frasson E, Moretto G, Beltramello A, et al: Neuropsychological and neuroimaging correlates in corticobasal degeneration. Ital J Neurol Sci 1998; 19:321–328Crossref, Medline, Google Scholar
6 Grimes DA, Lang AE, Bergeron CB: Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology 1999; 53:1969–1974Crossref, Medline, Google Scholar
7 Rebeiz JJ, Kolodny EH, Richardson EP Jr: Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol 1968; 18:20–33Crossref, Medline, Google Scholar
8 Boeve BF, Lang A, Litvan I: Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 2003; 54(suppl 5):S15-S19Google Scholar
9 Volicer L, McKee A, Hewitt S: Dementia. Neurol Clin 2001; 19:867–885Crossref, Medline, Google Scholar
10 DePold Hohler A, Ransom BR, Chun MR, et al: The youngest reported case of corticobasal ganglia degeneration. Parkinsonism Relat Disord 2003; 10:47–50Crossref, Medline, Google Scholar
11 Litvan I, Agid Y, Goetz C, et al: Accuracy of the clinical diagnosis of corticobasal degeneration: a clinicopathologic study. Neurology 1997; 48:119–125Crossref, Medline, Google Scholar
12 Cummings JL, Litvan I: Neuropsychiatric aspects of corticobasal degeneration. Adv Neurol 2000; 82:147–152Medline, Google Scholar
13 Litvan I, Cummings JL, Mega M: Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry 1998; 65:717–721Crossref, Medline, Google Scholar
14 Kertesz A, Martinez-Lage P, Davidson W, et al: The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology 2000; 55:1368–1375Crossref, Medline, Google Scholar
15 Graham NL, Bak TH, Hodges JR: Corticobasal degeneration as a cognitive disorder. Mov Disord 2003; 18:1224–1232Crossref, Medline, Google Scholar
16 Hanna P, Doody R: Alien limb sign. Adv Neurol 2000; 82:135–145Medline, Google Scholar
17 Mendez MF: Corticobasal ganglionic degeneration with Balint’s syndrome. J Neuropsychiatry Clin Neurosci 2000; 12:273–275Link, Google Scholar
18 Monza D, Ciano C, Scaioli V, et al: Neurophysiological features in relation to clinical signs in clinically diagnosed corticobasal degeneration. Neurol Sci 2003; 24:16–23Crossref, Medline, Google Scholar
19 Savoiardo M, Grisoli M, Girotti F: Magnetic resonance imaging in CBD, related atypical parkinsonian disorders, and dementias. Adv Neurol 2000; 82:197–208Medline, Google Scholar
20 Ikeda K, Iwasaki Y, Ichikawa Y: Cognitive and MRI aspects of corticobasal degeneration and progressive supranuclear palsy (letter). Neurology 2000; 54:1878Crossref, Medline, Google Scholar
21 Groschel K, Hauser TK, Luft A, et al: Magnetic resonance imaging-based volumetry differentiates progressive supranuclear palsy from corticobasal degeneration. Neuroimage 2004; 21:714–724Crossref, Medline, Google Scholar
22 Moretti R, Ukmar M, Torre P, et al: Cortico-basal ganglionic degeneration: a clinical, functional and cognitive evaluation (1-year follow-up). J Neurol Sci 2000; 182:29–35Crossref, Medline, Google Scholar
23 Ukmar M, Moretti R, Torre P, et al: Corticobasal degeneration: structural and functional MRI and single-photon emission computed tomography. Neuroradiology 2003; 45:708–712Crossref, Medline, Google Scholar
24 Yamauchi H, Fukuyama H, Nagahama Y, et al: Atrophy of the corpus callosum, cortical hypometabolism, and cognitive impairment in corticobasal degeneration. Arch Neurol 1998; 55:609–614Crossref, Medline, Google Scholar
25 Wolters A, Classen J, Kunesch E, et al: Measurements of transcallosally mediated cortical inhibition for differentiating parkinsonian syndromes. Mov Disord 2004; 19:518–528Crossref, Medline, Google Scholar
26 Trompetto C, Buccolieri A, Marchese R, et al: Impairment of transcallosal inhibition in patients with corticobasal degeneration. Clin Neurophysiol 2003; 114:2181–2187Crossref, Medline, Google Scholar
27 Hosaka K, Ishii K, Sakamoto S, et al: Voxel-based comparison of regional cerebral metabolism between PSP and corticobasal degeneration. J Neurol Sci 2002; 199:67–71Crossref, Medline, Google Scholar
28 Ishii K: Clinical application of PET for diagnosis of dementia. Ann Nucl Med 2002; 16:515–525Crossref, Medline, Google Scholar
29 Taniwaki T, Yamada T, Yoshida T, et al: Heterogeneity of glucose metabolism in corticobasal degeneration. J Neurol Sci 1998; 161:70–76Crossref, Medline, Google Scholar
30 Hirono N, Ishii K, Sasaki M, et al: Features of regional cerebral glucose metabolism abnormality in corticobasal degeneration. Dement Geriatr Cogn Disord 2000; 11:139–146Crossref, Medline, Google Scholar
31 Lutte I, Laterre C, Bodart JM, et al: Contribution of PET studies in diagnosis of corticobasal degeneration. Eur Neurol 2000; 44:12–21Crossref, Medline, Google Scholar
32 Garraux G, Salmon E, Peigneux P, et al: Voxel-based distribution of metabolic impairment in corticobasal degeneration. Mov Disord 2000; 15:894–904Crossref, Medline, Google Scholar
33 Laureys S, Salmon E, Garraux G, et al: Fluorodopa uptake and glucose metabolism in early stages of corticobasal degeneration. J Neurol 1999; 246:1151–1158Crossref, Medline, Google Scholar
34 Nagasawa H, Tanji H, Nomura H, et al: PET study of cerebral glucose metabolism and fluorodopa uptake in patients with corticobasal ganglia degeneration. J Neurol Sci 1996; 139:210–217Crossref, Medline, Google Scholar
35 Frisoni GB, Pizzolato G, Zanetti O, et al: Corticobasal degeneration: neuropsychological assessment and dopamine D2 receptor SPECT analysis. Eur Neurol 1995; 35:50–54Crossref, Medline, Google Scholar
36 Hossain AK, Murata Y, Zhang L, et al: Brain perfusion SPECT in patients with corticobasal degeneration: analysis using statistical parametric mapping. Mov Disord 2003; 18:697–703Crossref, Medline, Google Scholar
37 Zhang L, Murata Y, Ishida R, et al: Differentiating between progressive supranuclear palsy and corticobasal degeneration by brain perfusion SPET. Nucl Med Commun 2001; 22:767–772Crossref, Medline, Google Scholar
38 Okuda B, Tachibana H, Kawabata K, et al: Comparison of brain perfusion in corticobasal degeneration and Alzheimer’s disease. Dement Geriatr Cogn Disord 2001; 12:226–231Crossref, Medline, Google Scholar
39 Okuda B, Tachibana H, Kawabata K, et al: Cerebral blood flow in corticobasal degeneration and progressive supranuclear palsy. Alzheimer Dis Assoc Disord 2000; 14:46–52Crossref, Medline, Google Scholar
40 Okuda B, Tachibana H, Kawabata K, et al: Cerebral blood flow correlates of higher brain dysfunctions in corticobasal degeneration. J Geriatr Psychiatry Neurol 1999; 12:189–193Crossref, Medline, Google Scholar
41 Pirker W, Asenbaum S, Bencsits G, et al: [123I]β-CIT SPECT in multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Mov Disord 2000; 15:1158–1167Crossref, Medline, Google Scholar
42 Lai SC, Weng YH, Yen TC, et al: Imaging early-stage corticobasal degeneration with [99mTc]TRODAT-1 SPET. Nucl Med Commun 2004; 25:339–345Crossref, Medline, Google Scholar
43 Tedeschi G, Litvan I, Bonavita S, et al: Proton magnetic resonance spectroscopic imaging in progressive supranuclear palsy, Parkinson’s disease and corticobasal degeneration. Brain 1997; 120:1541–1552Crossref, Medline, Google Scholar
44 Abe K, Terakawa H, Takanashi M, et al: Proton magnetic resonance spectroscopy of patients with parkinsonism. Brain Res Bull 2000; 52:589–595Crossref, Medline, Google Scholar
45 Kizu O, Yamada K, Nishimura T: Proton chemical shift imaging in Pick complex. Am J Neuroradiol 2002; 23:1387–1392Medline, Google Scholar
46 Coulier IM, de Vries JJ, Leenders KL: Is FDG-PET a useful tool in clinical practice for diagnosing corticobasal ganglia degeneration? Mov Disord 2003; 18:1175–1178Crossref, Medline, Google Scholar



