Content-Specific Delusions From Right Caudate Lacunar Stroke: Association with Prefrontal Hypometabolism
Content-specific delusions can also result from disturbed frontal functions. False beliefs may emerge from disruption of inferior prefrontal mechanisms having to do with reality monitoring. 4 Evidence from studies of patients with Alzheimer’s disease suggests the inferior prefrontal cortex is involved in the expression of delusions through alterations in reality testing. 5 Consequently, we hypothesize that lacunar stroke interrupting prefrontal pathways as they transverse the caudate nucleus can result in similar content-specific delusions.
We report on occurrence of content-specific delusions among patients with newly discovered lacunar infarctions in the right caudate nucleus. These patients underwent neuropsychological testing and functional neuroimaging with positron emission tomography (PET). Their results were compared with individually matched controls. This study evaluated the possibility of greater alterations in frontal-executive functions and inferior prefrontal metabolism in the caudate patients with delusions compared to the controls.
METHOD
Subjects
All patients with caudate stroke presented to a Veterans Affairs Medical Center Memory Disorders Clinic (MDC) over a 4-year period with chief complaint of content-specific delusions (persecutory 6/8, theft 5/8, autobiographical 2/8) and had ischemic lesions in the right caudate nucleus detected by MRI including T1, T2, and Fluid-attenuated inversion recovery (FLAIR) sequences. Control patients were selected from all subjects presenting to the Memory Disorders Clinic during the same time period with new onset of memory complaints, but without stroke or other abnormality detected by MRI, and who did not meet either DSM-IV criteria for dementia or proposed criteria for mild cognitive impairment. 6 Control subjects were individually matched to those with caudate lesions within 5 years of age and within 5 years of formal education, as well as by ethnicity and gender. Patients without cortical or subcortical cerebral lesions were chosen for the comparison group in order to highlight the differences in cortical metabolism directly resulting from presence of caudate lesions and to eliminate any potentially confounding influence from the presence of other lesions. For all patients the clinical diagnoses were established prior to obtaining PET imaging. The initial clinical diagnoses, however, did incorporate the findings from MRI scans of the brain. None of the patients or controls had structural lesions on the MRI or cortical strokes.
The subjects were screened for treatable causes of cognitive impairment including vitamin B 12 deficiency, thyroid function abnormalities, neurosyphilis, and normal pressure hydrocephalus. All medical illnesses and medications were reviewed for cognitive effects. Patients with a medical or psychiatric disorder that could affect cognition were excluded from the study. Patients on psychoactive medications (including antidepressant, antipsychotic, or benzodiazepine medications), acetylcholinesterase inhibitors, or other medications that could affect cognition were also excluded from participation.
The patients underwent neuropsychological tests at the time of initial presentation. The measures included the Mini-Mental State Examination (MMSE); digit span forward and backward; serial threes; language assessment including verbal fluency, assessment of comprehension and repetition, the Mini-Boston Naming Test (MBNT), and brief reading comprehension and sentence writing tests; the Neurobehavior (ten-item) Auditory Verbal Learning Test (AVLT), constructions from the Consortium to Establish a Registry for Alzheimer’s disease (CERAD), simple arithmetic calculations including single and two digit addition, multiplication, and an algebra problem; and frontal-executive functions including interpretation of idioms and proverbs, category assignment, the Luria Hand Sequence test, the Go/NoGo Test, alternate tapping, and the Luria alternating programs. 1 , 7 , 8 Neuropsychological tests included in the battery were chosen to evaluate the cognitive abilities believed to be most vulnerable to decline in patients with caudate lesions. This study was conducted in accordance with a protocol approved by the Greater Los Angeles Veterans Affairs Medical Center review board.
Positron Emission Tomography (PET)
Cerebral uptake of intravenously administered [ 18 F]fluorodeoxyglucose (FDG, 10mCi) occurred while patients lay with eyes open in a dimly lit, quiet room. Emission scanning commenced 40 minutes following the injection of FDG, using a Siemens ECAT EXACT HR scanner (CTI PET Systems Inc., Knoxville, TN). Emission images were obtained with the canthomeatal plane of each patient’s head set parallel to the plane of the ring of detectors. Images were reconstructed using a calculated attenuation correction algorithm and displayed in axial and coronal orientations as contiguous planes of brain tissue.
Statistical Parametric Mapping (SPM)
Statistical parametric mapping analysis of PET data employed the software package SPM 2. 9 PET images were coregistered using a nine-parameter affine transform and spatially normalized to the standard coordinates of Talairach and Tournoux. Global normalization by proportional scaling was used. An 8 mm full width at half-maximum, three-dimensional Gaussian smoothing filter was applied to all images.
Statistical Comparison
Categorical demographic variables and cognitive test results were compared between the groups using chi-square tests. Mean values for continuous demographic variables and cognitive test results were compared between groups using two-tailed t tests. Analyses were performed using SPSS, Version 12.0 for Windows.
Illustrative Case Report
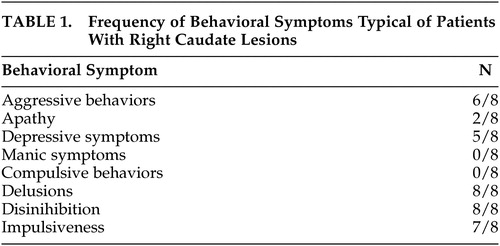
Patient 1 was typical for those with right-sided caudate lesions ( Table 1 ). He was a 76-year-old man with past medical history significant for hypertension and type 2 diabetes, brought by his wife to the emergency room for changes in personality and behavior. On interview, the patient’s wife described his acute development of paranoid delusions of family members, including his wife, wanting to harm or steal from him. The patient also developed aggressive behavior including engaging in physical altercations with strangers, leaving the house without clothing, gambling to the point of losing their entire life savings, forgetting items said to him during conversation, and misplacing items such as his wallet and car keys. There was no report of motor or sensory change. Neurological examination revealed only bilateral absent ankle jerk reflexes, diminished patellar reflexes, decreased vibration sensation in the lower extremities at the toes, ankles and knees, and mildly wide-based gait, all consistent with a peripheral neuropathy. Computed tomography (CT) of the head performed in the emergency room showed only mild generalized brain atrophy. MRI performed during the course of the patient’s hospital stay showed lacunar infarction of the right caudate, measuring less than 9 mm in diameter. PET imaging showed inferior prefrontal lobe hypometabolism. On follow-up examination approximately 1 year later, the patient’s symptoms were improved by a scheduled evening dose of quetiapine 100 mg.
 |
RESULTS
All subjects in the study were male, likely a result of the Veterans Affairs population in which the study was conducted. No significant difference between groups was detected for mean age (caudate stroke = 74.3 ± 8.0; controls = 70.9 ± 11.9) and years of formal education (caudate stroke = 14.8 ± 2.8; controls = 11.3 ± 1.5), or handedness (caudate stroke = 8 right and 0 left; controls = 7 right and 1 left) and ethnicity (caudate stroke and control groups were both comprised of 6 Caucasian and 2 African-American patients) distributions, confirming the validity of the matching process.
Two of the patients with caudate stroke refused to participate in any cognitive testing, and an additional patient refused portions of the examination. Compared to controls, subjects with caudate stroke performed less well on MMSE and tests of abstract reasoning, memory, and frontal-executive functions ( Table 2 ). Worse performance on delayed recognition of word list items in the patients with caudate stroke compared to the controls resulted from increased frequency of false positive responses, possibly related to decreased self-monitoring and resulting confabulation. Patients with caudate stroke and controls did not differ significantly on measures of attention, mental control, language, visuospatial constructions, and calculations. Patients with caudate stroke also demonstrated a trend to performing less well on more complex calculations of multiplication and algebra, but not for one and two-digit addition.
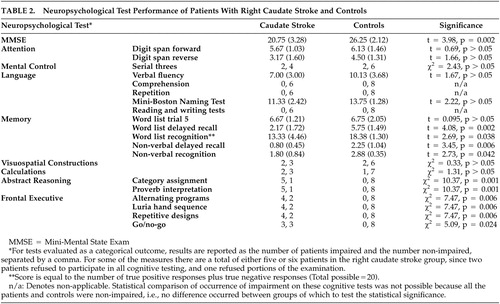 |
Figure 1 illustrates specific pixels with significantly less metabolic activity among patients with caudate stroke compared to controls (p<0.05). These pixels correspond to a region in the inferior frontal gyrus, including the pars opercularis and pars triangularis.
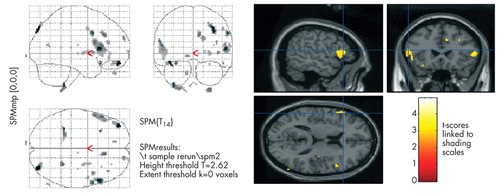
The maps reflect regions with significantly lower cerebral metabolism in patients with caudate stroke compared to controls.
DISCUSSION
The patients with lacunar infarction in the caudate nucleus and delusions had greater frontal-executive impairments on neuropsychological measures and decreased metabolism of the inferior prefrontal cortex on PET imaging. These findings support the hypothesis that development of delusions was consequent to disruption of inferior prefrontal lobe functions, which may include alterations in reality monitoring and testing.
The results of this study corroborate previous associations between alterations in behavior and cognition and basal ganglia ischemic lesions. An early case series described 12 patients with psychiatric symptoms and frontal-executive deficits due to acute caudate strokes. 10 Subsequently, other investigators have corroborated these findings. 11 , 12 Behavior changes resulting from vascular lesions have resulted from subcortical small lesions involving the caudate nucleus, globus pallidus, or thalamus, perhaps via disruption of frontal-subcortical circuits. 13 Additionally, PET studies have shown frontal hypometabolism with basal ganglia lesions, 14 and bilateral striatal infarctions have been associated with frontal hypometabolism and clinical presentations mimicking frontotemporal dementia. 15
The clinical syndrome described in caudate stroke patients includes dramatic changes in personality, as well as development of psychiatric symptoms, and cognitive deficits particularly in areas of attention, memory, and executive function. Sudden development of disinhibition and impulsiveness are perhaps the most striking component of this syndrome. 1 , 10 One report found that 46% of all patients with basal ganglia lesions presented with behavioral disorders, especially abulia, disinhibition, or depression. 16 Furthermore, among these basal ganglia lesions, those involving the caudate nuclei are most likely to cause behavioral alterations such as apathy, disinhibition, and major affective disturbances. 1 , 10 – 12 , 17 – 20
Distinct patterns of personality change may depend on the location of the caudate lesion. 1 Right caudate lesions are particularly associated with behavioral and psychiatric symptoms. Manic-like behavior with grandiose delusions may occur after lesions involving the right caudate nuclei, 21 and abulia or loss of psychic activation and episodic dyscontrol occur after right caudate or bilateral lesions, 17 , 18 , 22 especially in the elderly. 23 Localization within the caudate nuclei also influences the occurrence of specific behaviors. Dorsolateral caudate lesions, regardless of laterality, tend to produce apathy and reduced spontaneity and initiative. 10 In contrast, lesions in the ventromedial caudate are associated with the development of disinhibition, disorganization, and impulsiveness. 10 In sum, the behavioral disorders of caudate strokes are similar to those of Huntington’s disease, another disorder affecting the caudate nuclei. 10
Frontal-executive deficits constitute another important aspect of this syndrome. 11 Impairments in sustained attention span, sequencing and planning, abstract reasoning, and problem solving are common with caudate strokes. 1 Many patients also have memory dysfunction, characterized by a retrieval deficit and possibly explained by frontal difficulty in organization for retrieval. 1 These cognitive deficits can be long lasting. Bokura and Robinson reported that caudate lesions result in long-term cognitive impairment in areas of executive functions and consolidation of memories and that a significant number of patients deteriorate in their intellectual function between one and 2 years after their caudate stroke. 24
The behavioral and cognitive symptoms from caudate strokes correspond to disturbances in the five parallel frontal-subcortical circuits or loops that traverse the caudate nuclei. 3 These loops are named for the cortical locations in which they originate and include: 1) the anterior cingulate circuit, 2) inferior temporal circuit, 3) medial orbitofrontal circuit, 4) dorsolateral prefrontal circuit, and 5) the lateral frontal circuit. All of the circuits pass through the basal ganglia and thalamus before returning to the cortex. The caudate nuclei, through which fibers comprising the frontal lobe circuits pass, mediate prefrontal behaviors through these spatially and functionally well-defined sets of reciprocal connections. 3 The topographical organization of these loops may account for distinct behavioral disorders related to distinct lesion locations. 3 , 25 Among these circuits, a disturbance in the ventromedial one is most likely responsible for causing disinhibition, personality, and mood changes. 2 , 3 , 10 , 26 , 27
Results from this study support a relationship among right caudate lesions, frontal-executive cognitive deficits, and delusional thoughts that is mediated via dysfunction of the inferior prefrontal cortex. When compared to controls, patients with caudate lacune and delusions had reduced metabolism in the inferior frontal gyrus on voxel-based quantitative group analysis, and no patient had structural lesion in the frontal cortex. Changes in cortical metabolism in specific regions of the inferior prefrontal cortex have been suggested to involve the expression of delusions in patients with Alzheimer’s disease. 5 The patients reported in this study support the proposed role of altered inferior prefrontal lobe functioning in the development of delusions potentially due to disruption of self-corrective functions and dysregulation of the experience of familiarity. 4 Disruption of the inhibitory control of other brain regions by the prefrontal cortex may also be critical to the development of psychosis and delusions. 28 Other cognitive functions mediated by the prefrontal and temporal cortices include attentional bias, attributional bias, and theory of mind deficits that may contribute to development of persecutory delusions. 29
Left caudate lesions, by comparison, are less likely than right-sided ones to induce personality and behavioral changes and more likely to produce impairments in drive, executive control, attention, and memory. 27 The behavioral exceptions to this laterality appear to be depression and compulsions. Left caudate lesions, but not right sided ones, predispose to poststroke depression. 30 Thobois et al. describe severe compulsive behavior (e.g., using words with exactly 10 letters) after a left caudate hemorrhage and discuss a frontal cortex deafferentation mechanism. 31 This compulsive behavior may be worse if the caudate lesions are bilateral. 32
In conclusion, this study extends knowledge of reciprocal metabolic changes in patients with ischemic lesions in the basal ganglia by demonstrating inferior prefrontal lobe hypometabolism associated with anatomically distant right caudate nucleus ischemic lesions. The clinical features manifested by these patients suggest an etiological relationship between alterations in inferior prefrontal lobe functions of reality monitoring and testing, and the development of content-specific delusions. Future efforts will focus on determining the relationship between lesion location within the caudate nucleus, clinical symptoms, and changes in cortical metabolism. This information can be beneficial for clinicians and others caring for patients affected by basal ganglia ischemic lesions.
1 . Mendez MF, Cummings JL: Dementia: A Clinical Approach, 3rd ed. Philadelphia, Elsevier, 2003Google Scholar
2 . Alexander GE, DeLong MR, Strick PL: Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 1986; 9:357–381Google Scholar
3 . Mega MS, Cummings JL: Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 1994; 6:358–370Google Scholar
4 . Richardson ED, Malloy PF: The frontal lobes and content-specific delusions, in The Frontal Lobes and Neuropsychiatric Illness, Edited by Salloway SP, Malloy PF, Duffy JD. Washington, D.C., American Psychiatric Publishing, 2001, pp 215–232Google Scholar
5 . Sultzer DL, Brown CV, Mandelkern MA, et al: Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer’s disease. Am J Psychiatry 2003; 160:341–349Google Scholar
6 . Winblad B, Palmer K, Kivipelto M, et al: Mild cognitive impairment—beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med 2004; 256:240–246Google Scholar
7 . Folstein MF, Folstein SE, McHugh PR: Mini-mental state: a practical method for grading cognitive states of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
8 . Morris JC, Heyman A, Mohs RC, et al: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part 1: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989; 9:1159–1165Google Scholar
9 . Wellcome Department of Imaging Neuroscience: Statistical Parametric Mapping Software. Available at www.fil.ion.ucl.ac.uk/spm/doc/Google Scholar
10 . Mendez MF, Adams NL, Lewandowski KS: Neurobehavioral changes associated with caudate lesions. Neurology 1989; 39:349–354Google Scholar
11 . Petty RG, Bonner D, Mouratoglou V, et al: Acute frontal lobe syndrome and dyscontrol associated with bilateral caudate nucleus infarctions. Br J Psychiatry 1996; 168:237–240Google Scholar
12 . Wang PY: Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei) 1991; 47:199–203Google Scholar
13 . Mori E: Impact of subcortical ischemic lesions on behavior and cognition. Ann NY Acad Sci 2002; 977:141–148Google Scholar
14 . Sultzer DL, Mahler ME, Cummings JL, et al: Cortical abnormalities associated with subcortical lesions in vascular dementia: clinical and PET findings. Arch of Neurol 1995; 52:773–780Google Scholar
15 . Nishio Y, Nakano Y, Matsumoto K, et al: Striatal infarcts mimicking frontotemporal dementia: a case report. Eur J Neurol 2003; 10:457–460Google Scholar
16 . Bhatia KP, Marsden CD: The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain 1994; 117:859–876Google Scholar
17 . Degos JD, da Fonseca N, Gray F, et al: Severe frontal syndrome associated with infarcts of the left anterior cingulate gyrus and the head of the right caudate nucleus: a clinico-pathological case. Brain 1993; 116:1541–1548Google Scholar
18 . Rodier G, Tranchant C, Mohr M, et al: Neurobehavioral changes following bilateral infarct in the caudate nuclei: a case report with pathological analysis. J Neurol Sci 1994; 126:213–218Google Scholar
19 . Croisile B, Tourniaire D, Confavreux C, et al: Bilateral damage to the head of the caudate nuclei. Ann Neurol 1989; 25:313–314Google Scholar
20 . Krishnan KR, Figiel GS: Neurobehavioral changes with caudate lesions. Neurology 1989; 39:1410–1411Google Scholar
21 . Starkstein SE, Mayberg HS, Berthier ML, et al: Mania after brain injury: neuroradiological and metabolic findings. Ann Neurol 1990; 27:652–659Google Scholar
22 . Perry RG, Bonner D, Mouratoglou V, et al: Acute frontal lobe syndrome and dyscontrol associated with bilateral caudate nucleus infarctions. Br J Psychiatry 1996; 168:237–240Google Scholar
23 . Nagaratnam N, Bou-Haidar P, Leung H: Confused and disturbed behavior in the elderly following silent frontal lobe infarction. Am J Alzheimer Dis Other Dem 2003; 18:333–339Google Scholar
24 . Bokura H, Robinson RG: Long-term cognitive impairment associated with caudate stroke. Stroke 1997; 28:970–975Google Scholar
25 . Middleton FA, Strick PL: Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn 2000; 42:183–200Google Scholar
26 . Caplan LR, Schmahmann JD, Kase CS, et al: Caudate infarcts. Arch Neurol 1990; 47:133–143Google Scholar
27 . Benke T, Delazer M, Bartha L, et al: Basal ganglia lesions and the theory of fronto-subcortical loops: neuropsychological findings in two patients with left caudate lesions. Neurocase 2003; 9:75–80Google Scholar
28 . Suzuki M, Zhou SY, Takahashi T, et al: Differential contributions of prefrontal and temporolimbic pathology to mechanisms of psychosis. Brain 2005; 128:2109–2122Google Scholar
29 . Blackwood NJ, Howard RJ, Bentall RP, et al: Cognitive neuropsychiatric models of persecutory delusions. Am J Psychiatry 2001; 158:527–539Google Scholar
30 . Vataja R, Leppävuori A, Pohjasvaara T, et al: Poststroke depression and lesion location revisited. J Neuropsychiatry Clin Neurosci 2004; 16:156–162Google Scholar
31 . Thobois S, Jouanneau E, Bouvard M, et al: Obsessive-compulsive disorder after unilateral caudate nucleus bleeding. Acta Neurochir (Wien) 2004; 146:1027–1031Google Scholar
32 . Croisile B, Tourniaire D, Confavreux C, et al: Bilateral damage to head of the caudate nuclei. Ann Neurol 1989; 25:313–314Google Scholar



