Relationship Between Corpus Callosum Abnormalities and Schneiderian First-Rank Symptoms in Antipsychotic-Naïve Schizophrenia Patients
Abstract
The corpus callosum (CC), has been hypothesized to be implicated in the pathogenesis of schizophrenia; however, the findings from magnetic resonance imaging studies are conflicting. Moreover, the relationship between first-rank-symptoms (FRS) and CC abnormalities in schizophrenia is yet to be examined. The authors examined CC morphometry, based on Witelson's method, in antipsychotic-naïve-schizophrenia patients in comparison with matched healthy-control subjects. Patients had significantly smaller CC, splenium, and isthmus areas than control subjects. A novel finding of the study is that only those without FRS differed from control subjects, but not those with FRS. Study findings support a neuro-developmental hypothesis and possible connectivity abnormalities in symptom-genesis.
Schizophrenia has been proposed as a neurodevelopmental disorder involving dysfunctional interhemispheric transfer.1 The corpus callosum (CC), the anatomical mediator of interhemispheric transfer,2 closely linked to brain neurodevelopment,3 has been a region of interest in schizophrenia. Early postmortem studies have indicated an increased thickness of CC in affected patients.4,5 However, later studies did not support the above findings.6,7 Magnetic resonance imaging (MRI) has the advantage of studying CC in vivo and avoids potential confounders associated with postmortem studies.8 Nonetheless, MRI studies have yielded conflicting results regarding structural differences in CC. Although most of the studies have reported a decrease in the midsagittal area of CC, a substantial number of studies found no difference in size.9 Some studies examined the shape of CC and reported increased curvature, with midbody, genu, and splenium being the most affected regions.10,11 An earlier meta-analysis showed a decrease in the size of CC in patients with schizophrenia, as compared with control subjects.8 A recent meta-analysis, in addition to confirming earlier finding of decrease in area, reported that the difference is more pronounced at first presentation and becomes less prominent as the illness becomes chronic.12 Some studies have also reported a difference based on patients' gender; the first study to examine the gender difference, by Nasrallah and colleagues,13 reported that only female patients with schizophrenia, but not male patients, had increased middle and anterior callosal thickness, as compared with controls. However, results of later studies were inconclusive.14–16
These inconsistencies in findings might have been secondary to differences in subject characteristics (for example, sex, handedness, age at onset (AAO) of psychosis, as well as CC measurement methods).17 Another important confounding factor is the effect of antipsychotic medications, because psychotropic medications can have a neuroprotective role in schizophrenia by either preventing neuronal loss or by protecting against the pathophysiologic effect of the disease process.18 Also, antipsychotic medications produce variations in regional white-matter volume.19 Both typical and atypical antipsychotics cause hyperprolactinemia, which, in turn, increase the oligodendrocyte number in CC and could increase the CC volume.20 Examining drug-naïve patients is important to control for these confounding factors, but only a few studies have investigated drug naïve patients.12 Use of low-resolution MRI machines to acquire images can also confound the results because of poor image quality. Studies examining CC have used MRI of strength 0.5 to 1.5 Tesla, and it is preferable to use an MRI of higher strength for better resolution and image quality.
Few studies have examined subregions of CC in schizophrenia, but they differ in their results. Some of them report involvement of anterior regions of CC involving genu and body.8,12 However, other evidence points toward involvement of posterior regions, also; in one study, a decrease in density of the inferior part of CC was reported and was found to correlate with the reality-distortion score.21 Also, a case report of a schizophrenia patient with a lipoma of the splenium of CC supported the involvement of posterior regions.22 In a recent study using MRI tractography, aberrant myelination was seen in both the genu and splenium.23
Two pathogenetic mechanisms are proposed to account for these callosal abnormalities. One view is that failure of elimination of synapses leads to abnormality in CC; this is known as the “hyperconnectivity hypothesis.”24 An alternate view is that too many synapses are eliminated during adolescence; this is known as the “hypoconnectivity hypothesis.”25,26 As noted above, reduction in CC volume supports the hypoconnectivity hypothesis, as a smaller CC might be due to either a loss or decrease in thickness of interhemispheric axons or a reduction in the diameter of their myelin sheaths. On the other hand, electrophysiological and neuropsychological studies have shown an increased EEG interhemispheric coherence, enhanced Stroop effect, and a decreased interhemispheric transfer time to ipsilateral and contralateral stimulus. These findings are incompatible with the hypoconnectivity hypothesis and support the hyperconnectivity hypothesis.27 Preliminary data suggest that first-rank symptoms (FRS) could be related to hyperconnectivity and hyperactivation of the right parietal lobe.28 However, no study has examined the relationship of FRS with CC.
In this study, we examined CC in antipsychotic-naïve schizophrenia patients (N=30) as a factor of age, sex, education, socioeconomic status, and handedness, matched with healthy-control subjects (N=30). Also, we examined the relationship between FRS and CC abnormalities—whether patients with FRS differ from those without FRS.
Our primary aim was to assess the CC area in antipsychotic-naïve schizophrenia patients as compared with healthy controls, and our secondary aim was to assess the relationship between FRS and CC sub-areas. On the basis of earlier reports, our pre-hoc primary hypothesis was that schizophrenia patients have smaller CC than controls. Our secondary hypothesis was that there will be a positive correlation between FRS score and posterior CC areas (i.e., isthmus and/or splenium—the CC areas that transmit interparietal fibers).
MATERIALS AND METHODS
Subjects
The study sample consisted of 30 drug-naïve schizophrenia patients and 30 healthy controls. Patients meeting the DSM-IV diagnostic criteria for schizophrenia were recruited from the outpatient department of the National Institute of Mental Health And Neurosciences (NIMHANS), Bangalore, India. The diagnosis was established by applying the Mini-International Neuropsychiatric Inventory (MINI).29 An experienced psychiatrist confirmed the diagnosis through independent clinical interview. Patients had neither history of medical illness nor comorbid psychiatric illness, including substance dependence. None of them were exposed to any kind of psychotropics or electroconvulsive therapy before or at the time of assessments. We carefully ascertained relevant clinical information pertinent to illness onset and antipsychotic-naïve status by reliable information obtained from the patient and family members. We assessed psychopathology by the Scale for the Assessment of Positive Symptoms (SAPS)30 and Scale for the Assessment of Negative Symptoms (SANS).31 In a seminal study, Mellor32 gave an exhaustive list of 11 FRSs and detected the prevalence of 72%.32 In this study, a qualified psychiatrist assessed Schneiderian FRS by comprehensive clinical interview as per Mellor's definition of first-rank symptoms.
Age-, sex-, and education-matched healthy controls who volunteered for the participation in the study were recruited by word-of-mouth. None of them had psychiatric disorder as confirmed by MINI.29 They had neither history of medical illness nor substance dependence. There was no family history of psychiatric illness, including alcohol dependence syndrome in their first-degree relatives. We assessed handedness using Annett's questionnaire;33 all 60 subjects were right-handed. We excluded those who were left-handed or ambidextrous. Written informed consent was taken from all subjects before assessment. The NIMHANS Ethics Committee approved the study.
Scanning Protocol
MRI was done with a 3.0-Tesla scanner; a T1-weighted three-dimensional magnetization prepared rapid-acquisition gradient echo sequence was performed (TR=8.1 msec.; TE=3.7 msec.; rotation angle: 8°; FOV=256 mm, slice-thickness: 1 mm without interslice gap; NEX=1, matrix = 256 × 256), yielding 165 sagittal slices.
Morphometric Analysis
The CC measurements were done in the midsagittal section, using the principles of Witelson's method.34 The midsagittal section was chosen from the set of T1-weighted three-dimensional MP-RAGE sagittal images, using the following criteria:35 1) a distinct outline of the CC; 2) an easily-identified cerebral aqueduct; 3) clear visibility of cortical gyral crests, both anterior and posterior to CC; 4) absence of visible intrusion into gray and white matter. These midsagittal images were numerically coded to facilitate blinded analysis of CC morphometry.
The midsagittal section of the CC was measured by use of the Scion Image–Windows© version of NIH Image software, which has been used to measure CC areas in children, adolescents, and adults.36 The semi-automated segmentation method used in this software has optimal reliability and validity, and this method correlated highly with the point-counting stereological approach.37 CC was divided into rostrum, genu, body, isthmus, and splenium on a valid neuroanatomical basis, as per Witelson's method.34
The steps for measuring the CC using the Scion Image Software were as follows:38 The CC in the midsagittal section was segmented automatically, using the Scion Image Software, with the CC highlighted in red.
A straight line was drawn antero-posteriorly using the Scion Image Software (from left to right) over the CC, passing through the point of maximum curvature of the inner border of the genu (Figure 1). Using the Scion Image Software, four perpendicular lines were automatically drawn on the CC. Finally, the fifth perpendicular line was drawn by the software at the point where the straight line cuts the inner body of the genu of the CC. These five perpendicular lines divided the CC into rostrum, genu, rostral body, anterior mid-body, posterior mid-body, isthmus, and splenium, as per Witelson's validated neuroanatomical basis.34 The exact positions of these line placements with respect to the proportions of the CC area that they divide are shown in Figure 1. In this study, as per Keshavan et al.,36 the rostral body, the anterior mid-body, and the posterior mid-body (i.e., subdivisions III, IV, and V in Figure 1) were added together to form the body of the CC.
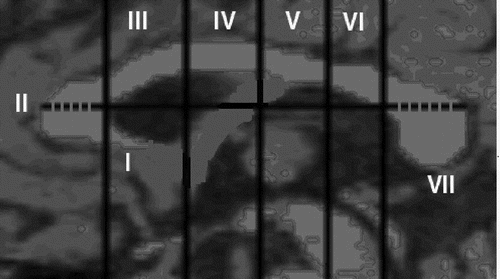
Interrater Reliability and Blinded Ratings
Two authors (NPR, GVS) performed an interrater reliability exercise by analyzing 10 coded images. The interrater reliability analysis revealed excellent concurrence (i.e., intraclass correlation coefficient of >0.8) for all measurements, namely, intracranial area, rostrum, body, genu, isthmus, and splenium. All images were coded to blind the rater, and the single rater (NPR) was blind to subject status as well as clinical data measuring CC areas.
Data Analysis
The sociodemographic and clinical data were compared by independent-samples t-test. We compared morphometric data using analysis of covariance (ANCOVA), with intracranial area and age as covariate and sex as fixed factor. We used Spearman's correlation analysis to examine the relationship between the morphometric measurements and psychopathology related variables, namely, SANS and SAPS.
RESULTS
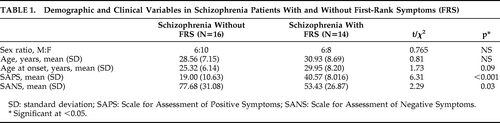
Age (mean [standard deviation {SD}]) of schizophrenia patients (29.6 [7.86] years) did not differ significantly from that of healthy controls (29.4 [8.02] years; p=0.8). There was no significant difference in sex ratio (M:F) of Patients (12:18) versus Controls (14:16; χ2=0.27; p=0.4). Monthly family income of Patients ($2,496.55 [4,731.08]) and Controls ($2,758.62 [2,181.99]) did not differ (p=0.7). Also, there was no difference in educational status; Patients and Controls were matched on years of education (Patients: 7.13 [4.94]; Controls: 7.06 [5.10]; p>0.95] and also the educational level (Patients: illiterate, 6; grade-school, 21; college, 3; Controls: illiterate, 6; grade-school, 22; college, 2; χ2 p>0.85). The age at onset of psychosis was 27.45 (7.43) years, and the duration of untreated psychosis was 41.06 (36.52) months. Psychopathology scores were available in all 30 patients; total SAPS score was 29.06 (14.39), and SANS score was 66.36 (31.22).
As a group, 13 of the 14 patients had auditory hallucinations, and 4 patients had somatic passivity. The frequency of FRS were as follows: thought broadcast: 2; third-person auditory hallucinations: 13; running-commentary auditory hallucinations: 3; made to act: 1; somatic passivity: 4. The demographic and clinical variables between those with FRS and without FRS are shown in Table 1.
 |
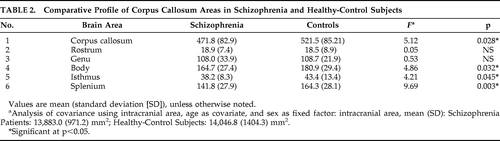
There was no significant difference in intracranial area (mean [SD]; mm2) between the Patients (13,883 [971.24]) and that of the Controls (14,046.0 [1,404.32]; p>0.6). The mean [SD] of respective areas (mm2) of total CC, rostrum, genu, body, isthmus, and splenium in Patients and Controls are shown in Table 1. On ANCOVA, total CC area, body, isthmus, and splenium areas were significantly smaller in schizophrenia patients than in healthy subjects, but the areas of rostrum and genu did not differ significantly between two groups (Table 2).
 |
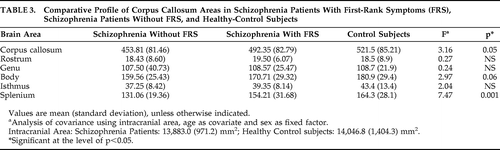
We examined the difference in CC areas between three groups, namely, schizophrenia Patients with first-rank symptoms (FRS), without FRS, and Controls, using ANCOVA with age and intracranial area as covariates and sex as a fixed factor. There was no significant difference in intracranial area (mean [SD]; mm2) of the Patients with FRS (13,781.28 [979.77]) and those without FRS (13,972.06 [986.75]) and that of the Controls (14,046.0±1,404.32; p>0.79). There was a significant effect of status of FRS in the splenium area and a trend toward significance in body and total CC area (Table 3). Post-hoc analysis revealed those without FRS to have significantly smaller splenium than Controls (p=0.001). Schizophrenia Patients with FRS, although intermediate, did not differ significantly from the Controls (p=0.75). There was a trend-level significance between those without FRS and with FRS (p=0.07; Figure 1). There was no significant correlation between SANS and SAPS scores and callosal areas.
 |
DISCUSSION
To the best of our knowledge, this is the first study to use 3-Tesla MRI to examine corpus callosum in antipsychotic-naïve schizophrenia patients. In this study, patients had significantly smaller corpus callosum area, splenium, isthmus, and body. A novel finding of the study is that only those patients without FRS differed significantly from control subjects; FRS-positive patients did not.
Our findings are in accord with earlier studies. Earlier studies including a recent meta-analysis reporting significant decrease in CC area12 and smaller splenium.10,11,39–41 Also, lower fractional anisotrophy in the splenium was seen on DTI.23,42,43 Involvement of the posterior region of CC is also supported by a study examining tachistoscopic functions, which need the integrity of the posterior callosum.44 In an earlier study, men had smaller splenium than women, similar to our finding, and authors attributed these changes to differences in hormonal influences and differences in cerebral asymmetry.39
In our study, there was no difference in the genu and anterior portion of CC. This is in agreement with some earlier studies.9,45,46 However other studies have reported contrasting findings.8,12 Because schizophrenia is a heterogeneous disorder, differences in AAO of psychosis could be the reason, as those with earlier AAO had smaller anterior CC in an earlier study.47 As mean AAO in the study population was 27.4 years, our population represents late-onset schizophrenia, which possibly explains the absence of anterior CC abnormality.
The CC develops through a combination of target-aimed growth, exuberance, and pruning at different stages. These processes are partially under control of many epigenetic factors, and some of the epigenetic factors, like perinatal ischemia, maternal alcohol use, epilepsy, and sensory deprivation alter development of CC. Interestingly these factors are also implicated in the development of schizophrenia.
Neurodevelopmental aberrations can lead to dysregulated connectivity between the hemispheres,3 and, indeed, deficient interhemispheric transfer has been documented in schizophrenia.48 Hence, the presence of smaller CC regions in schizophrenia, as observed in this study, suggests interhemispheric hypoconnectivity and is in tune with the “hypoconnectivity” hypothesis of schizophrenia, which suggests that a reduced number of interhemispheric axons result in smaller CC.27
Interestingly, hyperconnectivity might be postulated to underlie the genesis of Schneider's first-rank symptoms (FRS), as supported indirectly by the functional-imaging studies. Positron emission tomography (PET) and functional MRI studies have implicated hyperactivation of right-parietal area as neuroanatomical substrates of FRS.28,49–51 Also, when normal controls were led to believe that another person was controlling their actions, there was activation of the inferior parietal lobule.52 Thus, FRSs in schizophrenia are associated with hyperactivity in the parietal cortex.52–54 Fibers from parietal cortex implicated in FRS will traverse in the splenium.3 Our results suggest that those with FRS had larger splenium than those without FRS and were closer to controls and probably have adequate connectivity through splenium regions; this would support the hyperconnectivity hypothesis.
As discussed earlier, both hypoconnectivity and hyperconnectivity hypotheses are proposed in pathogenesis of schizophrenia. These connectivity abnormalities in schizophrenia might be more complex and might have differential relationships with the symptom clusters. Also, connectivity abnormalities might be reflected by the age at onset, as observed in the current study, in which there was a significant positive correlation between age at onset and FRS score. That is, those with FRS had later age at onset and bigger splenium, representing a hyperconnectivity model, and those without FRS had smaller splenium, representing a hypoconnectivity model. These findings have potential clinical implications. The presence of neuroanatomical correlates for first-rank symptoms would help in easier identification of valid subtypes, and further studies are needed to look for differences in outcome, response to treatment, and side effects.
In addition to hypo- and hyperconnectivity abnormalities, callosal abnormalities can result from faulty connections between different hemispheres: “dysconnectivity.” This idea is supported by enhanced redundancy-gain in schizophrenia, which could correlate with callosal dysfunction functionally.55 Also, Nasrallah earlier proposed that FRS could be due to a state of impaired interhemispheric awareness, in which each hemisphere regards the input from the other as non-self, or alien information, from an external source.56 The right hemisphere becomes an “alien intruder,” and the patient with schizophrenia harbor both self- (contents of left-hemisphere consciousness) and “non-self” (contents of right-hemisphere consciousness). Interhemispheric integration defect as evidenced by corpus callosum abnormality in our study supports the above hypothesis. In this context, it is important to note that the other interhemispheric commissures (anterior as well as posterior commissures) may also play a role in interhemispheric hyper- or hypoconnectivity; this needs to be evaluated in future studies.
Our study has several methodological strengths, importantly, examining antipsychotic-naïve patients avoided potential confounding effects of neuroleptic treatment. Also, use of high-resolution 3-Tesla MRI scan, assessment by single rater blind to subject status as well as clinical data, and use of objective criteria to define midsagittal section,35 are other rigorous methodological factors.
However, the following are the potential limitations of our study. Recruitment of control subjects was by word-of-mouth, which may have skewed the sample and affected generalizability from this sample. However, we matched Controls and Patients in terms of age, sex, education, and handedness to avoid potential confounding factors. The sample was moderately ill, with predominantly negative symptoms, and had a longer duration of untreated psychosis, and, thus, the results need to be replicated to be applicable for better generalizability.
In summary, our study demonstrated antipsychotic-naïve schizophrenia patients to have a significantly smaller area of CC, splenium, isthmus, and body, as compared with matched healthy-control subjects. Also, there were significant positive correlations between splenium of the CC and FRS, as well as between FRS and age at onset. Together, these findings suggest neurodevelopmentally-mediated hypo- and hyperconnectivity in schizophrenia.
1. : Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology 1996; 14:1–17Crossref, Medline, Google Scholar
2. : Schizophrenia and the corpus callosum: developmental, structural, and functional relationships. Behav Brain Res 1994; 64:203–211Crossref, Medline, Google Scholar
3. : Agenesis of the corpus callosum: genetic, developmental, and functional aspects of connectivity. Nat Rev Neurosci 2007; 8:287–299Crossref, Medline, Google Scholar
4. : Quantitative brain measurements in chronic schizophrenia. Br J Psychiatry 1972; 121:259–264Crossref, Medline, Google Scholar
5. : Corpus callosum thickness in chronic schizophrenia. Br J Psychiatry 1983; 142:284–287Crossref, Medline, Google Scholar
6. : A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res 1983; 8:251–260Crossref, Medline, Google Scholar
7. : Axonal counts of the corpus callosum of schizophrenic patients. J Neuropsychiatry Clin Neurosci 1989; 1:391–393Link, Google Scholar
8. : Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry 1995; 58:457–461Crossref, Medline, Google Scholar
9. : A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1–52Crossref, Medline, Google Scholar
10. : Shape and size of the corpus callosum in schizophrenia and schizotypal personality disorder. Schizophr Res 2000; 42:193–208Crossref, Medline, Google Scholar
11. : Mapping morphology of the corpus callosum in schizophrenia. Cereb Cortex 2000; 10:40–49Crossref, Medline, Google Scholar
12. : Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophr Res 2008; 101:124–132Crossref, Medline, Google Scholar
13. : A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry 1986; 21:274–282Crossref, Medline, Google Scholar
14. : Corpus callosum dimensions measured by magnetic resonance imaging in bipolar affective disorder and schizophrenia. Biol Psychiatry 1989; 26:659–668Crossref, Medline, Google Scholar
15. : Structural and functional characteristics of the corpus callosum in schizophrenics, psychiatric controls, and normal controls: a magnetic resonance imaging and neuropsychological evaluation. Arch Gen Psychiatry 1990; 47:1060–1064Crossref, Medline, Google Scholar
16. : The size and fibre composition of the corpus callosum with respect to gender and schizophrenia: a post-mortem study. Brain 1999; 122(Pt 1):99–110Crossref, Medline, Google Scholar
17. : Abnormalities of the corpus callosum in first-episode, treatment-naïve schizophrenia. J Neurol Neurosurg Psychiatry 2002; 72:757–760Crossref, Medline, Google Scholar
18. : Effects of antipsychotics on brain structure. Curr Opin Psychiatry 2006; 19:145–150Crossref, Medline, Google Scholar
19. : State-related changes in cerebral white matter may underlie psychosis exacerbation. Psychiatry Res 2004; 130:71–78Crossref, Medline, Google Scholar
20. : Callosal oligodendrocyte number in postpartum Sprague-Dawley rats. Brain Res 2009; 1267:18–24Crossref, Medline, Google Scholar
21. : A voxel-based method for the statistical analysis of gray- and white-matter density applied to schizophrenia. Neuroimage 1995; 2:244–252Crossref, Medline, Google Scholar
22. : Decreased multi-band posterior interhemispheric coherence with a lipoma on the corpus callosum: a case report of a possible association. Clin Electroencephalogr 1997; 28:155–159Crossref, Medline, Google Scholar
23. : Abnormal brain connectivity in first-episode psychosis: a diffusion MRI tractography study of the corpus callosum. Neuroimage 2007; 35:458–466Crossref, Medline, Google Scholar
24. : Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res 1982; 17:319–334Crossref, Medline, Google Scholar
25. : Schizophrenia: a disconnection syndrome? Clin Neurosci 1995; 3:89–97Medline, Google Scholar
26. : Brain disconnection and schizophrenia. Br J Psychiatry 1973; 123:661–662Crossref, Medline, Google Scholar
27. : Schizophrenia, neurodevelopment, and corpus callosum. Mol Psychiatry 2003; 8:261–274Crossref, Medline, Google Scholar
28. : A PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusions of alien control). Brain 1997; 120(Pt 11):1997–2011Crossref, Medline, Google Scholar
29. : The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(Suppl 20):22–33; quiz: 34–57Medline, Google Scholar
30. : The Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, Iowa, University of Iowa, 1984Google Scholar
31. : The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, Iowa, University of Iowa, 1983Google Scholar
32. : First-rank symptoms of schizophrenia, I: the frequency in schizophrenics on admission to hospital; II: differences between individual first-rank symptoms. Br J Psychiatry 1970; 117:15–23Crossref, Medline, Google Scholar
33. : The binomial distribution of right-, mixed-, and left-handedness. Q J Exp Psychol 1967; 19:327–333Crossref, Medline, Google Scholar
34. : Hand and sex differences in the isthmus and genu of the human corpus callosum: a postmortem morphological study. Brain 1989; 112(Pt 3):799–835Crossref, Medline, Google Scholar
35. : A computerized magnetic resonance imaging study of corpus callosum morphology in schizophrenia. Psychol Med 1993; 23:45–56Crossref, Medline, Google Scholar
36. : Development of the corpus callosum in childhood, adolescence, and early adulthood. Life Sci 2002; 70:1909–1922Crossref, Medline, Google Scholar
37. : A comparison of stereology and segmentation techniques for volumetric measurements of lateral ventricles in magnetic resonance imaging. Psychiatry Res 1995; 61:53–60Crossref, Medline, Google Scholar
38. : Corpus callosum abnormalities associated with greater externalizing behaviors in subjects at high risk for alcohol dependence. Psychiatry Res 2007; 156:209–215Crossref, Medline, Google Scholar
39. : Characterization of sexual dimorphism in the human corpus callosum. Neuroimage 2003; 20:512–519Crossref, Medline, Google Scholar
40. : Corpus callosum development in childhood-onset schizophrenia. Schizophr Res 2003; 62:105–114Crossref, Medline, Google Scholar
41. : Spatial relationships of neuroanatomic landmarks in schizophrenia. Psychiatry Res 1996; 67:81–95Crossref, Medline, Google Scholar
42. : Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 2000; 68:242–244Crossref, Medline, Google Scholar
43. : A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med 2008; 38:877–885Crossref, Medline, Google Scholar
44. : Tachistoscopic tests of colour-naming and matching in schizophrenia: evidence for posterior callosum dysfunction? Psychol Med 1987; 17:621–630Crossref, Medline, Google Scholar
45. : Magnetic resonance imaging in schizophrenia: altered brain morphology associated with P300 abnormalities and eye-tracking dysfunction. Biol Psychiatry 1991; 30:753–769Crossref, Medline, Google Scholar
46. : A magnetic resonance imaging study of corpus callosum size in familial schizophrenic subjects, their relatives, and normal controls. Schizophr Res 2000; 41:397–403Crossref, Medline, Google Scholar
47. : [An MRI morphometric study of corpus callosum and cerebellar vermis in schizophrenia] A study on the effects of age at onset and sex on phenomenology, neurological soft signs, minor physical anomalies, and structural brain abnormalities in patients with first-episode schizophrenia, in Psychiatry. Bangalore, India, National Institute of Mental Health and Neurosciences (NIMHANS), 2001Google Scholar
48. : Reduced interhemispheric transmission in schizophrenia patients: evidence from event-related potentials. Neurosci Lett 2002; 320:57–60Crossref, Medline, Google Scholar
49. : Cerebral blood flow changes associated with Schneiderian first-rank symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci 2002; 14:277–282Link, Google Scholar
50. : Schneiderian first-rank symptoms and right-parietal hyperactivation: a replication using fMRI. Am J Psychiatry 2005; 162:1545Crossref, Medline, Google Scholar
51. : Reduced volume of parietal and frontal association areas in patients with schizophrenia characterized by passivity delusions. Psychol Med 2005; 35:783–789Crossref, Medline, Google Scholar
52. : Experiencing oneself vs. another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage 2002; 15:596–603Crossref, Medline, Google Scholar
53. : Attention, motor control, and motor imagery in schizophrenia: implications for the role of the parietal cortex. Schizophr Res 2004; 70:241–261Crossref, Medline, Google Scholar
54. : Schizophrenia and the inferior parietal lobule. Schizophr Res 2007; 97:215–225Crossref, Medline, Google Scholar
55. : Enhanced redundancy gain in schizophrenics: a correlate of callosal dysfunction? Neuropsychologia 2008; 46:2808–2815Crossref, Medline, Google Scholar
56. : The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry 1985; 26:273–282Crossref, Medline, Google Scholar



