Brains of Optimistic Older Adults Respond Less to Fearful Faces
Abstract
The authors examined the neural correlates of emotion processing and how they relate to individual differences in optimism among older adults. Brain response during processing of fearful faces was measured by functional magnetic resonance imaging in 16 older adults and was correlated with level of optimism. Greater optimism was associated with reduced activation in the fusiform gyrus and frontal regions, which may reflect decreased salience of negative emotional information or better emotion regulation among optimistic individuals. Relationships persisted after taking into account cortical thickness, amygdala volume, and resting perfusion. Findings have potential implications for the promotion of successful aging.
Numerous empirical studies have demonstrated that optimism has benefits for physical health, emotional well-being, and longevity.1 Empirical evidence from cross-sectional studies suggests greater optimism in older age.2 Although the mechanism linking optimism to its many benefits is unknown, explanations include better emotional regulation among older adults with higher levels of optimism;3 optimists’ tendency to use more effective, active coping strategies in stressful situations, such as problem-solving;4 associations between optimism and internal locus of control;5 and optimists’ tendency to engage in more positive health practices.1 Regardless of the precise mechanism, optimists may live longer and healthier lives.
Despite the clear benefits of optimism on physical and emotional well-being, little is known about the neural correlates of optimism, and we know of no published reports using functional magnetic resonance imaging (fMRI) to examine individual differences in optimism among older adults and how such differences relate to brain structure and function. Previously published reports assessing aspects of well-being other than optimism have demonstrated that, among older adults, increased thickness of prefrontal regions was associated with increased extraversion and less neuroticism.6 Also, a recent fMRI study reported that higher self-reported life satisfaction (as measured by the Satisfaction With Life Scale [SWLS7]) was associated with lower activation in prefrontal regions during the encoding of positive images.8 Given that activation was assessed only for those items that were successfully encoded, the authors argued that lower activation might represent enhanced neural efficiency among older adults reporting higher satisfaction.
Processing of facial expressions is an important aspect of emotional behavior, and the abilities to identify and to discriminate among facial displays of various emotions are important components of emotion-processing and social communication.9 Evidence from neuropsychology and neuroimaging studies suggests that emotional face perception involves a distributed network involving various regions, including the amydgala, fusiform gyrus, insula, orbitofrontal cortex, medial prefrontal cortex, thalamus, and occipitotemporal regions.9 Studies examining age-related differences in the neural correlates of emotion-processing have found that, when compared with young individuals, older adults tend to activate frontal regions to a greater degree and the amygdala and fusiform gyrus to a lesser extent.10–12 Despite growing research on both successful aging13 and the neuroscience of social cognition,14 there have been few studies integrating these fields to examine effects of aging on neural correlates of emotion-processing.
We chose to focus on processing of fearful faces and how that relates to individual differences in level of optimism. There are benefits to examining specific emotions, rather than global negative or positive affect, given that specific emotional dispositions differ in their influence on perception of risk and decision-making.15 Dispositional optimism has been conceptualized as a personality characteristic involving generalized positive expectancies for the future.4 Within this framework, a major component of optimism is expecting the best to happen in the future, and, conversely, a key part of pessimism involves expecting that the worst will happen. As such, optimists may be relatively less fearful, particularly about the likelihood of negative events (although evidence suggests that optimism is not identical to lack of worry in terms of predicting outcomes).4
The present study aimed at elucidating neural correlates of emotion-processing for fearful faces and how it relates to individual differences in optimism among older adults. We hypothesized that 1) older adults would activate a widespread network, including fusiform, amygdala, and prefrontal regions during the processing of fearful faces; and 2) greater optimism would be linked to decreased task-related activation in these regions during fearful-face processing, even after accounting for potentially confounding variables, including cortical thickness, amygdala volume, and resting cerebral blood flow.16
Methods
Participants
Participants included 17 healthy older adults (age range: 64 to 86 years) enrolled in ongoing studies, who reported average or higher levels of optimism on modified versions of the Life Orientation Test-Revised (LOT-R)4 administered as part of a “Successful Aging Questionnaire” developed by the Stein Institute for Research on Aging at the University of California, San Diego, or part of questionnaires administered to participants of the San Diego, CA, site of the National Institutes of Health-funded Women’s Health Initiative.17 Potential participants were excluded for history of neurological disorders, psychiatric disorders, substance use disorders, head injury, or untreated hypertension; left-handedness; weight over 250 pounds; and MRI contraindications. Study participation involved a clinical interview, neuropsychological evaluation, administration of measures of emotional well-being, and a neuroimaging exam. One participant was excluded from all analyses for excessive motion, resulting in 16 participants in the final sample. This study was conducted in accordance with UCSD Institutional Review Board-approved procedures and with the guidelines of the Helsinki Declaration. All the participants provided written informed consent.
Assessment of Emotional and Cognitive Functioning
Participants completed multiple standardized self-report measures of emotional functioning. Measures of positive affect included the LOT-R,4 designed to assess expectancies for positive versus negative outcomes; the SWLS,7 a measure of global life satisfaction; and the Positive and Negative Affect Schedule (PANAS18), a measure of emotional well-being that assesses positive and negative affect. Although individuals with a history of psychiatric disorders, including mood and anxiety disorders, were excluded form the present study, the Geriatric Depression Scale (GDS19) and the Beck Anxiety Inventory (BAI20) were administered to assess subthreshold symptoms of depression and anxiety, respectively. Also, participants completed a neuropsychological battery, including standardized measures of global cognition, memory, executive functions, working memory, and language, in order to ensure that they were cognitively normal. All mean demographically-corrected neuropsychological scores were within the average range or higher (standard scores >100; T-scores >50; z-scores >0), indicating that participants were not cognitively impaired.
Facial Affect-Matching Scanner Task
The facial affect-matching task used during scanning was adapted from the work of Hariri and colleagues.21 Participants were instructed to match the affective facial expression of a target face (which appeared at the top of the screen) to one of two faces at the bottom of the screen and respond via a hand-held button box. Each block consisted of six consecutive trials of one of three affect conditions: happy, angry, and fearful. During control trials, participants matched a target geometric form (i.e., an oval or circle), which appeared on the top of the screen, to one of two geometric forms presented at the bottom of the screen. Each trial lasted 5 seconds. Each run lasted 8 minutes and 32 seconds, and consisted of three blocks each of happy, angry, and fearful faces, and three blocks of sensorimotor control trials. A fixation cross appeared at the beginning and end of the task and between blocks. Participants completed two runs of the task. Accuracy and response time were calculated.
Scanning Procedure
Scans were performed on a General Electric Signa Infinity EXCITE 3.0-Tesla, whole-body imager. Functional images were collected with a gradient-recalled echoplanar imaging sequence with the following parameters: 23-cm FOV; 64 × 64 matrix; 3.594 mm × 3.594 mm × 4.0 mm resolution; TR=2,000 msec; TE=32 msec; flip angle=90°; Bandwidth=250 (ramped), with 256 brain images collected. Thirty 2.6-mm thick oblique slices with a 1.4-mm gap were acquired. Field maps were obtained to correct for distortions in EPI images due to inhomogeneities in the static magnetic field. CBF data were not collected for four participants. Linear interpolation was used to estimate CBF values for those participants. (See Bangen et al.22 for parameters of the high-resolution anatomical image acquired using a spoiled gradient-echo [SPGR] pulse sequence and the resting state pulsed arterial spin labeling scan obtained using a modified flow-attenuated inversion-recovery pulse sequence23 to measure cerebral blood flow [CBF]).
Data Analysis
Functional Neuroimaging Data
The Analysis of Functional Neuroimaging (AFNI) software package24 was used to analyze the functional imaging data. Detailed information about the functional data processing has been previously published.22 The association between measured BOLD signal and the emotion-processing task was calculated with multiple regression analysis using the AFNI 3dDeconvolve program. Linear contrasts between fearful faces versus shape were calculated from these models and were designed to selectively identify regions of the brain related to fear and face-processing while controlling for more general cognitive processes, including visual attention and motor response. Results from the two runs were averaged. Voxel-wise, task-related, whole-brain response was examined, using within-group t-tests, with the fit coefficient from the two averaged runs serving as the dependent variable. Regions were considered activated if each voxel was significant at p <0.01 and the cluster contained at least 15 contiguous voxels. This threshold/voxel combination protected a whole-brain probability of false positives of p <0.01. Additional analyses were conducted for a priori regions of interest (ROIs), based on previous literature implicating them in fear-processing.25 These included amygdala, dorsolateral prefrontal cortex, and ventromedial prefrontal cortex. The ROIs were defined in AFNI by use of the Talairach Atlas and were then edited and resampled to the resolution of the functional data.
Structural Neuroimaging Data
Volumetric segmentation and cortical surface reconstruction were performed with FreeSurfer26 in order to account for cortical thickness and amygdala volume in the analyses correlating task-related activation and optimism. Mean cortical thickness was calculated by averaging the thickness of each hemisphere, with the respective contribution of each weighted by the surface area of the hemisphere. Amygdala volume was normalized by dividing each value by estimated total intracranial vault volume.
Cerebral Blood Flow Data
Perfusion data were acquired in order to account for CBF in the analyses correlating task-related activation and optimism. See Bangen et al.24 for detailed information on CBF data-processing.
Results
Participant Characteristics
Participants reported a relatively high degree of optimism, life satisfaction, and positive affect (as evidenced by mean scores in the upper half of the scale range on these measures, on which higher scores reflect more of the assessed construct); a lower degree of negative affect (as evidenced by a mean score on the PANAS Negative scale in the lower-fourth of the scale range on this measure, on which higher scores reflect more negative affect); and a minimal level of depression and anxiety (Table 1).
| Variable | Mean (SD) or N (%) |
|---|---|
| Demographics | |
| Age, years | 72.3 (6.3) |
| Education, years | 16.9 (2.4) |
| Women/men | 7/9 |
| Emotional functioning | |
| Life Orientation Test–Revised | 25.1 (3.0) |
| Satisfaction With Life Scale | 28.3 (3.8) |
| Positive and Negative Affect Scale–Positive | 37.1 (5.8) |
| Positive and Negative Affect Scale–Negative | 13.6 (5.7) |
| Geriatric Depression Scale | 2.1 (2.2) |
| Beck Anxiety Inventory | 3.3 (3.9) |
| Bilateral cortical thickness, mm | 2.4 (0.1) |
| Left hemisphere cortical thickness, mm | 2.4 (0.1) |
| Right hemisphere cortical thickness, mm | 2.4 (0.1) |
| Normalized amygdala volumea | 1.7 (0.3) |
| Left amygdala volumea | 0.8 (0.1) |
| Right amygdala volumea | 0.9 (0.2) |
| Whole-brain CBF (ml blood/100 g tissue/min) | 42.7 (8.2) |
| Left hemisphere CBF | 43.4 (8.6) |
| Right hemisphere CBF | 42.0 (7.8) |
| Scanner task performance | |
| Fearful-face matching accuracy, % correct | 63.1 (11.9) |
| Shape-matching accuracy, % correct | 62.0 (16.9) |
| Fearful-face matching response time, msec | 1,424.0 (312.0) |
| Shape-matching response time, msec | 1,491.6 (321.7) |
fMRI Data
Whole-Brain Analysis
When a whole-brain, voxel-wise, within-group t-test analysis was performed for fearful-face processing versus the shape-matching control condition, there were several regions of significant, task-related brain response (Figure 1). Specifically, participants showed significant response in regions including bilateral superior, middle, and inferior frontal gyrus; medial frontal gyrus; fusiform gyrus; insula; claustrum; thalamus; lentiform nucleus; middle temporal gyrus; parietal cortex; occipital cortex; and cerebellum. There were no areas for which they showed less response (deactivation) during the processing of fearful faces compared with the control condition. Of note, Wilcoxon signed-rank tests revealed no differences in accuracy or response time between fearful face and shape conditions (accuracy: Z = −0.53, NS; response time: Z = −0.34; NS; Table 1).
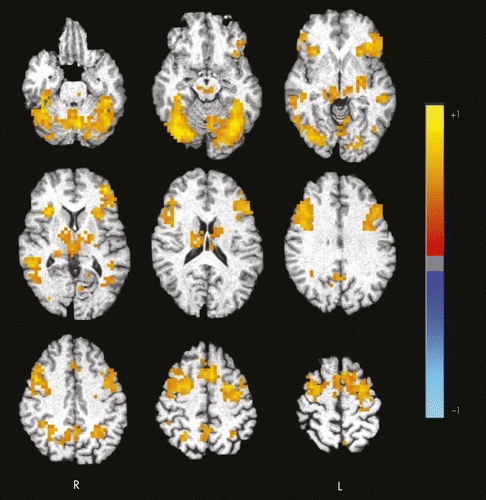
Color intensity represents the effect size (η2) for the fear versus shape contrast. Axial slices span from 21 inferior (top left) to 59 superior (bottom right) in 10-mm increments. Results have been clustered and thresholded so as to protect a whole-brain probability of false positives ≤0.01. Images are presented in radiological view. R: right; L: left.
Region of Interest (ROI) Analysis
For each of the three a priori ROIs, left and right hemispheres were examined separately so that there were a total of six ROIs, including left and right amygdala, dorsal medial prefrontal cortex, and ventral medial prefrontal cortex. Both bilateral amygdala and dorsal medial prefrontal cortex demonstrated positive task-related activation for the contrast of fearful face versus shape processing (mean effect size [eta2]=0.14 for amygdala, and 0.17 for dorsal medial prefrontal cortex). In contrast, ventral medial prefrontal cortex showed essentially no task-related response (mean eta2 = −0.01).
Correlation of Brain Response With Optimism and Other Measures of Well-Being
When task-related brain response for regions identified through the within-group, whole-brain analyses was correlated with emotional well-being measures administered outside of the scanner, there was a negative association between optimism and activation for clusters with peak intensity in the right fusiform gyrus (r = −0.61; p=0.01) and right inferior frontal gyrus (r = −0.54; p=0.03). The cluster with peak activation in the right fusiform gyrus also included voxels located in right hemisphere superior and middle temporal gyri, occipital lobe, and cerebellum. The cluster with peak activation in the right inferior frontal gyrus also included voxels in the right insula and claustrum. Activation between additional clusters identified through the whole-brain analyses did not correlate with optimism (all other p values NS).
Optimism was negatively associated with task-related activation in a priori ROIs, including bilateral ventral medial prefrontal cortex (VMPFC; left: r = −0.54; p=0.03; right: r = −0.59; p=0.02) and dorsal medial prefrontal cortex (DMPFC; left: r = −0.56; p=0.03; right: r = −0.55; p=0.03). The significant correlations between task-related response identified though the within-group, whole-brain analysis (right fusiform and right inferior frontal gyrus clusters) and a priori ROIs (VMPFC and DMPFC) are shown in Figure 2. In contrast, task-related activation was not significantly associated with any other measures of well-being (SWLS, PANAS, GDS, BAI).
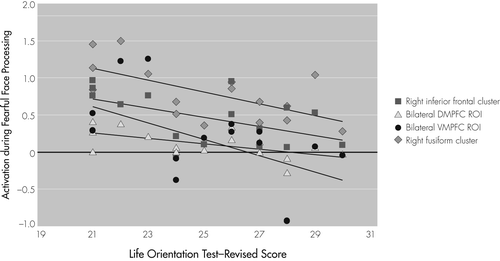
Clusters with peak activation in right fusiform gyrus and right inferior frontal gyrus were identified through whole-brain analyses. Dorsal medial prefrontal cortex (DMPFC) and ventral medial prefrontal cortex (VMPFC) a priori regions of interest (ROI) were selected on the basis of the existing literature.
Correlations of Brain Response With Structural Neuroimaging, Resting CBF, Task Performance, and Demographic Variables
Task-related brain response was not significantly related to the potentially confounding variables of cortical thickness, amygdala volume, or resting CBF (all p values NS). To more fully interpret our findings, we examined the relationships of fMRI task performance and brain response. Task-related brain response determined from both the whole-brain and ROI analyses was not related to accuracy or reaction time for fearful-face processing on the scanner task (all p values NS). Furthermore, optimism was not associated with any demographic, structural, or perfusion measures (all p values NS).
Discussion
In summary, results support our two predictions related to the neural correlates of fearful-face processing and the association between task-related activation and optimism in older adults. First, during the processing of fearful faces, healthy older adults activated a widespread network, including frontal regions and fusiform gyrus. Additional analyses conducted using a priori ROIs based on previous literature implicating them in emotion-processing revealed positive task-related activation in the amygdala and dorsal medial prefrontal cortex. Second, greater optimism was significantly associated with decreased task-related activation in fusiform gyrus, inferior frontal gyrus, ventral medial prefrontal cortex, and dorsal medial prefrontal cortex. Optimism was not related to structural neuroimaging, resting CBF, fearful-face matching performance, demographic, or cognitive variables, suggesting that these factors may not be mediating the relationship between increased optimism and decreased task-related brain response.
The present findings are consistent with those of Waldinger and colleagues,8 who reported that higher life satisfaction was correlated with lower activation in prefrontal regions during encoding of positive images. The authors interpreted these findings as reflecting greater neural efficiency in older adults with higher satisfaction. Reductions in neural activity are often observed on tasks measuring repetition priming.27 Taken together, the observed pattern may represent optimistic individuals’ ability to process emotional items in a more efficient manner than their less-optimistic peers and without needing to engage prefrontal and fusiform regions to the same degree.
Other possible explanations for the results include the idea that optimistic individuals find negative information less salient; are better able to regulate their emotions; use more effective, active coping strategies in stressful situations; and tend to have an internal locus of control. As Waldinger and colleagues note, a discrepancy in salience of positive versus negative information in a subgroup of older adults would corroborate findings from behavioral studies, which have reported that older adults tend to remember positive rather than negative images28 and to gaze toward happy faces and away from angry faces.29 Furthermore, individuals with anxiety disorders have consistently been reported to demonstrate greater response during the processing of emotional stimuli within regions including the medial prefrontal cortex and amygdala.30,31 It is possible that negative stimuli may be less salient; responses to them are better regulated; or more effective coping strategies are used, resulting in reduced task-related activation during the processing of fearful faces for optimistic older adults, a subgroup who have more positive expectancies for the future and who may be relatively less fearful themselves.
In the present study, amygdala activation was not detected during the whole-brain analysis and did not correlate with optimism level. The latter finding supports previous research reporting no link between task-related activation in the amygdala and well-being.8 Although it is generally thought that the amygdala plays an important role in the processing of fear-related information,32 it is also involved in vigilance33 and relevance detection.34 Fear-related stimuli may be less salient for optimistic older adults, resulting in either no or relatively little task-related activation during the processing of fearful faces for this subgroup of older adults. Of note, ROI analyses did reveal positive task-related activation in the amygdala, suggesting a small degree of activation that was not large enough to be detected during whole-brain analyses.
The present findings highlight the relationship between emotion-processing and optimism among older adults. In addition to its links with physical health, optimism has been linked to lower incidence of depression35 and enhanced response to depression treatment among older adults.36 Although the mechanism through which optimism influences mood remains unclear, there are several hypotheses, including optimistic older adults’ tendency to focus on positive expectations and future goals and thereby avoiding negative cognitive patterns; optimism is associated with improved coping, resulting in less depression, or a common biological cause involving neurotransmitters or genetic processes that may predispose individuals with low levels of optimism to depression.36 Regardless of the precise mechanism or mechanisms, evidence suggests that as explanatory style becomes more optimistic, depression decreases.37 Cognitive-behavioral therapy and other interventions aimed at enhancing optimism by focusing on developing more positive explanatory style and improving coping mechanisms as well as those targeted directly to enhance emotion-processing might have a potential to reduce mood symptoms. Furthermore, given the benefits of optimism not only on mood but also on other aspects of emotional well-being and physical health, such interventions may promote successful aging among older adults in general.
The present study has limitations. Our sample size was relatively small. However, our ability to demonstrate significant results despite a relatively small sample size suggests that these findings are likely robust. Second, our sample was somewhat homogeneous, which may limit generalizability of the findings to more diverse groups. Given that we intentionally recruited older adults who reported average-or-higher levels of optimism, there was little variability in optimism scores (i.e., optimism scores only ranged from 21 to 30 on a scale ranging from 0 to 32), resulting in a truncated range for statistical analyses. Nonetheless, we observed some important relationships despite this truncated range. Findings suggest that even within healthy individuals who range in optimism from average to above-average levels, variations in the degree of positive outlook are significantly related to the brain’s emotional processing response. Because of the strong association between pessimism and depressed mood, it is possible that the relationship between optimism and brain response to emotional processing might be obscured or might differ in individuals reporting higher levels of pessimism. A larger sample size and increased variability in optimism level may reveal additional relationships between optimism and brain response to emotional stimuli. Such findings may have important clinical implications. Finally, our use of multiple statistical tests might increase our rate of Type I errors.
In sum, the present study demonstrated that level of optimism was related to neural processing of emotional information among healthy older adults. Greater levels of optimism were associated with reduced task-related activation during emotional face processing in fusiform gyrus and frontal regions. This pattern of findings may reflect greater efficiency in neural processing, decreased salience of negative emotional information, better emotional regulation, more effective coping strategies, and/or an internal locus of control among optimistic individuals. These explanations are not necessarily mutually exclusive. It is possible that older adults with higher levels of optimism have less response to fearful stimuli, resulting in less perceived stress and a tendency not to get overwhelmed by stress or negative emotional information. This in turn may lead to better emotional and physical health. The present findings extend previous research in this area by administering standardized measures of emotional and cognitive functioning to more fully characterize participants, and examining the potential influence of age-related structural brain changes and resting hypoperfusion on results. Taken together, interventions aimed at enhancing optimism may have positive effects on mood, physical well-being, and emotion-processing among older adults. The present findings have potential implications for the elucidation, treatment, and prevention of conditions including late-life mood disorders and for the promotion of successful aging.
1 : Effect of optimism on psychological and physical well-being: theoretical overview and empirical update. Cogn Ther Res 1992; 16:201–228Crossref, Google Scholar
2 : Optimism, satisfaction and time perspective in the elderly. Int J Aging Hum Dev 2000; 51:167–181Crossref, Medline, Google Scholar
3 : Rapid emotion regulation after mood induction: age and individual differences. J Gerontol B Psychol Sci Soc Sci 2009; 64:733–741Crossref, Medline, Google Scholar
4 : Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol 1994; 67:1063–1078Crossref, Medline, Google Scholar
5 : Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health Psychol 1985; 4:219–247Crossref, Medline, Google Scholar
6 : Neuroanatomical correlates of personality in the elderly. Neuroimage 2007; 35:263–272Crossref, Medline, Google Scholar
7 : The Satisfaction With Life Scale. J Pers Assess 1985; 49:71–75Crossref, Medline, Google Scholar
8 : Neural activity, neural connectivity, and the processing of emotionally valenced information in older adults: links with life satisfaction. Cogn Affect Behav Neurosci 2011; 11:426–436Crossref, Medline, Google Scholar
9 : Human neural systems for face recognition and social communication. Biol Psychiatry 2002; 51:59–67Crossref, Medline, Google Scholar
10 : Age-related differences in brain activation during emotional face processing. Neurobiol Aging 2003; 24:285–295Crossref, Medline, Google Scholar
11 Age-related differences in brain regions supporting successful encoding of emotional faces. Cortex 2010; 46:490–497Google Scholar
12 : Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res 2005; 139:9–18Crossref, Medline, Google Scholar
13 : Recent advances in research on successful or healthy aging. Curr Psychiatry Rep 2007; 9:7–13Crossref, Medline, Google Scholar
14 : Principles, processes, and puzzles of social cognition: an introduction for the special issue on social cognitive neuroscience. Neuroimage 2005; 28:745–756Crossref, Medline, Google Scholar
15 : Fear, anger, and risk. J Pers Soc Psychol 2001; 81:146–159Crossref, Medline, Google Scholar
16 : Functional magnetic resonance imaging of compensatory neural recruitment in aging and risk for Alzheimer’s disease: review and recommendations. Dement Geriatr Cogn Disord 2009; 27:1–10Crossref, Medline, Google Scholar
17 : The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003; 13(Suppl):S107–S121Crossref, Medline, Google Scholar
18 : Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988; 54:1063–1070Crossref, Medline, Google Scholar
19 : Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982-1983; 17:37–49Crossref, Medline, Google Scholar
20 : Beck Anxiety Inventory Manual. San Antonio, TX, The Psychological Corporation, Harcourt Brace& Company, 1993Google Scholar
21 : Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport 2000; 11:43–48Crossref, Medline, Google Scholar
22 : Compensatory brain activity during encoding among older adults with better recognition memory for face-name pairs: an integrative functional, structural, and perfusion imaging study. J Int Neuropsychol Soc 2012; 18:402–413Crossref, Medline, Google Scholar
23 : Quantifying CBF with pulsed ASL: technical and pulse sequence factors. J Magn Reson Imaging 2005; 22:727–731Crossref, Medline, Google Scholar
24 : AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
25 : Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 2009; 34:418–432Medline, Google Scholar
26 : FreeSurfer. Neuroimage 2012; 62:774–781Crossref, Medline, Google Scholar
27 : Specificity of priming: a cognitive neuroscience perspective. Nat Rev Neurosci 2004; 5:853–862Crossref, Medline, Google Scholar
28 : Goal-directed memory: the role of cognitive control in older adults’ emotional memory. Psychol Aging 2005; 20:554–570Crossref, Medline, Google Scholar
29 : Use of gaze for real-time mood regulation: effects of age and attentional functioning. Psychol Aging 2009; 24:989–994Crossref, Medline, Google Scholar
30 : Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488Crossref, Medline, Google Scholar
31 The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35:169–191Crossref, Medline, Google Scholar
32 Hariri AR, Whalen PJ: The amygdala: inside and out. F1000 Biology Reports 2011; 3:2Google Scholar
33 : The amygdala: vigilance and emotion. Mol Psychiatry 2001; 6:13–34Crossref, Medline, Google Scholar
34 : The human amygdala: an evolved system for relevance detection. Rev Neurosci 2003; 14:303–316Crossref, Medline, Google Scholar
35 : Dispositional optimism and the risk of depressive symptoms during 15 years of follow-up: the Zutphen Elderly Study. J Affect Disord 2006; 91:45–52Crossref, Medline, Google Scholar
36 : Optimism, response to treatment of depression, and rehospitalization after coronary artery bypass graft surgery. Psychosom Med 2012; 74:200–207Crossref, Medline, Google Scholar
37 : Explanatory style change during cognitive therapy for unipolar depression. J Abnorm Psychol 1988; 97:13–18Crossref, Medline, Google Scholar



