Irritability in a Prospective Cohort of Huntington’s Disease Mutation Carriers
Abstract
A key neuropsychiatric symptom of Huntington’s disease, irritability, contributes to a decline in functioning and to great distress in both patients and their caregivers. To identify mutation carriers prone to the development of irritability, this study aimed to investigate the course and temporal relationships between irritability and other neuropsychiatric symptoms. A cohort of 90 mutation carriers was followed for 2 years. Using the Irritability Scale, the incidence of irritability was 23%, whereas irritability persisted in 70% of the mutation carriers with irritability at baseline. An increase in irritability was strongly associated with an increase in apathy.
Neuropsychiatric symptoms, motor disturbances, and cognitive impairment are core symptoms of Huntington’s disease (HD),1–3 an autosomal dominant, neurodegenerative disorder resulting from an expanded trinucleotide cytosine-adenine-guanine repeat in the HTT gene on chromosome 4 that codes for the mutant protein huntingtin. Although under normal circumstances, huntingtin is present ubiquitously, its physiologic role is not fully elucidated. The mutant huntingtin probably confers a toxic gain of function, mainly resulting in striatal cell loss. The age of onset is widespread, ranging from early childhood to senescence, and is generally about 40 years. The duration between diagnosis of motor symptoms and death is around 20 years. Neuropsychiatric symptoms, such as irritability, apathy, and depressed mood, often precede the onset of motor symptoms.
Irritability is a common and key neuropsychiatric symptom of HD,4–6 which can be characterized as a mood state predisposing toward anger, hostile appraisals, impatience, intolerance, poorly controlled anger, and overt aggression.7,8 Reduced control over temper frequently occurs, resulting in verbal or behavioral outbursts. However, irritability may also be present without observable manifestation. Irritability should be distinguished from the DSM-IV-TR diagnosis of intermittent explosive disorder, because the latter has to meet strict, observable criteria and is severe in its phenotype and consequences, whereas irritability may not even be visible to an observer. The subjective experience of irritability may be either brief or prolonged. In contrast to justifiable anger, verbal or behavioral outbursts originating from irritable mood are nonadaptive, complicate the interaction between the patient and his environment, and are always unpleasant for the individual. Irritability can occur early in the course of HD, e.g., up to 10 years before the onset of motor symptoms,9 and often contributes to a decline in personal and occupational functioning.10 Moreover, irritability of mutation carriers may also cause great distress among others, affecting the well-being of their families andpossibly contributing to the necessity of institutionalization.11
The prevalence of irritability ranges from 35% to 73%, depending on its definition, the assessment tools used, and the study populations.2,9,12 In REGISTRY (a European multicenter prospective observational study including both manifest and premanifest HD mutation carriers), a prevalence of 19.1% of overt disruptive or aggressive behavior among was found among 1468 mutation carriers.13 Two prospective studies conducted among 12 premotor symptomatic14 and 111 motor symptomatic15 HD mutation carriers showed that irritability increased over time.
Cross-sectional studies of irritability in motor symptomatic mutation carriers have found associations with other neuropsychiatric symptoms including impulsivity,4 obsessive compulsive symptoms,16 and suicidal thoughts.17 Using the 14-item Irritability Scale,18,19 we earlier demonstrated an association between irritability and a low Total Functioning Capacity score, use of benzodiazepines, and more often being married or living together.2
Because irritability can occur before the onset of motor symptoms, our cohort included both premotor symptomatic and motor symptomatic mutation carriers. Although cross-sectional associations with irritability have been investigated before,2,4,16,17 to our knowledge, this is the first study investigating predictors of incident and persistence of irritability in HD; also, this is the first study investigating correlates of change in irritability over time. Identification of mutation carriers prone to the development and persistence of irritability is required to provide adequate treatment and support of HD mutation carriers and their families. Therefore, this study investigates the course and temporal relationships between irritability and other neuropsychiatric symptoms in a cohort of 90 HD mutation carriers followed for 2 years.
Methods
Participants
HD mutation carriers and first-degree noncarriers were recruited between May 2004 and August 2006 from the outpatient departments of Neurology and Clinical Genetics of Leiden University Medical Centre and from a regional nursing home specialized in HD. All participants underwent genetic testing and were considered HD mutation carriers with a trinucleotide cytosine-adenine-guanine repeat length ≥36 repeats. A second measurement was conducted 2 years after the first measurement and a third measurement 2 years thereafter. The study design has been described in detail elsewhere.20
In the present longitudinal analysis, only the data of mutation carriers who participated in the second and third waves of this study are included, because the Irritability Scale2 was introduced halfway during the first measurement. Here, the second wave is referred to as the baseline measurement and the third wave as the follow-up measurement. A total of 121 mutation carriers completed the Irritability Scale at baseline, and irritability was also assessed in 90 mutation carriers at follow-up. In total, 32 mutation carriers dropped out (Figure 1).
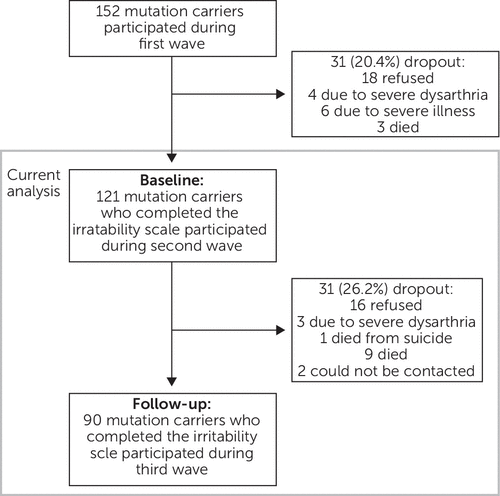
FIGURE 1. Flow Chart of Inclusion of the Study Participants for the Present Analysis
The study was approved by the medical ethical committee of the Leiden University Medical Centre, and informed consent was obtained from all participants.
Instruments
Assessment of irritability.
Irritability was assessed with the self-rated Irritability Scale (appendix I) that consists of 14 questions addressing the presence of various phenomena of irritability in the 2 weeks prior to the interview, rated on a four-point Likert scale.18 The total sum score of the Irritability Scale ranges from 0 to 42 points, with higher scores indicating more severe irritability. We used a cutoff score of >13 points as indicative for the presence of irritability; this score was selected based on our previous cross-sectional study among 130 HD mutation carriers (largely overlapping with baseline data of the current study) where this cutoff provided optimal sensitivity and specificity according to receiver-operating characteristic analyses.2 Cronbach’s α of the irritability scale is 0.90, and its sensitivity and specificity for detecting irritability in HD mutation carriers is 0.69 and 0.81, respectively.2 We preferred the use of the Irritability Scale over the Problem Behaviors Assessment (PBA) irritability subscale because the Irritability Scale was designed specifically for the assessment of irritability in neurodegenerative disease. Also, the psychometric properties of the Irritability Scale are better than those of the PBA irritability subscale (Cronbach’s α of 0.90 versus 0.67, respectively). Because no cutoff score for the PBA irritability subscale for the presence of irritability exists, a direct comparison is not possible. In our previous study, however, we found that with a cutoff score on the PBA irritability factor of >15 points, a cutoff score of >13 points on the irritability score yielded a sensitivity of 0.69 and a specificity of 0.81.
Assessment of other neuropsychiatric characteristics.
The PBA was used to assess depression. The PBA is a semistructured interview assessing the frequency and severity of 36 potential behavioral problems in HD in the month before the interview.4 The interrater reliability of the Dutch version of the PBA is 0.82 for severity scores and 0.73 for frequency scores. We used the symptom factor depression (range 0–80) that was previously estimated by factor analysis of the PBA.5 Apathy was assessed with the Apathy Scale (appendix II)21; this scale consists of 14 questions, measuring different features of apathy in the 2 weeks before the interview. Caregivers’ information and the interviewers’ judgment were included in rating the Apathy Scale, because patients with apathy may lack adequate insight into their own symptoms. The Apathy Scale was chosen above the apathy factor of the PBA because, although no clear preference arises from the literature, the Apathy Scale has better psychometric properties than the PBA apathy subscale (Cronbach’s α of 0.89 versus 0.84, respectively) and because the former was specifically designed for the assessment of apathy.
Sociodemographic and clinical characteristics.
Information on sociodemographic and clinical characteristics (including alcohol consumption, use of psychotropic medication, and information about the household) was collected in a standardized manner. Global daily functioning was assessed using the Total Functional Capacity scale22 of the Unified Huntington’s Disease Rating Scale (UHDRS).23 Motor symptoms were assessed using the motor section of the UHDRS (UHDRS-m) by trained neurologists. Mutation carriers with an UHDRS-m confidence level 0–1 were considered premotorsymptomatic and mutation carriers with UHDRS-m confidence level >1 were considered motorsymptomatic. Global cognitive functioning was assessed with the Mini-Mental State Examination (MMSE).24 Executive cognitive functioning was assessed with the Symbol Digit Modalities Test,25 the Verbal Fluency Test,26 and Stroop tests.27 Because there is a high level of multicolinearity between most tests (Pearson’s r>0.80), an aggregate variable for executive functioning, ExCog, was constructed by computing the mean of the standardized scores of the Symbol Digit Modalities Test, Verbal Fluency Test, and Stroop tests.
Statistical analyses.
Data are presented as n (%), mean±standard deviation, or median (interquartile range) when appropriate. Associations between sociodemographic, clinical, and neuropsychiatric characteristics on the one hand and incident and persistent irritability on the other were determined using univariate logistic regression; odds ratios were computed for the incidence or persistence of irritability. For the assessment of incident irritability, we identified HD mutation carriers without irritability at baseline who were irritable at follow-up. Similarly, for the assessment of persistent irritability we identified HD mutation carriers who were irritable at baseline and remained irritable at follow-up. A multivariate logistic regression (adjusting for sex and age that were entered into the model) was conducted with variables with p<0.10 in the initial univariate analyses. To assess associations between temporal changes of clinical and neuropsychiatric characteristics on the one hand and irritability on the other, we conducted an additional multivariate linear regression analysis. Absolute changes (i.e., Δ values) in all clinical and neuropsychiatric characteristics between baseline and follow-up were calculated. Linear regression analysis was used to assess whether changes in scores of clinical or neuropsychiatric characteristics as independent variables were associated with changes in the Irritability Scale score as the dependent variable. Because our investigation is of a explorative nature and we are not aware of known correlates of changes in irritability score, we decided to let a forward selection procedure select variables to be included in our final model based on statistical strength. In the final model, the most robust relationships with changes in both irritability, and its correlates were further analyzed using regression analysis, with adjustment for sex, age, and use of antipsychotics. All tests were two-tailed with p<0.05 denoting statistical significance. The SPSS 20.0 Package (SPSS, Chicago, IL) was used for the statistical analyses.
Results
The 90 HD mutation carriers in the current study had an average age of 49 years (standard deviation, 1.7 years), and 41 (46%) were men. Their mean Total Functioning Capacity score was 8.6 (standard deviation, 4.4), and their mean UHDRS-m score was 25.3 (standard deviation, 2.47). Twenty-five (28%) mutation carriers were premotor symptomatic and 64 (71%) were motor symptomatic. UHDRS-m score and motor symptomatic status were missing in one mutation carrier. The score on the Irritability Scale between baseline and follow-up showed no significant change: average increase, 1.56 points; 95% confidence interval, −0.23 to 2.89.
At baseline, of the 90 mutation carriers, 33 (37%) were irritable. During the 2-year follow-up, of the 57 nonirritable HD mutation carriers at baseline, 13 (23%) developed irritability and 23 (70%) with irritability at baseline were persistently irritable. Compared with mutation carriers who completed the follow-up, dropouts had on average a lower Total Functioning Capacity score (5.8 versus 8.6, p=0.004), higher UHDRS-m score (40.4 versus 25.1, p=0.007), lower MMSE score (23.7 versus 26.9, p=0.001), and worse executive cognitive functioning (−0.53 versus −0.13, p=0.04); however, there was no difference in their Irritability Scale score (10.1 versus 10.6, p=0.78).
Of the 57 nonirritable HD mutation carriers at baseline, the 13 mutation carriers with incident irritability at follow-up had, at baseline, a longer mean trinucleotide cytosine-adenine-guanine repeat length, a higher UHDRS-m score, a lower MMSE score, lower scores on all executive cognitive measures including the ExCog variable, and more frequently used benzodiazepines compared with HD mutation carriers who did not develop irritability. In a logistic regression models adjusting for sex and age, a longer trinucleotide cytosine-adenine-guanine repeat length, a higher UHDRS-m score, a higher use of benzodiazepines, a lower MMSE score, and worse cognitive functioning at baseline were all associated with incident irritability at follow-up (Table 1). Next, we built a final multivariate logistic model adjusting for all these significant correlates. In this model, all determinants lost their significance (Table 1).
| Characteristic | No Irritability at Follow-Up (N=44) | Incident Irritability (N=13) | p Valueb | Adjusted for Sex and Age | Fully Adjusted | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | p Value | OR | (95% CI) | p Value | ||||
| Sociodemographic characteristics | |||||||||
| Sex | 19 (43) | 7 (54) | 0.50 | ||||||
| Age in years | 49.6±1.7 | 50.29±2.32 | 0.82 | ||||||
| Married or living together | 26 (59) | 8 (62) | 0.87 | 1.09 | (0.30; 3.90) | 0.90 | |||
| Clinical characteristics | |||||||||
| CAG repeats | 43.0±0.4 | 45.00±1.21 | 0.04 | 1.34 | (1.05; 1.72) | 0.02 | 1.20 | (0.84; 1.73) | 0.32 |
| Motor score | 20.1±3.4 | 38.54±7.91 | 0.03 | 1.03 | (1.00; 1.06) | 0.03 | 1.00 | (0.96; 1.05) | 0.85 |
| Antidepressants | 13 (30) | 7 (54) | 0.11 | 3.20 | (0.85; 12.03) | 0.09 | |||
| Antipsychotics | 8 (18) | 5 (39) | 0.14 | 3.41 | (0.79; 14.83) | 0.10 | |||
| Benzodiazepines | 3 (7) | 4 (31) | 0.03 | 6.32 | (1.17; 34.03) | 0.03 | 5.00 | (0.74; 33.79) | 0.10 |
| Neuropsychiatric characteristics | |||||||||
| PBA depression | 6.86±2.04 | 9.69±4.94 | 0.54 | 1.02 | (0.98; 1.06) | 0.41 | |||
| Apathy score | 5.41±1.26 | 8.62±1.93 | 0.22 | 1.04 | (0.97; 1.12) | 0.24 | |||
| MMSE score | 27.7±0.5 | 24.4±1.3 | 0.01 | 0.81 | (0.68; 0.95) | 0.01 | 0.89 | (0.68; 1.16) | 0.40 |
| VFT | 25.0±2.0 | 15.4±3.6 | 0.03 | 0.93 | (0.88; 0.99) | 0.02 | |||
| SDMT | 37.7±2.9 | 24.4±6.1 | 0.047 | 0.96 | (0.92; 1.00) | 0.027 | |||
| Stroop color | 60.4±3.5 | 42.3±6.4 | 0.02 | 0.96 | (0.93; 0.99) | 0.02 | |||
| Stroop word | 77.9±3.7 | 51.5±8.0 | 0.01 | 0.96 | (0.93; 0.99) | <0.001 | |||
| Stroop interference | 34.6±2.3 | 24.0±3.9 | 0.04 | 0.94 | (0.90; 0.99) | 0.02 | |||
| ExCog | 0.10±0.13 | –0.63±0.26 | 0.02 | 0.33 | (0.14; 0.76) | 0.01 | 0.92 | (0.14; 5.84) | 0.93 |
TABLE 1. Predictors of Incident Irritability in Patients With Huntington's Disease Without Irritability at Baselinea
There were no significant differences in mutation carriers who showed remission of irritability compared with those with persistent irritability (Table 2). In the final model, we adjusted for the use of antidepressants, Symbol Digit Modalities Test score, and Stroop color score, because these variables had a correlation with p<0.10 in the univariate analyses. In this model, there were no significant associations.
| Characteristic | No Irritability at Follow-Up (N=10) | Persistent Irritability (N=23) | p Value* | Adjusted for Sex and Age | Fully Adjusted | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | p Value | OR | (95% CI) | p Value | ||||
| Sociodemographic characteristics | |||||||||
| Sex | 2 (20) | 11 (48) | 0.15 | ||||||
| Age in years | 42.9±2.8 | 50.4±2.8 | 0.13 | ||||||
| Married or living together | 8 (80) | 17 (74) | 0.71 | 0.61 | (0.09; 4.03) | 0.61 | |||
| Clinical characteristics | |||||||||
| CAG repeats | 44.1±1.1 | 45.0±0.7 | 0.46 | 1.26 | (0.94; 1.70) | 0.13 | |||
| Motor score | 19.1±7.2 | 29.3±4.3 | 0.23 | 1.02 | (0.98; 1.07) | 0.34 | |||
| Antidepressants | 1 (10) | 10 (44) | 0.09 | 4.69 | (0.46; 47.29) | 0.19 | 3.23 | (0.28; 37.31) | 0.35 |
| Antipsychotics | 0 (0) | 7 (30) | 0.23 | 2.94 | (0.28; 30.89) | 0.37 | |||
| Benzodiazepines | 3 (30) | 7 (30) | 0.98 | 0.89 | (0.16; 5.04) | 0.90 | |||
| Neuropsychiatric characteristics | |||||||||
| PBA depression | 12.9±4.1 | 11.1±2.8 | 0.71 | 0.98 | (0.92; 1.04) | 0.57 | |||
| Apathy score | 8.00±1.76 | 13.30±2.05 | 0.13 | 1.09 | (0.95; 1.25) | 0.22 | |||
| MMSE score | 28.0±0.6 | 26.4±0.8 | 0.26 | 0.73 | (0.48; 1.10) | 0.13 | |||
| VFT | 24.9±4.5 | 22.1±3.6 | 0.64 | 0.98 | (0.93; 1.03) | 0.41 | |||
| SDMT | 38.5±3.8 | 28.9±3.3 | 0.10 | 0.96 | (0.90; 1.02) | 0.15 | 1.00 | (0.90; 1.11) | 0.94 |
| Stroop color | 62.3±6.9 | 46.9±4.3 | 0.07 | 0.97 | (0.93; 1.01) | 0.12 | 0.51 | (0.91; 1.05) | 0.42 |
| Stroop word | 76.4±7.1 | 60.2±5.6 | 0.11 | 0.97 | (0.94; 1.01) | 0.11 | |||
| Stroop interference | 33.9±3.7 | 25.4±3.1 | 0.13 | 0.96 | (0.91; 1.02) | 0.21 | |||
| ExCog | 0.11±0.23 | –0.38±0.18 | 0.14 | 0.48 | (0.17; 1.32) | 0.15 | |||
TABLE 2. Predictors of Persistent Irritability in Patients With Huntington's Disease Subjects With Irritability at Baselinea
We analyzed the association between changes in the clinical and neuropsychiatric characteristics with the change in Irritability Scale score. In univariate analyses of these changes, we found that an increase in UHDRS-m score, an increase in the Apathy Scale, and the use of both antipsychotics and of benzodiazepines were associated with an increase in the Irritability Scale score over time. Next, we built a final multivariate regression model, comprising sex and age at baseline, increase in UHDRS-m score, Apathy Scale score, and the change in use of both antipsychotics and of benzodiazepines. We found that the association between change in Irritability Scale score and change in Apathy Scale score persisted after adjustment for confounders (Table 3, Figure 2). Furthermore, the continuous use of antipsychotics was independently associated with an increase in Irritability Scale score.
| Changes in Apathy Scale score | Changes in Irritability Scale score | |
|---|---|---|
| β (95% CI) | p value | |
| Crude | 0.29 (0.08; 0.49) | 0.01 |
| Adjusted for sex and age | 0.29 (0.09; 0.49) | 0.01 |
| Fully adjustedb | 0.41 (0.19; 0.62) | <0.001 |
TABLE 3. Associations Between Changes in Irritability Scale Score and Changes in Apathya
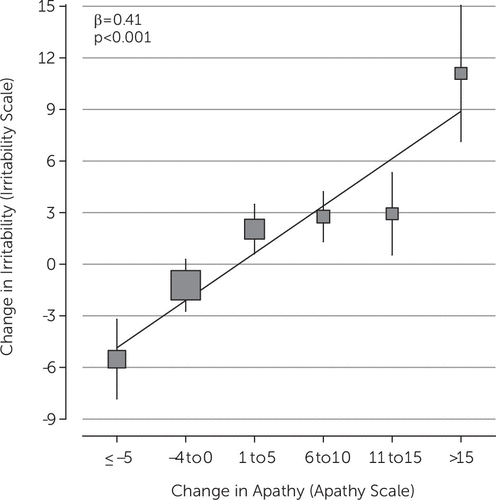
FIGURE 2. Fully Adjusted Multivariate Linear Regression of Changes in Irritability Scale Score on Changes in Apathy as Assessed With the Apathy Scale
Discussion
In this study, we investigated the course of irritability and its associations with clinical and neuropsychiatric symptoms in a cohort of 90 HD mutation carriers who were followed for 2 years. An increasing severity of apathy and the continuous use of antipsychotics were associated with increasing severity of irritability over 2 years.
A recent longitudinal study of neuropsychiatric symptoms followed 111 patients with HD for a mean of 5 years.15 At five moments of assessment during follow-up, a minimum of 49% of patients with HD had one or more symptom of irritability and a maximum of 83% had one or more symptom of irritability. However, the proportions of any of these symptoms cannot be translated easily into a prevalence rate of the syndrome of irritability, which we used in our analyses. Therefore, our incidence of 23% may be a good representation of new-onset irritability at 2-year follow-up in individuals with HD.
We found a link between apathy and irritability in HD, reflected by our finding that an increase in apathy coincided with an increase in irritability over a 2-year period. At first glance, this may seem paradoxical. Apathy is a disorder of motivation that is mainly characterized by a lack of goal-directed behavior and cognition and by decreased emotional responsiveness. Conversely, irritability has been characterized as a mood state predisposing toward negative or hostile appraisals and to the experience of anger. Although irritability may lead to the overt expression of anger, it may also only bother the patient himself without overt expression of irritability. In this paradigm, an individual with apathy may well experience irritability, a hypothesis that is supported by our study. An explanation for this comorbidity of apathy and irritability in HD may be that they have a related pathophysiology because both are caused by neural degeneration in subcortical and frontal circuits,28,29 which is a key feature of HD, in particular the anterior cingulate cortex and the orbitofrontal cortex. The anterior cingulate cortex plays an important role in affect regulation,30 as well as in decision making.31 Lesions in the anterior cingulate cortex, or in the neuronal circuitry connected to it, have been associated with apathy,28 as well as behavioral inhibition and aggression.28 The orbitofrontal cortex is involved in impulse inhibition, and lesions in the area of the orbitofrontal cortex are associated with behavioral disinhibition and emotional lability.28 In addition, hypoactivation of the orbitofrontal cortex, as well as the anterior cingulate cortex, is associated with impulsive aggression.29 Although emotional lability and impulsive aggression may be components of overt, observable irritability, these symptoms are absent in irritability that remains out of awareness, and to our knowledge, the role of subcortical circuitry has not been investigated in this type of irritability. Therefore, the comorbidity of apathy and irritability may only be partly due to a related pathophysiology. Another factor to be considered is the possibility between construct overlap of irritability and apathy as measured by the Irritability and the Apathy Scales, respectively. When looking at the items of both scales, there are no obvious similarities or overlapping items. Using Spearman’s correlation analyses, we found that the largest correlation coefficient was 0.44 between any of the 14 items of the Irritability Scale and any of the 14 items of the Apathy Scale. This rather low correlation on any two single items suggest that phenomenological or scale reasons cannot account for the associations we found over time between irritability and apathy.
We found an association between the use of antipsychotics and increasing irritability. Although selective serotonin reuptake inhibitors are recommended as a first-step treatment, antipsychotics are frequently prescribed for irritability and related behaviors.32 Several underlying mechanisms may explain the association between the used of antipsychotics and irritability. On the one hand, antipsychotics may have been prescribed to treat (among others) irritability, likely resulting in confounding by indication as the treated group may on average still be more irritable than the untreated group. On the other hand, antipsychotics may have caused irritability or some other third factor may underlie the relationship. Furthermore, in some patients antipsychotics (both first generation and second generation) are known to cause akathisia, a state of psychomotor agitation.33 Patients experiencing psychomotor agitation associated with akathisia may either become irritable or may have reported positively on certain items on the Irritability Scale due to some overlap between the two constructs.
We assessed irritability using the Irritability Scale because this scale was used to assess irritability in our earlier cross-sectional study.2 On this scale, our previously validated cut-off score of 14 points for the presence of irritability enabled us to investigate the occurrence and correlates of incident and persistent irritability.
The strengths of this study are its prospective design, a relatively large cohort of HD mutation carriers, and the use of validated instruments for the assessments of determinants and outcomes. A few limitations should also be addressed. First, although we validated the use of the Irritability Scale in our previous study, there is no gold standard for the assessment of irritability. Second, a relatively high number of mutation carriers dropped out and therefore did not participate in the current analysis; irritability at follow-up may have been a reason not to participate. Selection bias may account for an underestimation of the incidence of irritability and for the underestimation of the predictive value of some predictors because of insufficient power. Third, we cannot exclude the possibility that cognitive dysfunction in our HD patients has distorted the reliability and sensitivity of scales that assessed of neuropsychiatric characteristics.
In conclusion, we found an unexpected association between irritability and apathy. HD mutation carriers that become more apathetic over time also seem to become more irritable. The association between irritability and apathy may imply a shared underlying pathophysiology between these neuropsychiatric symptoms in HD. Because apathy may mask irritability, we recommend asking mutation carriers with apathy and their caregivers about the symptoms of irritability to provide adequate support and treatment.
1 : Neuropsychiatric aspects of Huntington’s disease. J Neurol Neurosurg Psychiatry 2001; 71:310–314Crossref, Medline, Google Scholar
2 : Irritability in Huntington’s disease. Psychiatry Res 2012; 200:813–818Crossref, Medline, Google Scholar
3 : Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci 2007; 19:441–448Link, Google Scholar
4 : Behavioral changes in Huntington Disease. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:219–226Medline, Google Scholar
5 : Behavioural problems in Huntington’s disease using the Problem Behaviours Assessment. Gen Hosp Psychiatry 2008; 30:155–161Crossref, Medline, Google Scholar
6 : Factor analysis of behavioural symptoms in Huntington’s disease. J Neurol Neurosurg Psychiatry 2011; 82:411–412Crossref, Medline, Google Scholar
7 : The Irritability Questionnaire: a new scale for the measurement of irritability. Psychiatry Res 2008; 159:367–375Crossref, Medline, Google Scholar
8 : Irritability: definition, assessment and associated factors. Br J Psychiatry 1985; 147:127–136Crossref, Medline, Google Scholar
9 : Psychiatric disorders in preclinical Huntington’s disease. J Neurol Neurosurg Psychiatry 2007; 78:939–943Crossref, Medline, Google Scholar
10 : Behavioural abnormalities contribute to functional decline in Huntington’s disease. J Neurol Neurosurg Psychiatry 2003; 74:120–122Crossref, Medline, Google Scholar
11 : Predictors of nursing home placement in Huntington disease. Neurology 2003; 60:998–1001Crossref, Medline, Google Scholar
12 : Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci 2007; 19:441–448Link, Google Scholar
13 : Observing Huntington’s Disease: the European Huntington’s Disease Network’s REGISTRY. PLoS Curr 2010; 2:RRN1184Medline, Google Scholar
14 : Longitudinal personality changes among presymptomatic Huntington disease gene carriers. Neuropsychiatry Neuropsychol Behav Neurol 2002; 15:192–197Medline, Google Scholar
15 : Longitudinal evaluation of neuropsychiatric symptoms in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2012; 24:53–60Link, Google Scholar
16 : Comorbidities of obsessive and compulsive symptoms in Huntington’s disease. J Nerv Ment Dis 2010; 198:334–338Crossref, Medline, Google Scholar
17 : Suicidal ideation in Huntington disease: the role of comorbidity. Psychiatry Res 2011; 188:372–376Crossref, Medline, Google Scholar
18 : A comparison of self-report and caregiver assessment of depression, apathy, and irritability in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2005; 17:378–383Link, Google Scholar
19 : Irritability in pre-clinical Huntington’s disease. Neuropsychologia 2010; 48:549–557Crossref, Medline, Google Scholar
20 : Cross-sectional study on prevalences of psychiatric disorders in mutation carriers of Huntington’s disease compared with mutation-negative first-degree relatives. J Clin Psychiatry 2008; 69:1804–1810Crossref, Medline, Google Scholar
21 : Apathy following cerebrovascular lesions. Stroke 1993; 24:1625–1630Crossref, Medline, Google Scholar
22 : Huntington disease: clinical care and evaluation. Neurology 1979; 29:1–3Crossref, Medline, Google Scholar
23 : Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord 1996; 11:136–142Crossref, Medline, Google Scholar
24 : “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
25 : The Symbol Digit Modalities Test: a neuropsychological test for economic screening of learning and other cerebral disorders. Learn Disord 1968; 3:83–91Google Scholar
26 : Initiation: Verbal Fluency Tests. Cognitive Assessment for Clinicians. Oxford, UK, Oxford University Press, 2003, pp 118–119Google Scholar
27 : Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18:643–662Crossref, Google Scholar
28 : Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci 2007; 9:141–151Medline, Google Scholar
29 : Language and the modulation of impulsive aggression. J Neuropsychiatry Clin Neurosci 2008; 20:261–273Link, Google Scholar
30 : Anterior cingulate cortex: unique role in cognition and emotion. J Neuropsychiatry Clin Neurosci 2011; 23:121–125Link, Google Scholar
31 : The functional neuroanatomy of decision-making. J Neuropsychiatry Clin Neurosci 2012; 24:266–277Link, Google Scholar
32 : Treatment of Irritability in Huntington’s Disease. Curr Treat Options Neurol 2010; 12:424–433Crossref, Medline, Google Scholar
33 : Akathisia: an updated review focusing on second-generation antipsychotics. J Clin Psychiatry 2009; 70:627–643Crossref, Medline, Google Scholar



