Capgras Syndrome and Phantom Vest Following Traumatic Brain Injury
To the Editor: Delusional misidentification syndromes, including Capgras delusion, are uncommon but potentially disabling consequences of traumatic brain injury (TBI).1 Tactile hallucinations have classically been described in delirium tremens, stimulant intoxication, and schizophrenia2 but are not usually associated with TBI.3 We present a case of severe TBI with episodic Capgras delusions and tactile hallucination of clothing (i.e., a “phantom vest”), the latter of which, to our knowledge, has not been reported previously.
Case Report
A left-handed man in his 30s experienced a severe TBI in a motor vehicle accident. His past medical history included hypertension and moderate alcohol use disorder but no other psychiatric problems. His initial injury presentation included a right subdural hematoma, producing right to left midline shift and necessitating a hemicraniectomy and external ventricular drain (EVD) placement. The patient developed seizures 4 weeks postinjury in the context of EVD malfunction. After correction of that malfunction and transitioning from levetiracetam to phenytoin, he remained free of clinically apparent seizures. The patient underwent a cranioplasty 5 weeks postinjury; his family reported that during this period, he was oriented consistently to time, place, and the etiology of his deficits.
On examination in the inpatient rehabilitation setting 6 weeks after the injury, the patient and his wife reported daily episodes (of approximately 1-hour duration) during which the patient stated that his wife had been replaced by an impostor. The episodes began a few days after he developed clinically apparent seizures and appeared to be triggered by his wife standing in his left hemispace. By contrast, the patient did not experience his wife as an impostor when he spoke to her over the phone. He also endorsed a constant sensation of wearing a vest, which he described as tightly covering his torso and extending onto his upper arms. This sensation began a few days after the cranioplasty and was not linked to his episodes of delusional misidentification. The “vest” sensation was consistent in character and intensity on the front, back, and sides of his torso and did not vary in relation to the presence or absence of clothing (i.e., it was not an illusion triggered by actual clothing). Three weeks into his rehabilitation hospitalization, the patient reported momentary relief from the vest sensation while showering with hot water and when his wife kissed his chest; the sensation otherwise persisted.
A neurological examination demonstrated motor aprosodia, normal visual fields, mild left lower facial and extremity hemiparesis, normal elementary sensory function, and left-sided extinction to double simultaneous stimuli. The patient scored 28 of 30 points on the Orientation Log,4, reflecting recovery of orientation; he scored 17 of 18 (z score=−1.2) on the Frontal Assessment Battery,5 reflecting mild executive dysfunction.6 Line bisection and circle cancellation tests were consistent with left egocentric neglect. The patient drew a picture of himself using his nondominant hand and demarcated the location of the vest (Figure 1A). He correctly identified which side of the drawing was his left and right sides, suggesting relative preservation of left- and right-sided body representation. The patient was able to correctly identify pictures of familiar and unfamiliar faces. When presented with famous faces (from a set customized to his general knowledge and interests), he correctly matched 83% of faces and names.
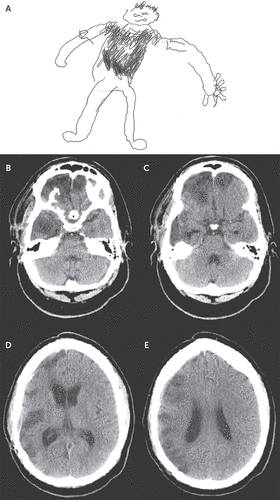
FIGURE 1. Pictorial Representation and Imaging Results for a Patient With Capgras Syndrome and Phantom Vest After Traumatic Brain Injury
[A] The patient’s pictorial representation of his body and the location of the vest. The right and left sides of his body are indicated. [B–E] Axial computed tomography images of the brain in ascending order.
Computed tomography (CT) performed during the patient’s rehabilitation stay revealed hypodensities in the gray and white matter structures of the bilateral straight and orbitofrontal gyri (right greater than left); the frontal polar region bilaterally; the right dorsolateral prefrontal (DLPF) region; the right anterior temporal polar region and the anterior and midinferior, middle, and superior temporal gyri; and the right supramarginal and angular gyri and right inferior and superior parietal lobules (Figure 1 B–E). CT imaging of the patient’s chest with contrast was negative for fracture or other pathology. Routine electroencephalography performed during the patient’s rehabilitation stay did not reveal epileptiform abnormalities, and his serum free phenytoin level was within normal limits during treatment with this medication.
Discussion
The Capgras delusion is predicated on disconnection between the visual facial processing regions of the temporal lobe and emotional input from limbic structures1,7,8 and aberrant, left-hemisphere-mediated interpretation of that disconnection.7,8 With respect to this anatomy of Capgras delusion, our patient sustained CT-evident injury to the right temporal pole, right DLPF region, and bilateral frontal poles. The lateral aspect of the right temporal pole supports facial recognition.9 Damage to this area and to the limbic-paralimbic connections therein disrupts integration of visual, emotional/visceral input (in our patient, the integration of the visual percept of his wife with its appropriate social-emotional affects, such as affection, familiarity, and acceptance). The combination of injury to the right DLPF region and bilateral frontal poles provides an anatomic basis for aberrant error detection and correction in relation to impaired right temporal polar integration of visual and emotional/visceral information.
Consistent with the argument offered by Devinsky,8 injury to these (and related) right-hemisphere structures in the context of relative preservation of left hemispheric (especially language-related) structures “releases” the left hemisphere to create dual categories and accompanying narrative for that information (i.e., the invention of a duplicate or impostor to resolve conflicting information). In this particular case, injury to the frontal poles (Brodmann area 10p), supramodal structures involved in the processing of internal states, memory retrieval, and the coordination overall of information processing across multiple cognitive operations10 may compound these integrative failures and contribute to impaired insight into the narrative of an “impostor wife” (i.e., the Capgras delusion).
The combination of right temporal polar, bilateral frontal polar, and right parietal injuries may also have contributed to the patient’s sensory experience we characterized as a “phantom vest.” The right parietal lobe is a critical node in body representation; damage to this area, combined with the sensory-limbic integrative deficits arising from damage to other right-hemisphere structures and impaired monitoring attendant to bilateral frontal polar injury, may underlie this unusual sensory perceptual/experiential phenomenon. The bilaterality of this phenomenon remains unexplained by this patient’s anatomy of injury. Nonetheless, this novel clinical observation is presented here as a phenomenon in need of replication and further anatomic characterization.
1 : Capgras syndrome: a novel probe for understanding the neural representation of the identity and familiarity of persons. Proc Biol Sci 1997; 264:437–444Crossref, Medline, Google Scholar
2 : Tactile hallucinations: conceptual and historical aspects. J Neurol Neurosurg Psychiatry 1982; 45:285–293Crossref, Medline, Google Scholar
3 : Psychosis following traumatic brain injury. Int Rev Psychiatry 2003; 15:328–340Crossref, Medline, Google Scholar
4 : Validity of the Orientation Log, relative to the Galveston Orientation and Amnesia Test. J Head Trauma Rehabil 2000; 15:957–961Crossref, Medline, Google Scholar
5 : The FAB: a Frontal Assessment Battery at bedside. Neurology 2000; 55:1621–1626Crossref, Medline, Google Scholar
6 : The Frontal Assessment Battery (FAB): normative values in an Italian population sample. Neurol Sci 2005; 26:108–116Crossref, Medline, Google Scholar
7 : Schizophrenia and monothematic delusions. Schizophr Bull 2007; 33:642–647Crossref, Medline, Google Scholar
8 : Delusional misidentifications and duplications: right brain lesions, left brain delusions. Neurology 2009; 72:80–87Crossref, Medline, Google Scholar
9 : The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 2007; 130:1718–1731Crossref, Medline, Google Scholar
10 : Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci 2004; 5:184–194Crossref, Medline, Google Scholar



