Skin Conduction Levels Differentiate Frontotemporal Dementia From Alzheimer’s Disease
Abstract
Patients with behavioral variant frontotemporal dementia (bvFTD) and Alzheimer’s disease (AD) differ in basic emotional tone. Skin conduction levels (SCLs), a measure of sympathetic tone, may be a sensitive test for discriminating these two dementias early in their course. Previous research has shown differences in resting SCLs between patients with bvFTD and AD, but no study has evaluated the discriminability of SCLs during different environmental conditions. The authors compared bvFTD patients (N=8), AD patients (N=10), and healthy control subjects (N=9) on SCL measures pertaining to real-life vignettes or scenarios differing in valence and emotional intensity. The SCLs among the bvFTD patients were decreased across all conditions, whereas the SCLs among the AD patients were increased compared with control participants. On analysis, the SCLs in response to emotional stimuli differentiated bvFTD from AD with an area under the receiver operator characteristic curve of 95.3%. At a cutoff ≤0.77 μS, emotional vignettes distinguished bvFTD from AD with a sensitivity of 86% and a specificity of 96%. These preliminary results indicate the potential utility of SCLs for differentiating bvFTD from AD early in their course, regardless of environmental condition.
Frontotemporal dementia is the second most common young-onset neurodegenerative dementia, after Alzheimer’s disease (AD).1,2 The differentiation of behavioral variant frontotemporal dementia (bvFTD) from young-onset AD has implications for prognosis, genetic considerations, and pharmacological management.3,4 Although these dementias usually have distinct clinical characteristics and criteria, involving early behavioral and language changes in bvFTD and episodic memory in AD, they can overlap and be difficult to distinguish. Examples include situations in which there is prominent memory impairment in bvFTD5,6 and in which there are neuropsychiatric features early in the course of AD.7,8 The overlap of clinical symptoms and the lack of definitive biomarkers confound the problem of differential diagnosis. There is clearly a need for an effective clinical test that can distinguish bvFTD from AD early in the course.
Differences in emotional or autonomic state in these two dementias can serve as the basis of a clinical test. Given mesiofrontal and anterior temporal pathology, emotional blunting is among the earliest and most characteristic features of bvFTD,9,10 and bvFTD patients have prominent impairments in empathy and sympathy.2 They may have particular problems in recognizing emotions with a negative valence as opposed to a positive valence.11,12 In sharp contrast to bvFTD, patients with early AD can have increased emotional reactivity and anxiety.13,14 Anxiety and an associated heightened sympathetic state may be particularly associated with young-onset AD15,16 and the first stages of the disease.17,18
Skin conductance levels (SCLs) reflect autonomic and emotional tone and could be a good clinical test for differentiating between bvFTD and AD early in their course. SCLs are a direct measure of ongoing, tonic sympathetic activity and a readily accessible psychophysiological index of emotional tone. Our prior research has shown that patients with bvFTD, compared with those with AD and healthy controls (HCs), have decreased resting SCLs, which correlate with emotional blunting. In addition, their average SCLs may be more likely to be abnormal than the more commonly assayed peak, phasic skin conduction responses to discrete events.19 In contrast, patients with AD may have increased sympathetic tone, possibly from retained insight or decreased sensorimotor gating associated with AD pathology in the entorhinal cortex.20
This study investigated average SCLs as a measure of emotional tone during video and audio presentation of real-life vignettes among patients with bvFTD and AD compared with HCs. Impairments on psychophysiological measures have varied with the valence (positive or negative outcomes) and emotionality (present or absent emotional paralinguistic cues) of stimuli.21,22 Investigating SCLs in response to scenarios varying in valence and emotion builds on our prior research showing baseline resting SCL differences between bvFTD and AD.19 In order to determine goodness of the SCLs as a predictive test for the binary classification of bvFTD versus AD, we determined the area under the receiver operating characteristic curve (auROC) for mean SCLs. We hypothesized that the bvFTD patients would have significantly lower SCLs, particularly in response to negative or emotional scenarios, and that the AD patients would have heightened SCLs in response to these vignettes. SCLs thus should distinguish the two dementias.
Methods
Participants
BvFTD and young-onset AD patients were recruited from the University of California, Los Angeles, Behavioral Neurology Program and Clinic, where they underwent clinical, neuropsychological, and neuroimaging assessments. Age-matched HCs were recruited from volunteers in the community. Under approval by the university’s institutional review board, this study enrolled 27 participants: eight patients with bvFTD, 10 patients with young-onset AD, and nine HCs. All participants and their caregivers gave informed consent.
The patients with bvFTD met International Consensus Criteria for clinically probable bvFTD.2 Clinical diagnoses were supported by predominant frontal or anterior temporal involvement on magnetic resonance imaging or fluorodeoxyglucose positron emission tomography (PET). Patients with AD met National Institute on Aging-Alzheimer’s Association criteria for clinically probable AD. HC participants did not have any known history of neurological or psychiatric disease.
Participants in all three groups were comparable in age: AD patients were in early stages of disease and age-matched with the bvFTD patients within 3 years. Disease severity was assessed with the Mini-Mental State Examination (MMSE [https://www.uml.edu/docs/Mini%20Mental%20State%20Exam_tcm18-169319.pdf]) and the Montreal Cognitive Assessment [http://www.mocatest.org/splash/]. In order to further document differences in socioemotional behavior between bvFTD and AD patients, we administered three behavioral scales that characterize the behavioral deficits of bvFTD: the Frontal Systems Behavior Scale, the Social Dysfunction Scale, and the Scale for Emotional Blunting. Exclusion criteria included the use of beta-blocker medications (because of the psychophysiological assessment) or the presence of complicating medical or psychiatric illnesses or medications at the time of participation.
Procedures
Presentation vignettes.
Four types of vignettes were designed to vary either in positive or negative outcomes (valence) or in emotional or unemotional presentations. The four vignettes were “Tumor” (“I saw my doctor today. Remember the tests I had done last week? Results came back and…”), “Accident” (“There was a big car accident on the freeway and grandpa’s car went off the bridge!”), “Missing” (“Jane didn’t come home from school yesterday. I looked for her everywhere.”), and “Soldier” (“I spoke with someone from the Army today. My [deployed] brother…lucky to not have any injuries…”). Each of these opening vignettes was followed by one of two potential outcomes (e.g., for “Tumor,” the test results were negative or positive for cancer). Each of these vignettes (opening and outcome) was delivered by an actor, with either an emotional presentation or a flat and nonemotional presentation.
The emotional versus unemotional delivery of these vignettes required variation in the methodology of delivery. The same stories were presented verbatim with either emotion (visual [video] and voice [audio]) or nonemotion (visual [neutral portrait] and voice [audio]) content. The emotional presentations included video (facial) paralinguistic cues portrayed by the actor, and the accompanying audio included (voice) emotional prosody. The unemotional presentations included a portrait of a neutral facial expression of the actor, and the accompanying audio included a flat voice with a flat emotional tone.
The experiments, with the presentation of vignettes and SCL measurements, were conducted in a quiet testing room. All vignettes were performed by the same actor (MMA) and programmed and presented through SuperLab Pro 4.0 software, with audio through headphones. Immediately prior to the experiment, participants were instructed to relax and remain still for a 2.5-minute baseline skin conductance recording.
Following this baseline period, the vignettes were presented on a television monitor to participants seated approximately three feet away. To minimize possible effects of attention and working memory deficits, we kept the vignettes short, at 30 seconds each. To control for order and carryover effects, we randomized the vignettes, using a random integer sequence generator (www.random.org), into three different counterbalanced presentation sequences. Each participant received one of the three counterbalanced presentation sequences. The experimenter directly monitored the visual gaze of each participant, in order to ensure that he or she was focused on the monitor. On debriefing, all participants were asked to identify the content of the vignettes and whether they were “emotional” or “unemotional.” There were no errors in vignette comprehension.
Psychophysiology
Skin conductance was continuously recorded through BIOPAC hardware (MP150 System) with the skin conductance module (GSR 100C) and BIOPAC software (AcqKnowledge v4.1). Electrodermal electrodes were placed on the palmar surface of the index and middle fingers of the participant’s right hand. Acquisition parameters were set at 5 µS/V, low pass filter 1 Hz with no high pass filter. The sampling rate was 31.25 Hz. The average SCLs (continuous changes in tonic SCLs) were determined for each individual during each vignette presentation and analyzed at one-second intervals. The average SCLs for the 30 seconds of exposure for each vignette were then computed for each individual.
Statistical Analysis
Statistical analysis was conducted with SPSS 23.0 software. Demographic descriptive statistics were generated for each group and compared with chi-square and analyses of variance (ANOVAs) for categorical and continuous variables, respectively. The SCLs were evaluated for normality with Shapiro-Wilks analysis; all between-groups and within-group SCLs were significantly positively skewed and lacked normality. Accordingly, group differences in SCLs across the three groups were analyzed with Kruskal-Wallis nonparametric analysis.
In addition, as recommended for obtaining normal distributions of skin conduction measures,23 the logs of the SCLs were obtained, and group differences across the three groups were further analyzed with ANOVA. Post hoc analyses of pairwise comparisons were done with Fisher’s least significant difference (LSD) and Tukey tests. We computed Spearman correlations to examine the relations between SCLs and behavioral scales. Finally, we determined the auROC values, which represent true positives (sensitivity) versus false positives (1-sensitivity) across the range of possible classification cutoffs. We compared the values compared using the confidence interval adjustment set at 95%. The Youdin index of maximum sensitivity determined the best SCL cutoff score for distinguishing bvFTD from AD.
Results
Participant Characteristics
There were no significant group differences on demographic variables of age, sex, or years of education (Table 1). The dementia groups did not differ on disease duration, the MMSE, or the Montreal Cognitive Assessment. As expected, both the bvFTD (p=0.024) and the AD (p=0.005) patients differed significantly from HCs on the MMSE. On further analysis, there were no significant correlations between the two dementia scales and the average SCLs, either within or across groups.
| bvFTD (N=8) | AD (N=10) | HC (N=9) | Statistical Comparison | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | Mean | SD | F | df | p |
| Age (years) | 60.25 | 11.81 | 61.10 | 5.93 | 54.22 | 10.59 | 1.41 | 2, 26 | n.s |
| Education (years) | 16.0 | 2.14 | 16.40 | 1.90 | 16.44 | 1.60 | 0.14 | 2, 26 | n.s. |
| Disease duration (years) | 4.50 | 3.74 | 3.40 | 1.65 | N/A | 0.70 | 1, 16 | n.s | |
| Mini-Mental State Examination | 26.00 | 4.72 | 25.20 | 3.22 | 29.78 | 0.44 | 5.25 | 2, 26 | 0.013b |
| Montreal Cognitive Assessment | 23.00 | 5.57 | 18.00 | 6.75 | N/A | 1.21 | 1, 16 | n.s. | |
| Frontal Systems Behavior Scale | 158.43 | 32.33 | 100.71 | 26.02 | N/A | 16.65 | 1, 16 | 0.001 | |
| Social Dysfunction Scale | 141.86 | 40.09 | 68.90 | 25.74 | N/A | 21.06 | 1, 16 | <0.001 | |
| Scale for Emotional Blunting | 11.33 | 8.00 | 1.78 | 2.24 | N/A | 11.91 | 1, 16 | 0.004 | |
TABLE 1. Demographic and Clinical Measures of Behavioral Variant Frontotemporal Dementia (bvFTD), Alzheimer’s Disease (AD), and Healthy Control (HC) Participantsa
Behavioral Assessments and Correlations
The bvFTD and AD groups differed significantly on the three behavioral scales—the Frontal Systems Behavioral Scale (p=0.001), the Social Dysfunction Scale (p<0.001), and the Scale for Emotional Blunting (p=0.004)—which indicates worse socioemotional behavioral function among the bvFTD patients (Table 1). There were no significant correlations between the socioemotional behavioral scale results and the average SCLs either within or across groups.
Psychophysiology
Part A.
The mean SCLs in μS for the three groups, and for their responses to vignettes differing in valence and emotional presentation, did not differ between the dementia patients and the HCs. Despite the smaller SCLs among the bvFTD patients compared with the AD patients, the mean SCL differences in total, positive valence, negative valence, emotional SCLs, and unemotional SCLs between the bvFTD and AD groups did not reach statistical significance (all Kruskal-Wallis tests, ns; Table 2).
| bvFTD | AD | |||
|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD |
| Positivea | 0.976 | 0.646 | 1.22 | 1.114 |
| Negativea | 0.97 | 0.642 | 1.317 | 1.301 |
| Emotionalb | 0.975 | 0.634 | 1.259 | 1.184 |
| Unemotionalb | 0.971 | 0.654 | 1.277 | 1.239 |
| Total | 0.957 | 0.646 | 1.244 | 1.185 |
TABLE 2. Skin Conduction Levels in Response to Vignette Conditions: Comparison of Behavioral Variant Frontotemporal Dementia (bvFTD) Compared With Alzheimer’s Disease (AD)
The mean logs of the SCLs differed significantly among the three groups (ANOVA; p<0.001; Table 3). On post hoc comparisons, there were significant differences between the bvFTD and AD groups (both LSD and Tukey p=0.037), AD and HC groups (both LSD and Tukey p=0.034), and bvFTD and HC groups (both LSD and Tukey p=0.038). There were no interactions among group, valence, and emotional content. Further analysis did not show differences among the four types of vignettes.
| Source | Type III Sum of Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|
| Corrected model | 0.957b | 11 | 0.087 | 0.969 | 0.474 |
| Intercept | 3.628 | 1 | 3.628 | 40.400 | 0.000 |
| Group | 0.949 | 2 | 0.475 | 5.284 | 0.005 |
| Valencec | 0.002 | 1 | 0.002 | 0.023 | 0.879 |
| Emotiond | 0.001 | 1 | 0.001 | 0.007 | 0.935 |
| Group by valencec | 0.003 | 2 | 0.002 | 0.019 | 0.982 |
| Group by emotiond | 0.000 | 2 | 0.000 | 0.001 | 0.999 |
| Valencec by emotiond | 0.000 | 1 | 0.000 | 0.002 | 0.964 |
| Group by valencec by emotiond | 0.001 | 2 | 0.000 | 0.005 | 0.995 |
| Error | 36.282 | 404 | 0.090 | ||
| Total | 40.761 | 416 | |||
| Corrected total | 37.239 | 415 |
TABLE 3. Analysis of Variance for Average Logs of the Skin Conduction Levelsa
Part B.
The auROC values were calculated from sensitivities and 1-specificities across all four conditions and for each of the four conditions separately (Figure 1). The areas under the curve ranged from 91.0% to 95.3%, with 93% for all conditions combined, and with the largest auROC elicited from emotional vignettes (Table 4). We reviewed auROC values for individual vignettes in order to determine which stimulus category rendered the most discriminating Youdin index. We calculated the Youdin indices, and a cutoff of ≤0.77–0.78 μS gave the highest value for emotional presentation (0.814). The other Youdin indices were as follows: positive valence (0.792), negative valence (0.711), and unemotional presentation (0.709). Overall, the maximum differentiation involved SCLs in response to vignettes with emotional presentations with a cutoff of ≤0.773 μS, which yielded a sensitivity of 0.857 and a specificity of 0.957 for differentiating bvFTD and AD.
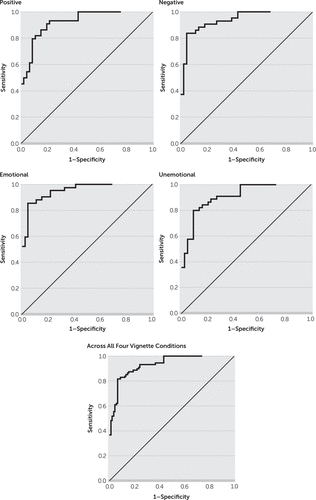
FIGURE 1. Areas Under the Receiver Operating Characteristic (ROC) Curve
| Stimuli | auROC | Standard Error | Asymptotic Significance | Asymptotic 95% CI | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Positive | 0.924 | 0.027 | 0.000 | 0.872 | 0.976 |
| Negative | 0.939 | 0.024 | 0.000 | 0.891 | 0.987 |
| Emotional | 0.953 | 0.020 | 0.000 | 0.914 | 0.992 |
| Unemotional | 0.910 | 0.030 | 0.000 | 0.851 | 0.968 |
| Across All Vignettes Conditions | 0.931 | 0.018 | 0.000 | 0.896 | 0.966 |
TABLE 4. Area Under the Receiver Operating Characteristic Curve (auROC) for Average Skin Conduction Levels During the Vignette Conditions
Discussion
BvFTD and AD, the two most common neurodegenerative dementias of the presenium, are easily distinguished with SCLs, a measure of sympathetic and emotional tone. Patients with bvFTD have prominent emotional blunting,24 and those with early AD may be prone to anxiety and increased emotional reactivity.15 Using this distinction, this preliminary study shows that SCLs in response to emotional vignettes can differentiate patients with bvFTD, who have low SCLs, from those with AD, who may have high SCLs, at a cutoff of ≤0.773 μS with a sensitivity of 86% and a specificity of 96%. Presenting an emotional scenario to patients with these young-onset neurodegenerative dementias while measuring SCLs is a promising, clinically accessible measure for differentiating bvFTD from AD.
The correct diagnosis of bvFTD or young-onset AD is critical not only for clinical management but also for clinical trial enrollment, genetic analysis, and the understanding of these disorders.3,4 The differential diagnosis may be difficult when the presentations vary from the established behaviorally predominant criteria for bvFTD2 and the cognitive features and criteria for AD.25 Clinicians may misdiagnose bvFTD as AD in the presence of significant deficits in episodic declarative memory,5,6 which is relatively spared in Consensus Criteria for bvFTD.2 Conversely, clinicians may misdiagnose AD as bvFTD in the presence of agitation or aggression, hallucinations, or delusions,7,8 particularly early in the course.
In one large neuropathological study, 22% of clinically diagnosed bvFTD patients were found to have AD on autopsy, having been misdiagnosed because of the presence of early neuropsychiatric symptoms.7 A third risk for misdiagnosis is the occurrence of an AD frontal variant, best characterized as the “behavioral/dysexecutive” variant of AD, a form of AD that often meets Consensus Criteria for clinically probable bvFTD.26 Finally, there are no definitive clinical tests for these disorders. Early in the course of bvFTD, fluorodeoxyglucose PET scans can be normal.27 In AD, amyloid PET may be costly and fail to correlate with the presence of disease state, whereas tau PET is not yet clinically available.
A readily accessible, noninvasive test of tonic emotional state, such as SCLs, can effectively discriminate between bvFTD and AD. Emotional blunting and related deficits characterize patients with bvFTD early in their course and correlate with their mesiofrontal-limbic and associated anterior insular and temporal pathology.9,10 Patients with bvFTD have impairments in emotion recognition and understanding;12,28–31 empathic concern;32 emotion regulation;33,34 and affective theory of mind, or the ability to infer the emotions and feelings of others.35–38 In contrast, patients with mild AD or mild cognitive impairment due to AD can have increased anxiety, emotional reactivity, or even affective disturbance, reflecting the beginning stages of their disease.15,18,39,40 These symptoms in AD may reflect the presence of the earliest Alzheimer’s pathology in the entorhinal cortex, with consequent impairment in sensorimotor gating.20
Although they did not have statistical significance, the largest auROC group differences occurred with emotional presentations. Valence represents the positive versus negative significance of an emotional presentation, and emotional intensity corresponds to the paralinguistic and prosody cues that convey emotion. It is not surprising that the emotional presentations showed the largest discriminability in SCLs between the patients with bvFTD and those with AD.
This study has limitations. First, the study included a relatively small number of participants, which raises concern for sampling bias or a lack of representativeness of the bvFTD and AD patients. The findings showing the value of SCLs for the differential diagnosis of these two dementias need replication in a larger investigation. Second, all the bvFTD and AD diagnoses were clinical; there was no neuropathological verification of the diagnosis. Third, there were no statistically significant differences across valence and emotional stimulus categories. Although the methodology did not show statistically worse SCLs for emotional versus unemotional presentations between bvFTD and AD, the fact that the largest auROC group differences occurred with emotional presentations supports the main hypothesis as well as the potential value of SCLs in differentiating these two young-onset dementias. Finally, the emotional presentations included video presentations of an actor demonstrating behavioral cues of emotion, whereas the unemotional presentations involved projecting a static, neutral portrait of the actor. Despite this design, there were no significant ANOVA differences when we compared the effects of emotional versus unemotional presentation on the SCLs.
In conclusion, SCL differences can be a simple clinical test for distinguishing patients with early bvFTD from those with early AD, particularly of young onset. SCLs are readily available, inexpensive, and noninvasive measures of emotional state, which is blunted in bvFTD and often exaggerated in early AD. Further research is necessary to corroborate these findings in a larger sample of participants with these dementias.
1 : The prevalence of frontotemporal dementia. Neurology 2002; 58:1615–1621Crossref, Medline, Google Scholar
2 : Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134:2456–2477Crossref, Medline, Google Scholar
3 : Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol 2011; 10:162–172Crossref, Medline, Google Scholar
4 : Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry 2007; 15:84–87Crossref, Medline, Google Scholar
5 : In vivo and post-mortem memory circuit integrity in frontotemporal dementia and Alzheimer’s disease. Brain 2012; 135:3015–3025Crossref, Medline, Google Scholar
6 : Episodic memory and the medial temporal lobe: not all it seems. Evidence from the temporal variants of frontotemporal dementia. J Neurol Neurosurg Psychiatry 2012; 83:1145–1148Crossref, Medline, Google Scholar
7 : Clinicopathologic differences among patients with behavioral variant frontotemporal dementia. Neurology 2013; 80:561–568Crossref, Medline, Google Scholar
8 : Alzheimer’s disease. N Engl J Med 2010; 362:329–344Crossref, Medline, Google Scholar
9 : Evaluation of emotional blunting in behavioral variant frontotemporal dementia compared to Alzheimer’s disease. Dement Geriatr Cogn Disord 2014; 38:79–88Crossref, Medline, Google Scholar
10 : Neuroanatomical correlates of emotional blunting in behavioral variant frontotemporal dementia and early-onset Alzheimer’s disease. J Alzheimers Dis 2014; 41:793–800Crossref, Medline, Google Scholar
11 : Discrete neural correlates for the recognition of negative emotions: insights from frontotemporal dementia. PLoS One 2013; 8:e67457Crossref, Medline, Google Scholar
12 : Emotional reactivity and emotion recognition in frontotemporal lobar degeneration. Neurology 2007; 69:148–155Crossref, Medline, Google Scholar
13 : The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord 2016; 190:264–271Crossref, Medline, Google Scholar
14 : Anxiety of Alzheimer’s disease: prevalence, and comorbidity. J Gerontol A Biol Sci Med Sci 1999; 54:M348–M352Crossref, Medline, Google Scholar
15 : Differences in anxiety among patients with early- versus late-onset Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 2014; 26:73–80Link, Google Scholar
16 : Frequency and characteristics of anxiety among patients with Alzheimer’s disease and related dementias. J Neuropsychiatry Clin Neurosci 2003; 15:180–186Link, Google Scholar
17 : The frequency and correlates of anxiety in patients with first-time diagnosed mild dementia. Int Psychogeriatr 2012; 24:1771–1778Crossref, Medline, Google Scholar
18 : Personality traits and behavioral and psychological symptoms in patients at an early stage of Alzheimer’s disease. Int J Geriatr Psychiatry 2013; 28:276–283Crossref, Medline, Google Scholar
19 : Skin conductance levels may reflect emotional blunting in behavioral variant frontotemporal dementia. J Neuropsychiatry Clin Neurosci 2014; 26:227–232Link, Google Scholar
20 : Prepulse inhibition of acoustic startle response in mild cognitive impairment and mild dementia of Alzheimer type. Psychiatry Clin Neurosci 2006; 60:55–62Crossref, Medline, Google Scholar
21 : Primary empathy deficits in frontotemporal dementia. Front Aging Neurosci 2014; 6:262Crossref, Medline, Google Scholar
22 : Loss of cells—loss of self: frontotemporal lobar degeneration and human emotion. Curr Dir Psychol Sci 2007; 16:289–294Crossref, Google Scholar
23 : Electordermal Activity, 2nd ed. New York, Springer, 2012Crossref, Google Scholar
24 : The Scale for Emotional Blunting in patients with frontotemporal dementia. Neurocase 2006; 12:242–246Crossref, Medline, Google Scholar
25 : The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:263–269Crossref, Medline, Google Scholar
26 : The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain 2015; 138:2732–2749Crossref, Medline, Google Scholar
27 : The added value of 18-fluorodeoxyglucose-positron emission tomography in the diagnosis of the behavioral variant of frontotemporal dementia. Am J Alzheimers Dis Other Demen 2014; 29:607–613Crossref, Medline, Google Scholar
28 : Emotion recognition in frontotemporal dementia and Alzheimer’s disease: a new film-based assessment. Emotion 2015; 15:416–427Crossref, Medline, Google Scholar
29 : Right limbic FDG-PET hypometabolism correlates with emotion recognition and attribution in probable behavioral variant of frontotemporal dementia patients. PLoS One 2015; 10:e0141672Crossref, Medline, Google Scholar
30 : Is the emotion recognition deficit associated with frontotemporal dementia caused by selective inattention to diagnostic facial features? Neuropsychologia 2014; 60:84–92Crossref, Medline, Google Scholar
31 : Emotion comprehension in the temporal variant of frontotemporal dementia. Brain 2002; 125:2286–2295Crossref, Medline, Google Scholar
32 : Orbitofrontal and limbic signatures of empathic concern and intentional harm in the behavioral variant frontotemporal dementia. Cortex 2016; 75:20–32Crossref, Medline, Google Scholar
33 : Emotion regulation deficits in frontotemporal lobar degeneration and Alzheimer’s disease. Psychol Aging 2010; 25:30–37Crossref, Medline, Google Scholar
34 : Executive functions and the down-regulation and up-regulation of emotion. Cogn Emotion 2012; 26:103–118Crossref, Medline, Google Scholar
35 : Differential cognitive and affective theory of mind abilities at mild and moderate stages of behavioral variant frontotemporal dementia. Cogn Behav Neurol 2015; 28:63–70Crossref, Medline, Google Scholar
36 : Cognitive and affective theory of mind in neurodegenerative diseases: neuropsychological, neuroanatomical and neurochemical levels. Neurosci Biobehav Rev 2012; 36:2147–2164Crossref, Medline, Google Scholar
37 : The role of social cognition in moral judgment in frontotemporal dementia. Soc Neurosci 2011; 6:113–122Crossref, Medline, Google Scholar
38 : The SEA (Social Cognition and Emotional Assessment): a clinical neuropsychological tool for early diagnosis of frontal variant of frontotemporal lobar degeneration. Neuropsychology 2012; 26:81–90Crossref, Medline, Google Scholar
39 : The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry 2013; 21:685–695Crossref, Medline, Google Scholar
40 : Behavioral symptoms in mild cognitive impairment as compared with Alzheimer’s disease and healthy older adults. Int J Geriatr Psychiatry 2013; 28:265–275Crossref, Medline, Google Scholar



