Complex Metal Ions: Neuropsychiatric and Imaging Features
Throughout human history, compounds (either natural or synthetic) have been used as poisons. The oldest known example is use of ricinoleic acid (from castor beans) in the late Stone Age, more than 20,000 years ago (5). A current definition of a poison (from Webster’s Dictionary) is “a substance that through its chemical action usually kills, injuries, or impairs an organism” (6). If the poison is produced spontaneously by a living organism, it is a toxin or venom (7, 8). Introduction of poisons into the human body can occur via ingestion through the gastrointestinal track, percutaneous absorption, injection, or inhalation. Exposures can be intentional or accidental. Critical elements that affect survival or long-term effects include preexisting medical illnesses, length of time and amount in each instance of exposure, number of exposures, and healthcare received after exposure. To some extent, each individual toxic compound has differing effects on both the nervous system and other organ systems. Within the neurosciences, poisons are traditionally divided into five categories: psychoactive drugs of abuse, complex metal ions, gases, heavy metals, and organic phosphates/solvents/pesticides. Some complex metal ions are essential for human life, such as hemoglobin; others are rapidly fatal. Frequent sources of human exposure to complex metal ions are in medical evaluations and treatments (prescription and over-the-counter), less commonly in occupational exposures (i.e., metal plating or rubber manufacturing industries), and rarely in homicide or suicide (9–11). Due to the vast numbers of compounds and heterogeneous nature of complex metal ions, a singular mechanism of injury cannot be defined or circumscribed. Examples will be given to highlight illustrative points for this class of compounds and how they interface with the nervous system.
The high metabolic activity of the basal ganglia (Figure 1) results in increased need for substrates (glucose and oxygen) making those nuclei highly vulnerable to a number of poisons (1, 12, 13).These structures perform critical roles in cognition, emotion, behavior, and motor control, making it is imperative to understand the basic tenants of how poisons and toxins affect the human brain (1, 10).
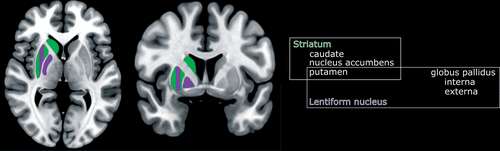
FIGURE 1. The essential clinical anatomy of the basal ganglia are illustrated on representative MRIs The major groupings are the striatum (green) and lentiform nuclei (purple). The putamen is considered part of both structures (striped) (1). These structures have high metabolic activity and oxygen consumption and are rich in mitochondria (1, 2), and thus they are particularly sensitive to poisons or toxins that affect aerobic respiration in the mitochondria. The anatomy of the basal ganglia sometimes includes the subthalamic nucleus and the substantia nigra.
Overview of Complex Metal Ions
A complex ion has a coordinating metal ion surrounded by other ions in Lewis acid-base interactions. The surrounding ligands form coordination bonds, such that all ligands donate a pair of electrons to the bond and thus function as Lewis bases. There is a very broad group of compounds that meet this definition with multitudes of human exposures, making generalizations difficult. The chemistry of complex metal ions encountered in medicine is diverse. For example, the chemotherapy agent cisplatinum [Pt(NH3)2Cl2] is considered simple. In contrast, cyclical chelates such as gadoteric acid [C16H25GdN4O8] are considered complex. Some agents, such as cyanide, only become a complex metal ion after introduction into living cells.
Cyanide
Few poisons are more infamous than cyanide. It is highly lethal, and survival can be limited in acute exposures (14). Cyanide compounds have very narrow medical use. Iatrogenic cyanide poisoning can occur with the intravenous administration of nitroprusside, particularly in patients with renal failure, as a result of the impaired excretion of thiocyanate (4, 15, 16). Tragic intentional uses of cyanide compounds include mass suicide (potassium cyanide was used in the Jonestown mass suicide) and homicide (hydrogen cyanide was used in the killing center of Auschwitz-Birkenau, the largest killing center of the Nazi regime) (17, 18). Occupational cyanide exposures include electroplating and fumigation (19). In Western countries, the most common source of cyanide poisoning is accidental due to smoke inhalation from fires (20). Hydrogen cyanide is produced when materials containing nitrogen (e.g., plastics, wool, vinyl, and wood) undergo combustion (21). This is frequently in combination with carbon monoxide exposure, a more widely recognized source of morbidity and mortality in smoke inhalation. Data support the role of cyanide in smoke inhalation injury, with toxic levels found in 33%−90% of victims who die in a closed-space fire (20).
When cyanide is introduced to the human body, it diffuses into cells and binds at the binuclear (iron[Fe]/copper[Cu]) center of cytochrome a3 complex IV in the electron transport chain (Figure 2) (3, 4). This leads to cessation of adenosine triphosphate (ATP) production, profound cellular hypoxia and a shift to glycolytic metabolism that results in lactic acidosis. Acute toxicity can be divided into early manifestations (within minutes) that include neurologic (anxiety, confusion, headache), respiratory (tachypnea), and cardiovascular (tachycardia) symptoms, and later manifestations occurring with the progression of hypoxia include seizures, coma, hypotension, arrhythmias, and death (4). Treatment includes supportive measures (i.e., oxygen administration and intubation) and administration of a cyanide antidote. Two approaches are approved by the Food and Drug Administration for acute cyanide poisoning (Figure 2). The cyanide antidote kit contains sodium and amyl nitrite and sodium thiosulfate. The nitrites induce the production of a preferential cyanide binding site (methemoglobin), while sodium thiosulfate combines with cyanide to generate thiocyanate which is then renally excreted. The more recently approved (in 2006) hydroxycobalamin reacts with cyanide to produce cyanocobalamin (vitamin B12) (4).
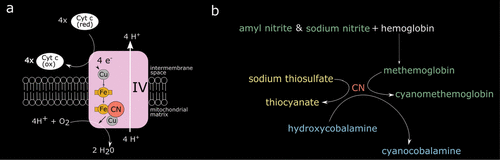
FIGURE 2. AND COVER. (Panel a) Regardless of the source, once in the body, cyanide (CN) is a rapidly acting toxin that acts on cellular respiration. It binds at the binuclear (iron[Fe]/copper[Cu]) center of complex IV in the electron transport chain (3, 4). (Panel b) Treatment of CN poisoning involves pulling CN away from the electron transport chain by binding into safer components. Two commercial products are available for treatment of CN poisoning. The kit contains nitrites (green) to induce conversion to the less toxic cyanomethemoglobin (green) and sodium thiosulfate (yellow) to promote conversion to thiocyanate (yellow) for renal excretion. Hydroxycombalamin is also a treatment option that results in the conversion of CN to cyanocobalamine (vitamin B12).
If patients survive the acute toxicity of cyanide, neuropsychiatric sequalae are predicated on injury to brain structures that have high oxygen and glucose requirements, such as the basal ganglia (Figures 1 and 3). Parkinsonism and dystonia are common sequalae in those that survive acute cyanide toxicity (13, 22, 23). Cyanide poisoning most commonly presents with hyperintensities in the basal ganglia (particularly in the bilateral putamen) on MRI. With more significant exposures, there can be hemorrhagic necrosis and cellular death in the cortex and cerebellum (12, 13, 24). Surprisingly, although the hippocampus is a highly oxygen-sensitive structure, published case reports have not commonly found hippocampal injury after acute cyanide toxicity (13, 22, 23, 25–29) The lesions may not appear immediately on computed tomography (CT) (Figure 3). They can take several weeks to months to appear as the acute initial edema from hypoxia resolves with some resolution of the subacute hypertensities in weeks to months (30). Of note, the severity and chronicity of the clinical symptoms do not always match to the imaging findings (31).
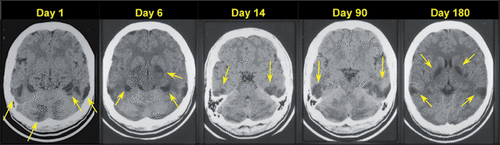
FIGURE 3. AND COVER. An Asian male in his late teens (still in high school) with adjustment difficulties after immigrating to the United States drank potassium cyanide obtained from his father’s jewelry and electroplating business. Shortly after the attempt he was found unconscious and 911 was called. He was taken to the emergency department and then hospitalized in the intensive care unit for weeks. Neuropsychiatry was consulted after extubation due to residual cognitive, affective, and motor (parkinsonian) symptoms. Serial CT scans demonstrate evolving course of leukoencephalopathy and subcortical involvement (yellow arrows), especially in the globus pallidus. Note the additional involvement in the posterior temporoparietal region and cerebellum.
Chronic, low-level cyanide exposure can result in toxicity as well. The cyanogenic shrub, Cassava (Manihot esculenta), is a food staple for hundreds of millions of humans world-wide, especially in Africa (32). Cyanogenic compounds can be removed from the cassava plant through processing methods. Inadequate processing in times of social or environmental adversity can result in retained cyanogenic compounds and thus human exposure when consumed (32). Two diseases in humans have been associated with Cassava consumption: tropical ataxic neuropathy (TAN) and konzo (meaning “tired legs” in Kiyaka, a dialect spoken in the Democratic Republic of Congo) (33, 34). Both are myeloneuropathies endemic throughout the tropics. Although chronic, low-level cyanide toxicity is a commonly proposed mechanism given the association with consumption of cassava (32, 33, 35), some authors have disputed the importance of cyanide in the pathophysiology of both diseases (36). TAN is a sensory-predominant myeloneuropathy which presents with distal neuropathic pain, severe loss of dorsal column sensory modalities, diminished deep-tendon reflexes in the legs, and preservation of strength (34). In contrast, konzo is a predominantly a motor myeloneuropathy with irreversible paraparesis (and rarely, tetraparesis), that may also be accompanied by paresthesias and visual loss that tend to improve over time (33). Cognitive deficits have also been documented in children with konzo (37). Interestingly, low-level cyanide toxicity has also been hypothesized to play a role in two visual disorders: tobacco amblyopia and Lieber’s hereditary neuropathy (38, 39).
Cisplatinum
Metal-based compounds are frequently used as antineoplastic agents. Cisplatinum is a prototypical metal-based antineoplastic agent that has served as the foundation for the synthesis of other organometallic compounds used in cancer therapy (40). It is a complex metal ion with a relatively simple structure composed of a central platinum molecule with two ammine molecules and two chlorine atoms attached. Once cisplatinum is transported into cells, it is hydrolyzed into an electrophilic compound that binds to purine residues on DNA, leading to inhibition of cellular division and apoptotic cellular death. This mechanism is responsible for both the therapeutic cytotoxic effects on neoplasms as well as the toxicity and side effects. It has been used alone or in combination with other chemotherapeutic agents and is an example of a complex metal ion that has significant life-saving medical uses. Nevertheless, adverse effects are common, with over 70% of patients experiencing gastrointestinal symptoms, and up to 50% experiencing neurologic symptoms (most commonly, peripheral neuropathy) over the course of their treatment (41, 42). Hepatic, renal, and cardiac toxicity are all well-described side effects of cisplatinum therapy (43).
Platinum-based agents, including cisplatinum, can lead to significant neurotoxicity (44). Sensory neuropathy is the most common dose-dependent neurotoxic effect. In a study of patients undergoing treatment for metastatic germ cell tumors, 11% had paresthesias after 3–4 cycles of chemotherapy with cisplatinium, with 65% experiencing paresthesias at 3 months (45). Fortunately, the majority of patients experienced improvement, with only 17% having persistent symptoms at one year after completion of therapy. Ototoxicity leading to significant hearing loss and tinnitus are well-described toxicities related to cisplatinum especially in children (46). As in neuropathy, ototoxicity is dose-dependent. In one study, 80% of adults with germ cell tumors experienced a hearing off of at least 20 dB (47). A study of children receiving cisplatinum for hepatoblastoma found 63% experienced of at least Brock grade 1 or higher loss (40 dB at a minimum of 8 kHz) (48).
CNS toxicity is much less common but has been described with cisplatinum. A subacute encephalopathy with headaches, severe hypertension, cortical blindness, and seizures has been described, and most likely represents posterior reversible encephalopathy syndrome (PRES; sometimes referred to as reversible posterior leukoencephalopathy syndrome) (49–55). The most common MRI findings are hyperintensities on T2 weighted images, likely indicating edema, primarily in the fiber tracts of the parietal lobe, occipital lobe, or periventricular areas. Other less commonly cited edematous areas include frontal and temporal lobes or the subcortical regions. Unlike the injury of cyanide, there is usually resolution of this syndrome with treatment and thus a decrease in the white matter edema. The MRI images show normalization and decreased hyperintense signal within a few weeks to months (51, 54). The incidence of PRES in patients treated with cisplatinum is not known. A review of all cases of PRES between 2005 and 2011 at one site (Memorial Sloan Kettering Cancer Center) found that 55% of patients (N=17/31) had received chemotherapy within the prior month (56). Of these, two had received carboplatin/paclitaxel combination therapy, two carboplatin monotherapy, and two oxaliplatin monotherapy. Data on the number of patients given a platinum-based compound who did not develop PRES is not available, but expected to be quite large, leading to a low estimated incidence.
Newer MRI techniques are beginning to be applied to examine the long-term effects of cisplatinum upon brain networks and cognitive function. One group used diffusion tensor imaging (DTI) and diffusion kurtosis imaging (DKI) in combination with neuropsychological testing to examine tissue microstructure in a cross-sectional study of testicular cancer (TC) patients with (N=27) and without (N=18) cisplatinum-based chemotherapy 14 years after treatment (57). Although the cisplatinum-treated group had both lower cognitive performance and higher radial kurtosis in multiple white matter areas (corona radiata, bilateral internal capsules, bilateral superior longitudinal fasciculi, corpus callosum) than the group without chemotherapy, imaging findings were not related to cognitive performance. A different group used DTI in combination with mathematical graph analysis to assess structural network characteristics in a longitudinal study of TC patients with (N=22) and without (N=42) cisplatinum-based chemotherapy at baseline (3 months after orchiectomy) and 6 months later (3 months post completion of the chemotherapy) (58). Results indicated that the cisplatinum group had decreased network and local efficiencies, as well as decreased small-worldness, all associated with decreased cognitive performance (58). However, in both these studies, it is challenging to affirm that the declining cognitive performance was solely due to the cisplatinum, as other agents in the cisplatinum-based therapy have been associated with possible cognitive effects (57).
Gadolinium-Based Contrast Agents
Gadolinium-based contrast agents (GBCAs) consist of a central gadolinium ion (Gd3+) that is chelated to produce a stable structure, an important feature as free gadolinium ions are highly toxic. GBCAs are examples of complex metal ions with significant medical diagnostic value and rare adverse effects (59). Gadolinium is a paramagnetic molecule that reduces T1-relaxation time, thereby generating high signal on T1-weighted MRI (60). GBCAs are characterized by their structure and charge: each compound can be either linear or macrocyclic and ionic or nonionic.
The most feared toxicity from GBCAs is nephrogenic systemic fibrosis (NSF; also known as nephrogenic sclerosing dermopathy), a rare but potentially fatal complication. The pathophysiology of NSF is driven primarily by fibroblast stimulation, leading to the characteristic changes to skin and injury to other organs (61). The skin thickens and hardens in the extremities, sometimes involving the trunk, but usually sparing the face. The morbidity is high, with contractures developing, and sometimes involving organ systems beyond the skin, including the muscle, heart, and lungs (62). In a multicenter retrospective study of 83,121 patients, 15 were identified with NSF, all of whom had received a greater than standard dose of GBCA (63). The incidence with high dose GBCA in patients on chronic hemodialysis was 0.4%, and 8.8% when restricted to those with an estimated glomerular filtration rate of less than 15 mL/min who were not in hemodialysis. More recently, a systematic review and meta-analysis of patients with stage 4 or 5 chronic kidney disease (pooled sample of 4931) who were administered a group II GBCA (e.g., gadobenate dimeglumine, gadobutrol), found zero patients with NSF. The upper bound of the 95% confidence interval was 0.07%, suggesting that incidence using group II GBCAs is quite low (64).
While the clinical significance remains in question, administration of GBCAs has been shown to deposit gadolinium in the brain, skin, and bones of individuals with normal renal function (65–68). Initial reports demonstrated gadolinium retention in deep gray matter structures (e.g., the globus pallidus and dentate nucleus) in human brain tissue based on MRI (65, 66). There have been several systematic reviews and an international conference led by the National Institutes of Health (69–71). Retention of gadolinium can be visualized on unenhanced T1-weighted MRI as increased signal intensity, most commonly in the dentate nucleus of the cerebellum and the globus pallidus, with some reports of much lesser wide-spread brain deposition (72). This increase is most commonly found after multiple infusions of the linear contrast agents, but also to a lesser extent with the macrocyclic agents. While the reasons for highest signal in the globus pallidus and dentate remain unclear, both structures are rich in iron and are associated with multiple metal-transporter-mediated conditions (72). Studies in rats have consistently implicated a greater degree of deposition for linear than macrocyclic GBCAs, although the human data has been less consistent (59). This is an area of active study with new clinical recommendations including decreasing the use of linear agents, limiting the dosage of gadolinium to the least amount needed, and caution in vulnerable populations (70).
Although “gadolinium-deposition disease” (GDD) was first proposed in 2016, it is not widely accepted as a distinct disease with a proven pathophysiology (73, 74). Proposed symptoms should begin within two months of GBCA administration, are broad and nonspecific, and include neuropathic pain, joint stiffness, muscle spasms, buzzing sensation, fatigue, clouded mentation, and skin thickening, discoloration, and pain, although the exact criteria remain poorly described and no validation studies of any criteria have been published (75). While GDD remains unproven, it nevertheless represents a significant medicolegal risk concern (75) and mitigation strategies have been proposed (59, 75).
Conclusions
In conclusion, complex metal ions are a very diverse group of compounds within the official classification of poisons. As noted above, these agents can have both life-saving and lethal consequences. As equally diverse as their index of lethality so are their effects within the CNS. Examples describes here ranged from cessation of mitochondrial respiration, particularly within the basal ganglia to inhibition of cellular division and apoptotic cellular death. Given the predilection of many poisons for the basal ganglia, neuropsychiatric symptoms often follow. Thus, it is necessary to include poison/toxin exposure in a neuropsychiatric assessment. Emerging imaging techniques such as diffusion tensor imaging or diffusion kurtosis may help to elicit a deeper understanding of not only the possible brain injuries occurring after a poisoning, but also further insight into healthy functional brain networks.
1 : Differential diagnosis for bilateral abnormalities of the basal ganglia and thalamus. Radiographics 2011; 31:5–30Crossref, Medline, Google Scholar
2 : Diagnostic approach in patients with symmetric imaging lesions of the deep gray nuclei. Neurologist 2003; 9:250–261Crossref, Medline, Google Scholar
3 : Biochemical mechanisms of cyanide toxicity, in Toxicology of Cyanides and Cyanogens: Experimental, Applied and Clinical Aspects. Edited by Hall AH, Isom GE, Hoboken, NJ, John Wiley and Sons, 2015, pp 70–81Crossref, Google Scholar
4 : A review of acute cyanide poisoning with a treatment update. Crit Care Nurse 2011; 31:72–81, quiz 82Crossref, Medline, Google Scholar
5 : Oldest poison pushes back ancient civilization 20,000 years. https://www.livescience.com/21961-oldest-poison-tools-stone-age-humans.htmlGoogle Scholar
6
7
8
9 : Occupational cyanide poisoning. BMJ Case Rep 2011; 2011:bcr0920114865Crossref, Medline, Google Scholar
10 : Occupational exposure to solvents: neuropsychiatric and imaging features. J Neuropsychiatry Clin Neurosci 2015; 27:1–6Link, Google Scholar
11 : Toxic leukoencephalopathy and hypokalemia due to exposure to trimethyltin. J Clin Neurol 2017; 13:298–299Crossref, Medline, Google Scholar
12 : Surviving acute cyanide poisoning: a longitudinal neuropsychological investigation with interval MRI. BMJ Case Rep 2014; 2014:bcr2013203025Crossref, Medline, Google Scholar
13 : MR changes after acute cyanide intoxication. AJNR Am J Neuroradiol 2002; 23:1398–1401Medline, Google Scholar
14 National Research Council (US) Subcommittee on Acute Exposure Guideline Levels: Hydrogen cyanide: acute exposure guideline levels, in Acute Exposure Guideline Levels for Selected Airborne Chemicals. Washington, DC, National Academies Press, 2002Google Scholar
15 : Sodium nitroprusside in 2014: a clinical concepts review. J Anaesthesiol Clin Pharmacol 2014; 30:462–471Crossref, Medline, Google Scholar
16 : Nitroprusside-related cyanide poisoning: time (long past due) for urgent, effective interventions. Chest 1992; 102:1842–1845Crossref, Medline, Google Scholar
17
18
19
20 : Focus on smoke inhalation: the most common cause of acute cyanide poisoning. Prehosp Disaster Med 2006; 21:s49–s55Crossref, Medline, Google Scholar
21 : Toxicity of fire smoke. Crit Rev Toxicol 2002; 32:259–289Crossref, Medline, Google Scholar
22 : Dystonic-Parkinsonian syndrome after cyanide poisoning: clinical and MRI findings. J Neurol Neurosurg Psychiatry 1988; 51:1345–1348Crossref, Medline, Google Scholar
23 : Cyanide-induced parkinsonism: a clinicopathologic report. Neurology 1985; 35:921–925Crossref, Medline, Google Scholar
24 : Visual loss caused by acute cyanide poisoning: a case report. Clin Toxicol (Phila) 2011; 49:121–123Crossref, Medline, Google Scholar
25 : Delayed onset generalised dystonia after cyanide poisoning. Clin Neurol Neurosurg 1995; 97:213–215Crossref, Medline, Google Scholar
26 : Sequelae of attempted suicide by cyanide ingestion: a case report. Int J Psychiatry Med 1990; 20:173–179Crossref, Medline, Google Scholar
27 : Experimental cyanide encephalopathy. AMA Arch Pathol 1959; 67:306–323Medline, Google Scholar
28 : Cyanide-induced parkinsonism: clinical, MRI, and 6-fluorodopa PET studies. Neurology 1989; 39:142–144Crossref, Medline, Google Scholar
29 : Neurological sequelae of cyanide intoxication: the patterns of clinical, magnetic resonance imaging, and positron emission tomography findings. Ann Neurol 1995; 38:825–828Crossref, Medline, Google Scholar
30 : The value of morphological neuroimaging after acute exposure to toxic substances. Toxicol Rev 2006; 25:87–98Crossref, Medline, Google Scholar
31 : Long-term neuropsychiatric sequelae in a survivor of cyanide toxicity patient with arterialization. Cureus 2020; 12:e8430Medline, Google Scholar
32 : Cyanide and the human brain: perspectives from a model of food (cassava) poisoning. Ann N Y Acad Sci 2016; 1378:50–57Crossref, Medline, Google Scholar
33 : Konzo: a distinct neurological disease associated with food (cassava) cyanogenic poisoning. Brain Res Bull 2019; 145:87–91Crossref, Medline, Google Scholar
34 : Tropical myeloneuropathies: the hidden endemias. Neurology 1985; 35:1158–1170Crossref, Medline, Google Scholar
35 : Health implications of cassava production and consumption. J. Agric. Soc. Res. JASR 2011; 11:118–125Google Scholar
36 : A new unifying hypothesis for lathyrism, konzo and tropical ataxic neuropathy: nitriles are the causative agents. Food Chem Toxicol 2011; 49:563–570Crossref, Medline, Google Scholar
37 : Neuropsychological effects of konzo: a neuromotor disease associated with poorly processed cassava. Pediatrics 2013; 131:e1231–e1239Crossref, Medline, Google Scholar
38 : Leber’s hereditary optic neuropathy with late disease onset: clinical and molecular characteristics of 20 patients. Orphanet J Rare Dis 2014; 9:158Crossref, Medline, Google Scholar
39 : Tobacco-alcohol amblyopia: a diagnostic dilemma. J Neurol Sci 2013; 327:41–45Crossref, Medline, Google Scholar
40 : Metal complexes in cancer therapy: an update from drug design perspective. Drug Des Devel Ther 2017; 11:599–616Crossref, Medline, Google Scholar
41 : Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: a retrospective evaluation. Oncol Rep 2013; 29:1285–1292Crossref, Medline, Google Scholar
42 : Overview of cisplatin-induced neurotoxicity and ototoxicity, and the protective agents. Food Chem Toxicol 2020; 136:111079Crossref, Medline, Google Scholar
43 : Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 2014; 740:364–378Crossref, Medline, Google Scholar
44 : Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol 2009; 63:761–767Crossref, Medline, Google Scholar
45 : Cisplatin neurotoxicity in the treatment of metastatic germ cell tumour: time course and prognosis. Br J Cancer 2001; 85:823–826Crossref, Medline, Google Scholar
46 : Mechanisms of cisplatin-induced ototoxicity and prevention. Semin Hear 2019; 40:197–204Crossref, Medline, Google Scholar
47 : Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J Clin Oncol 2016; 34:2712–2720Crossref, Medline, Google Scholar
48 : Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med 2018; 378:2376–2385Crossref, Medline, Google Scholar
49 : A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996; 334:494–500Crossref, Medline, Google Scholar
50 : Cisplatin neurotoxicity presenting as reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol 1998; 19:415–417Medline, Google Scholar
51 : The double-edged sword: neurotoxicity of chemotherapy. Blood Rev 2015; 29:93–100Crossref, Medline, Google Scholar
52 : Convulsions and transient cortical blindness after cisplatin. BMJ 1991; 302:416Crossref, Medline, Google Scholar
53 : Subacute encephalopathic toxicity of cisplatin. Lung Cancer 1995; 13:305–309Crossref, Medline, Google Scholar
54 : Reversible posterior leukoencephalopathy syndrome following combinatorial cisplatin and pemetrexed therapy for lung cancer in a normotensive patient: a case report and literature review. Oncol Lett 2016; 11:1512–1516Crossref, Medline, Google Scholar
55 : Cisplatin-induced posterior reversible encephalopathy syndrome and successful re-treatment in a patient with non-seminomatous germ cell tumor: a case report. J Med Case Reports 2012; 6:409Crossref, Medline, Google Scholar
56 : Posterior reversible encephalopathy syndrome in patients with cancer. Oncologist 2015; 20:806–811Crossref, Medline, Google Scholar
57 : Lower cognitive performance and white matter changes in testicular cancer survivors 10 years after chemotherapy. Hum Brain Mapp 2015; 36:4638–4647Crossref, Medline, Google Scholar
58 : Changes in brain structural networks and cognitive functions in testicular cancer patients receiving cisplatin-based chemotherapy. J Natl Cancer Inst 2017; 109:djx085Crossref, Google Scholar
59 :
60 : MRI contrast agents: basic chemistry and safety. J Magn Reson Imaging 2012; 36:1060–1071Crossref, Medline, Google Scholar
61 : Effect of different classes of gadolinium-based contrast agents on control and nephrogenic systemic fibrosis-derived fibroblast proliferation. Radiology 2010; 256:735–743Crossref, Medline, Google Scholar
62 : Nephrogenic systemic fibrosis. Am J Nephrol 2009; 29:1–9Crossref, Medline, Google Scholar
63 : Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology 2008; 248:807–816Crossref, Medline, Google Scholar
64 : Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: a systematic review and meta-analysis. JAMA Intern Med 2020; 180:223–230Crossref, Medline, Google Scholar
65 : Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 2014; 49:685–690Crossref, Medline, Google Scholar
66 : High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270:834–841Crossref, Medline, Google Scholar
67 : Gadolinium tissue deposition in brain and bone. Magn Reson Imaging 2016; 34:1359–1365Crossref, Medline, Google Scholar
68 : High levels of gadolinium deposition in the skin of a patient with normal renal function. Invest Radiol 2016; 51:280–289Crossref, Medline, Google Scholar
69 : Gadolinium deposition and the potential for toxicological sequelae: a literature review of issues surrounding gadolinium-based contrast agents. Br J Clin Pharmacol 2018; 84:2522–2534Crossref, Medline, Google Scholar
70 : Gadolinium retention: a research roadmap from the 2018 NIH/ACR/RSNA Workshop on Gadolinium Chelates. Radiology 2018; 289:517–534Crossref, Medline, Google Scholar
71 : The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity: a systematic review. PLoS One 2017; 12:e0171704Crossref, Medline, Google Scholar
72 : Gadolinium deposition in the brain: current updates. Korean J Radiol 2019; 20:134–147Crossref, Medline, Google Scholar
73 : Gadolinium deposition disease: initial description of a disease that has been around for a while. Magn Reson Imaging 2016; 34:1383–1390Crossref, Medline, Google Scholar
74 : Gadolinium-based contrast agents: What is the evidence for “gadolinium deposition disease” and the use of chelation therapy? Clin Toxicol (Phila) 2020; 58:151–160Crossref, Medline, Google Scholar
75 : Gadolinium deposition disease: a new risk management threat. J Am Coll Radiol 2020; 17:546–550Crossref, Medline, Google Scholar



