Cognitive and Behavioral Sequelae of Closed Head Injury in Older Adults According to Their Significant Others
Abstract
This study examined the neurobehavioral effects of closed head injury (CHI) in older adults according to their significant others. Informants of 17 mild and moderate CHI patients ≥50 years old when injured completed the Geriatric Evaluation of Relative's Rating Instrument, a questionnaire inquiring about the patient's cognition, affect, interpersonal relations, and daily activities. The significant others provided retrospective ratings of preinjury functioning and completed the same instrument an average of 4 and 13 months postinjury. The significant others of 10 community-residing, normal control subjects completed the questionnaire at comparable intervals between each rating. Compared with their preinjury functioning, and unlike the control subjects, patients showed declines in cognition and mood. The possible impact of these changes, including their effect on subjective burden in caregivers, is discussed.
The neurobehavioral effects of closed head injury (CHI) in young adults include alterations in cognition, mood, and social functioning that reduce the quality of life for both the patient and significant others.1–7 These changes often disrupt rehabilitation efforts, return to work, and family relationships.4,5,8,9 Significant others find the emotional and cognitive sequelae such as slowness, irritability, and memory deficit more stressful than the physical symptoms.8,10 Early research by Oddy et al.7 reported that relatives of severely injured patients who were 6 months postinjury described changes in the patients encompassing poor memory and concentration, as well as impatience and irritability. Many of these symptoms were present 7 years later.11 More recent studies have also confirmed the high incidence of behavioral and cognitive changes after CHI. Dikmen et al.12 had survivors of moderate to severe CHI rate their functioning at 1 month, 1 year, and 2 years postinjury. At 1 month, the greatest problems concerned fatigue, memory disturbance, dizziness, and concentration. Memory problems and irritability persisted at 1 and 2 years. Schalén et al.13 examined survivors of severe CHI who had good recoveries or moderate disabilities on the Glasgow Outcome Scale.14 At 5 to 8 years postinjury, relatives reported symptoms in the patients that included hostile feelings, poor memory, and fatigue.
These previous studies have characterized the outcome of predominantly young survivors of head injury. As a result, little is known about the neurobehavioral sequelae and their pattern of recovery in older patients who are in their fifth decade and beyond. Although the incidence of head injury is highest in persons 18 to 30 years of age, epidemiological studies reveal that head injury also poses a significant problem for older persons.15–17 Moreover, studies in young adults have tended to focus on patients with severe CHI. Although mortality following CHI rises with age,18,19 a number of older adults with mild and moderate injuries may survive and thus require postacute rehabilitation and ongoing family supervision. Thus, it is important to examine whether a similar pattern of behavioral changes is found in this older at-risk population.
In an earlier review of the neurobehavioral sequelae in older CHI patients,20 we described the findings regarding the cognitive and behavioral changes reported by significant others of 13 patients ≥50 years old who were in the initial 5 months of recovery following mild and moderate CHI. Significant others completed the Geriatric Evaluation by Relative's Rating Instrument (GERRI).21 Compared with their preinjury status, patients were rated as exhibiting significant declines in cognition, mood, and social functioning. The most salient changes included decreased memory, comprehension, and concentration as well as increased restlessness and irritability. In the current study, long-term follow-up to 2 years postinjury is described. We hypothesized that compared with a demographically similar group of control subjects without head injuries, the patients with mild and moderate CHI would still show significant postinjury impairments in cognition, mood, and social functioning.
METHODS
Subjects
The sample included 17 CHI patients (mean age=69.9 yr, SD=11.5; mean education=11.7 yr, SD=3.8). They were prospectively recruited from acute care neurosurgery services of hospitals affiliated with Emory University School of Medicine in Atlanta and the University of Maryland School of Medicine in Baltimore. To be included in the study, patients had to have ratings made at three time periods (preinjury and two follow-up occasions). Five patients sustained mild head injuries, defined as Glasgow Coma Scale (GCS)22 scores of 13–15 with loss of consciousness lasting less than 20 minutes and normal neurologic and neuroradiologic findings on computed tomography (CT) or magnetic resonance imaging. The remaining 12 patients sustained moderate injuries, classified as GCS scores of 9–12, or 13–15 if there was evidence on CT of focal neurologic deficits or brain lesions (contusions, hematomas). The primary mechanism of injury was motor vehicle accidents (n=11), 5 patients were injured in falls, and 1 patient was a pedestrian injured in a street accident. All of the patients were functioning independently at the time of their accidents, and none resided in personal care or nursing homes.
A comparison group of 10 community-residing control subjects (mean age=74.7 yr, SD=10.6; mean education=13.4 yr, SD=2.1) was recruited. There were no significant differences (P>0.05) between the patient and control groups in age, education, or distribution of gender.
Patients and control subjects did not have premorbid histories of drug or alcohol abuse, psychiatric disturbance, or neurologic illness including significant head injury. In addition, both the patients and the control subjects were free of preexisting dementia as assessed by family ratings on the Blessed Dementia Rating Scale,23 a questionnaire that inquires about changes in cognition, personality, and activities of daily living within the past 6 months. The scores were within normal limits for both groups (range 0–3.5 for patients, 0–2 for control subjects). The procedures were explained to all subjects prior to their participation, and informed consent was obtained by using protocols approved by the Human Investigations Committees of the participating centers.
Instrument
The Geriatric Evaluation by Relative's Rating Instrument was developed by Schwartz21 to measure cognitive and behavioral changes in community-residing outpatients in their fifth decade of life or older. The GERRI is completed by a significant other and contains 49 items, which are scaled from 1 (almost all the time) to 5 (almost never). In addition, a rating of 6 (does not apply) is given if the behavior is never performed (e.g., pays bills with checks). Specific items on the GERRI are combined to yield domains measuring cognition (20 items), mood (10 items), and social functioning (19 items). In a sample of patients with Alzheimer's disease, Schwartz21 found that the GERRI had high interrater reliability and validity in discriminating patients with mild, moderate, and severe dementia.
Procedures
The GERRI was completed by a significant other on three different occasions. Initially, the significant other was asked to rate the patient's preinjury behaviors in terms of their frequency of occurrence. The average time from injury to the preinjury GERRI rating was 40 days (SD=35.1). The questionnaire was then mailed to the same informant an average of 123 days postinjury (SD=71.3) and later at an average of 390 days (SD=88.5) postinjury. The informant was asked on these two follow-up occasions to provide ratings based on the patient's current functioning. Of 22 patients who had a preinjury and an initial follow-up GERRI, 17 (77%) also had a GERRI available at the last postinjury occasion. None of the patients were in the hospital at the time of their follow-up ratings.
A significant other for each control subject completed the GERRI based on the subject's current functioning. The GERRI was then mailed to the significant other at two follow-up intervals. The number of days separating the first and second ratings (mean±SD: 70.8±69.7 for patients, 32.7±22.9 for control subjects) and second and third ratings (240.2±81.5 for patients, 215.7±52.8 for control subjects) was comparable between the two groups, P>0.05.
RESULTS
Composite Scores
Items were combined to form domains representing cognition, mood, and social functioning, using the clusters delineated by Schwartz.21 Scoring of certain items was reversed to provide a uniform rating system. Separate repeated-measures analyses of variance (ANOVAs) were performed on the three GERRI domains, with group (CHI, Control) as the between-subjects factor and time (first, second, and third rating) as the within-subjects factor. Two-tailed levels of significance were used throughout.
Figure 1 shows the mean GERRI ratings for patients and control subjects as a function of time. Higher ratings indicate a worsening in performance. Analysis of the Cognitive subscale revealed a significant main effect of time (F=4.20, df=2,50, P<0.05), as well as a significant interaction between group and time (F=4.25, df=2,50, P<0.05). Analysis of the simple main effects demonstrated a significant difference among the mean ratings of patients (F=6.20, df=2,32, P<0.01). Post hoc paired t-tests revealed that the scores of patients were significantly higher (i.e., worse; P<0.05) at their initial postinjury and long-term follow-up assessments when compared with their preinjury functioning (first rating). In contrast, the scores of the control subjects were not significantly different among the three time periods (F=1.29, df=2,18, P>0.05), suggesting a relative stability of functioning. One-way ANOVAs comparing the patients and control subjects at each time period revealed significantly worse functioning for the patients at the initial postinjury rating (F=4.89, df=1,25, P<0.05), but not at the preinjury or long-term follow-up ratings.
A repeated-measures ANOVA performed on the Mood subscale indicated a significant main effect of time (F=5.81, df=2,50, P<0.01), with scores higher (i.e., worse) at the last assessment than at the first (P<0.05) and second (P<0.05) and a worsening (P=0.05) between the preinjury and initial postinjury scores. Despite the appearance in Figure 1 that the scores of patients but not control subjects tended to worsen over time, there was no significant interaction (F=1.74, df=2,50, P>0.05). However, analysis of each group separately did reveal a significant difference among the mean ratings for patients (F=5.72, df=2,32, P<0.01), but not for control subjects (F=0.47, df=2,18, P>0.05). Paired t-tests in the patient group indicated a significant difference for the mood ratings at preinjury (first occasion) versus both of the postinjury follow-up occasions (P<0.05), with a trend (P=0.08) for a continued worsening between the latter two occasions. The patients' mean GERRI ratings did not significantly differ from the control subjects' at any time period.
The analysis of the Social Functioning subscale revealed nonsignificant main effects of group and time, and no interaction between these factors (P>0.05). Analysis of the simple main effects for each group separately indicated a trend (P=0.08) for the patients' ratings to change over time compared with the control subjects' (P=0.78). However, the patients' mean GERRI ratings did not significantly differ from the control subjects' at any time period.
Responses to Individual Items
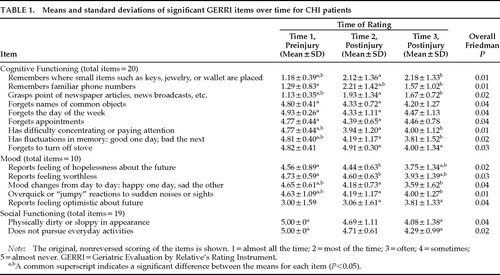
Individual GERRI items for patients comparing their preinjury and two postinjury ratings were analyzed with nonparametric Friedman tests to determine those behaviors that were most affected. Table 1 shows the overall significant items (P<0.05) and their mean values at each occasion. The original untransformed mean values are displayed. Items in the cognitive domain included declines in memory, comprehension, and attention. Mood changes were also noted by significant others, encompassing feelings of hopelessness and worthlessness. Changes in physical appearance and pursuit of activities were affected as well after the head injury. Follow-up comparisons (Wilcoxon signed-rank tests) of the significant item scores at the three occasions were conducted to examine the pattern of changes over time. A common superscript for each item in Table 1 indicates a significant (P<0.05) difference between two occasions. As seen, 8 of the 9 behaviors in the cognitive domain significantly worsened from the preinjury assessment to the immediate postinjury follow-up, and 2 of the 9 behaviors (remembers familiar phone numbers, forgets to turn off stove) worsened from the first to the second follow-up occasions. In contrast, 2 of the 5 mood behaviors and none of the 2 social functioning behaviors were rated as worse at the immediate postinjury occasion when compared with preinjury functioning. However, a worsening between the preinjury and the last postinjury occasion was noted for 6 of the 7 combined mood and social functioning items.
Table 2 displays the results of the analyses of differences in the GERRI items over time for the control subjects. Friedman tests revealed that only 5 items were rated as significantly different over time. As can be seen, however, none of these items were the same as those endorsed as changing in the CHI patients. In addition, post hoc Wilcoxon signed-rank tests indicated nonsignificant differences for any behaviors between the first and second ratings, unlike the findings for patients. Most of the differences occurred between the first and third or second and third ratings.
DISCUSSION
The results of this study indicate that older adults sustaining mild and moderate CHI show cognitive and behavioral changes, according to their significant others, that are similar to those seen in young adults following more severe head injury.7,8,10,11,13 Most of these sequelae encompass cognitive difficulties that we have previously documented via significant others' reports in the initial 5 months of recovery as well as objective neuropsychological evaluations in this older group.20,24 Changes in mood were also noted, including feelings of hopelessness and worthlessness. Other investigators2,4–6,25 have observed that these alterations in young survivors lead to considerable family stress and disruption of relationships. Although subjective burden was not measured in the current study, it is likely that similar stresses are found in the significant others of these older patients and that these stresses, in turn, affect their perceptions of the patients' functioning on the three GERRI domains.
Analysis of the specific items on the GERRI revealed different patterns of temporal onset in the patient group for the domains of cognition, mood, and social functioning. Extension of our previous study using the GERRI20 to a longer follow-up period indicated that some changes were not initially appreciated by significant others. Whereas many of the changes in cognition were rated as immediately noticeable after the injury, some of the mood and social functioning changes were not apparent until the last postinjury rating. In addition, feelings of hopelessness and worthlessness as well as lack of optimism about the future were rated as worsening from the initial to the longer postinjury follow-ups. This trend suggests that depression may have a delayed onset in this population, similar to findings in young survivors.26,27 Alternatively, the relatives' own levels of depression may increase over time, and this in turn may affect their ratings of the patient's mood. Further distinction of these two possibilities would require independent and objective evaluation of the patients' and control subjects' moods and the way in which these correlate with the GERRI ratings. A finding via independent ratings that patients' moods worsened over time would underscore the importance of assessing depression at several points in recovery following CHI in order to detect associations that may not be immediately apparent. Studies in young CHI patients demonstrate that depression is associated with poor cognitive recovery, diminished social relationships, and reduced quality of life.28–30 In our own work,31 we have documented a high frequency of depression, approximately 30%, in older adults on the basis of a self-report inventory (Geriatric Depression Scale32). In addition, higher scores on the Geriatric Depression Scale, indicative of greater depression, predict attentional and memory functioning an average of 7 and 13 months postinjury, but not at the initial 1-month assessment.33
The postinjury changes reported by the significant others of the CHI patients were actually quite mild when compared with estimates of the patients' preinjury status. Although there were clear alterations in functioning following the head injury, an inspection of the mean values indicated that significant others noted rather slight repercussions. Moreover, with the exception of the cognitive domain score, the patients were not rated as being significantly more impaired than the control subjects, even though there were significant changes over time in the overall scores of the patients. The lack of more pronounced changes could be related to our inclusion of patients with mild/moderate as opposed to severe injuries. As we noted above, most studies in young adults have focused on relatives' ratings of patients with severe CHI. In addition, our patients were carefully screened for preexisting major health problems, depression, and cognitive impairments, and thus they comprised a rather well-functioning group prior to their injuries. Finally, the GERRI ratings, as with any self-report measures, depend on the accuracy of the informants. In a sample of patients with head injuries or orthopedic injuries sustained 5 to 10 years previously, we found that relatives tended to underestimate the extent of the patients' current cognitive problems when compared with objective evaluation of these patients.34 The significant others in the current study may likewise have minimized the extent of alterations in patient functioning.
On a methodological note, our findings highlight the importance of including a control group for comparison with a head-injured sample. Item analyses indicated that variations in functioning over time were also reported for the control subjects, although the specific GERRI items differed between the groups. In a study of subjective complaints after mild head injury, Dikmen et al.35 found that control subjects without head injury reported a considerable number of physical and behavioral symptoms. These trends, also noted in other research, suggest that a failure to incorporate non–head-injured control subjects may provide an inaccurate assessment of initial outcome and recovery.
In summary, the results of this study reveal parallels between the types of neurobehavioral changes reported by significant others of older adults and the findings in outcome from more severe CHI in young persons. Although our sample is not representative of all older adults sustaining CHI, our findings do paint a more positive picture as opposed to the generally pessimistic portrayal in the literature.36 Future studies should examine the impact of these alterations of older adults' capacity for independent functioning. In addition, assessment of the impact of these alterations on caregiver stress and burden should be investigated to identify possible supportive needs and interventions.
ACKNOWLEDGMENTS
This research was supported by National Institute on Disability and Rehabilitation Research Field-Initiated Research Grant H133G30051 and by NS21889, the Javits Neuroscience Investigator Award.
 |
 |

FIGURE 1. Mean ratings on the Geriatric Evaluation by Relative's Rating Instrument for the cognitive, mood, and social functioning domains as a function of group and time of rating.
1 Brooks DN, McKinlay W: Personality and behavioural change after severe blunt head injury: a relative's view. J Neurol Neurosurg Psychiatry 1983; 46:336–344Crossref, Medline, Google Scholar
2 Brooks DN, Campsie L, McKinlay WW: The five year outcome of severe blunt head injury: a relative's view. J Neurol Neurosurg Psychiatry 1986; 49:764–770Crossref, Medline, Google Scholar
3 Grant I, Alves W: Psychiatric and psychosocial disturbances in head injury, in Neurobehavioral Recovery From Head Injury, edited by Levin HS, Grafman J, Eisenberg HM. New York, Oxford University Press, 1987, pp 232–261Google Scholar
4 Kreutzer JS, Gervasio AH, Camplair PS: Primary caregivers' psychological status and family functioning after traumatic brain injury. Brain Inj 1994; 8:197–210Crossref, Medline, Google Scholar
5 Kreutzer JS, Gervasio AH, Camplair PS: Patient correlates of caregivers' distress and family functioning after traumatic brain injury. Brain Inj 1994; 8:211–230Crossref, Medline, Google Scholar
6 Livingston MG, Brooks DN, Bond MR: Patient outcome in the year following severe head injury and relatives' psychiatric and social functioning. J Neurol Neurosurg Psychiatry 1985; 48:876–881Crossref, Medline, Google Scholar
7 Oddy M, Humphrey M, Uttley D: Subjective impairment and social recovery after closed head injury. J Neurol Neurosurg Psychiatry 1978; 4l:611–616Google Scholar
8 Jacobs HE: The Los Angeles Head Injury Survey: procedures and initial findings. Arch Phys Med Rehabil 1988; 69:425–431Medline, Google Scholar
9 Prigatano GP: Personality and psychosocial consequences of brain injury, in Neuropsychological Rehabilitation After Brain Injury, edited by Prigatano GP, Fordyce DJ, Zeener HK, et al. Baltimore, Johns Hopkins University Press, 1986, pp 29–50Google Scholar
10 Brooks DN: Head injury and the family, in Closed Head Injury: Psychological, Social, and Family Consequences, edited by Brooks DN. Oxford, UK, Oxford University Press, 1984, pp 123–147Google Scholar
11 Oddy M, Coughlan T, Tyerman A, et al: Social adjustment after closed head injury: a further follow-up seven years after injury. J Neurol Neurosurg Psychiatry 1985; 48:564–568Crossref, Medline, Google Scholar
12 Dikmen S, Machamer T, Temkin N: Psychosocial outcome in patients with moderate to severe head injury: 2-year follow-up. Brain Inj 1993; 7:113–124Crossref, Medline, Google Scholar
13 Schalén W, Hansson L, Nordstrom G, et al: Psychosocial outcome 5–8 years after severe traumatic brain lesions and the impact of rehabilitation services. Brain Inj 1994; 8:49–64Crossref, Medline, Google Scholar
14 Jennett B, Bond M: Assessment of outcome after severe brain damage. Lancet 1975; 1:480–484Crossref, Medline, Google Scholar
15 Frankowski RF: Descriptive epidemiologic studies of head injury in the United States: 1974–1983. Adv Psychosom Med 1986; 16:153–172Crossref, Medline, Google Scholar
16 Kraus J: Epidemiology of head injury, in Head Injury, edited by Cooper PR. Baltimore, Williams and Wilkins, 1987, pp 1–19Google Scholar
17 Kraus JF, McArthur DL: Epidemiologic aspects of brain injury. Neuroepidemiol 1996; 14:435–450Google Scholar
18 Luerssen TG, Klauber MR, Marshall LF: Outcome from head injury related to patient's age: a longitudinal prospective study of adult and pediatric head injury. J Neurosurg 1988; 68:409–416Crossref, Medline, Google Scholar
19 Vollmer DG, Torner JC, Jane JJ, et al: Age and outcome following traumatic coma: why do older persons fare worse? J Neurosurg 1991; 75:537–549Google Scholar
20 Goldstein FC, Levin HS: Neurobehavioral outcome of traumatic brain injury in older adults: initial findings. Journal of Head Trauma Rehabilitation 1995; 10:57–73Crossref, Google Scholar
21 Schwartz GE: Development and validation of the Geriatric Evaluation by Relative's Rating Instrument (GERRI). Psychol Rep 1983; 53:479–488Crossref, Medline, Google Scholar
22 Teasdale G, Jennett B: Assessment of coma and impaired consciousness: a practical scale. Lancet 1974; 2:81–84Crossref, Medline, Google Scholar
23 Blessed G, Tomlinson BE, Roth M: The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 1968; 114:797–811Crossref, Medline, Google Scholar
24 Goldstein FC, Levin HS, Presley RM, et al: Neurobehavioral consequences of closed head injury in older adults. J Neurol Neurosurg Psychiatry 1994; 57:961–966Crossref, Medline, Google Scholar
25 Brooks N, Campsie L, Symington C, et al: The effects of head injury on patient and relative within seven years of injury. Journal of Head Trauma Rehabilitation 1987; 2:1–13Crossref, Google Scholar
26 Fordyce DJ, Roueche JR, Prigatano GP: Enhanced emotional reactions in chronic head trauma patients. J Neurol Neurosurg Psychiatry 1983; 46:620–624Crossref, Medline, Google Scholar
27 Jorge RE, Robinson RG, Arndt SV, et al: Comparison between acute- and delayed-onset depression following traumatic brain injury. J Neuropsychiatry Clin Neurosci 1993; 5:43–49Link, Google Scholar
28 Bornstein RA, Miller HB, Van Schoor JT: Neuropsychological deficit and emotional disturbance in head-injured patients. J Neurosurg 1989; 70:509–513Crossref, Medline, Google Scholar
29 Holbrook TL, Hoyt DB, Anderson JP, et al: Functional limitation after major trauma: a more sensitive assessment using the Quality of Well-Being Scale—The Trauma Recovery Project. J Trauma 1994; 38:74–78Crossref, Google Scholar
30 MacNiven E, Finlayson MAJ: The interplay between emotional and cognitive recovery after closed head injury. Brain Inj 1993; 7:241–246Crossref, Medline, Google Scholar
31 Levin HS, Goldstein FC, MacKenzie E: Depression as a secondary condition following mild and moderate traumatic brain injury. Seminars in Clinical Neuropsychiatry 1997; 2:207–215Medline, Google Scholar
32 Yesavage JA, Brink TL, Lum O, et al: Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1983; 17:37–49Crossref, Medline, Google Scholar
33 Goldstein FC, Levin HS, Clark AN, et al: Depression and cognitive functioning in older adults with traumatic brain injuries. J Int Neuropsychol Soc 1998; 4:9Google Scholar
34 Goldstein FC, Clark A, McGuire C, et al: Detection of dementia: comparison of informant reports versus objective patient screening (abstract). J Neuropsychiatry Clin Neurosci 1997; 9:173Google Scholar
35 Dikmen S, McLean A, Temkin N: Neuropsychological and psychosocial consequences of minor head injury. J Neurol Neurosurg Psychiatry 1986; 49:1227–1232Crossref, Medline, Google Scholar
36 Mazzucchi A, Cattelani R, Missale G, et al: Head-injured subjects aged over 50 years: correlations between variables of trauma and neuropsychological follow-up. J Neurol 1992; 239:256–260Medline, Google Scholar



