Correlates of Executive Function in Multiple Sclerosis:
Abstract
Proton magnetic resonance spectroscopy (MRS) was performed in a group of patients with multiple sclerosis (MS) and matched control subjects to examine the relationship between frontal lobe pathology and performance on tests of executive function. The N-acetyl aspartate/creatine ratio (NAA/Cr) was significantly reduced in frontal lesions and/or normal-appearing white matter in the patient group compared with the control group, but choline/creatine ratios did not differ. Although MRS abnormalities and executive deficits were not correlated for MS patients as a group, a few patients with more severe abnormalities of NAA/Cr ratio performed worse than other patients on the spatial working memory test, suggesting that subtle frontal neuropathological abnormalities detected by MRS may contribute to executive deficits. Further investigation is warranted to determine the value of MRS as an index of the pathophysiological processes leading to cognitive deficit.
Studies using proton magnetic resonance spectroscopy (MRS) in the characterization of plaques in multiple sclerosis (MS) have reported a reduction in the N-acetyl aspartate/creatine ratio (NAA/Cr) in both acute and chronic lesions.1–4 NAA is an abundant amino acid in the human brain, not only present within the cell body, but also distributed throughout the axon and proximal dendrites.5 It is thus considered to be a marker of neuronal integrity. MRS abnormalities have also been demonstrated in normal-appearing white matter (NAWM) of MS patients with a reduction of NAA/Cr ratios.6 It has been suggested that these MRS abnormalities may be due to microscopic pathology7 and that MRS is therefore potentially sensitive to the presence of subtle white matter abnormalities.
Cognitive deficits in the areas of attention, memory, and executive function, with relative preservation of language functions,8–10 have been reported in MS. Correlations have also been reported between MRI indices such as ventricular brain ratios, T2-weighted lesion load, and size of corpus callosum and neuropsychological impairment.11,12 Correlations between specific neuropsychological deficits and detectable pathology in appropriate regions of the brain12,13 have been more problematic, and a recent study by our group14 has highlighted the difficulty of this approach in the presence of widespread brain pathology.
At best, the correlation between lesion load and neuropsychological deficits is only modest, and it may be argued that conventional MRI is not sensitive enough to distinguish between different pathological processes such as edema, gliosis, and axonal and myelin loss, which are likely to have different functional effects. The use of other techniques, such as MRS, that are potentially sensitive to neuronal integrity and subclinical demyelination is therefore of considerable interest.
To date, only a few studies have used proton MRS to examine the biochemical correlates of neuropsychological performance in patients with various types of brain disease, and the results have been conflicting. One study of patients with right temporal lobectomy for epilepsy reported that those who had impaired verbal memory had decreased NAA/(choline+creatine) ratios on proton MRS in the contralateral temporal lobe,15 and another study suggested that temporal creatine levels correlated with memory deficits in schizophrenia.16 However, others have found no such associations in patients with systemic lupus erythematosus17 or in cognitively impaired HIV-seropositive patients.18
The aim of our study was to examine the value of MRS as compared to conventional MRI in examining neuropathological changes in the frontal lobes and their relationship to executive function. A battery of executive tests similar to that used in our previous study14 was administered to all subjects.
METHODS
Subjects
Twenty-five patients (10 male, 15 female) with clinically definite MS according to the criteria of Poser et al.19 were recruited from the outpatient clinics and the neurorehabilitation unit at the National Hospital of Neurology and Neurosurgery. Their ages ranged between 24 and 50 years. Patients were excluded from the study if their visual acuity was less than 6/12 or if they were unable to use a computer touchscreen. Patients were also excluded if they were experiencing a relapse (defined as the development of new signs or worsening of existing signs within the past month) or if they were significantly depressed on clinical interview. The same patients participated in a parallel study investigating the correlation between neuropsychological deficits and lesion load.14
Thirty-eight healthy control subjects (18 male, 20 female) were chosen to match the patient group as closely as possible with respect to age, gender, and estimated premorbid IQ. Any subject whose alcohol intake exceeded the recommended levels (21 units for males and 14 units for females per week) was excluded from the study. Informed consent was obtained from all subjects.
Assessment of Physical Disability and Psychiatric Symptoms
Physical disability was assessed on the Kurtzke Expanded Disability Status Scale (EDSS).20
The Hospital Anxiety and Depression Scale (HAD)21 was administered to all subjects. This self-rating scale has subscales for anxiety and depression (range 0–21 for each). HAD scores greater than 10 on either scale were considered indicative of “caseness.”
MRI and MRS
MRI and MRS were performed on a Signa 1.5-tesla GE scanner using a standard quadrature head coil. The study commenced with a T2-weighted fast spin-echo sequence (TR=3,000 ms, TEf=80 ms). A series of 36 contiguous, axial slices (3 mm thickness) was selected for measurement of lesion volume.
After imaging was completed, a volume of interest (VOI) ranging between 3.5 ml and 6 ml was prescribed from the left frontal white matter in a position anterior to the anterior horn of the left lateral ventricle to avoid any partial volume effects from gray matter and ventricle. In the patient group, the VOI incorporated a high-signal lesion and an area of normal-appearing white matter. Large lesions were chosen in order to minimize partial volume effects. If no lesions could be identified on imaging, spectra were collected from a VOI of frontal NAWM. Water-suppressed spectra were obtained by using a stimulated echo acquisition mode (STEAM) sequence.22 Acquisition parameters were TR 2,000 ms, TE 135 ms, and TM 12 ms. A total of 256 averages were collected by using an 8-step phase cycle in about 9 minutes. A total of 1,024 points were collected with a spectral width of 750 Hz. Shimming to a line width of about 1.5 Hz and water suppression were reoptimized for each new location. In the control group, spectra were obtained exclusively from an area of left frontal white matter.
The three resonances visible at an echo-time of 135ms were assigned as follows: N-acetyl groups at 2.02 ppm and 2.6 ppm, creatine/phosphocreatine at 3.04 ppm, and choline-containing compounds at 3.2 ppm.23,24 Data processing included 1.5-Hz line broadening for filtering and baseline correction (cubic spline). Peak areas were determined by using a line-fitting program (SA/GE, G.E., Milwaukee, WI). Peaks were fitted to Gaussian functions by use of a Marquardt fitting procedure. The peak area ratios of NAA and choline relative to creatine were calculated.
Neuropsychological Assessment
Premorbid IQ was estimated by using the National Adult Reading Test (NART).25 Current intellectual ability was assessed with the Advanced Progressive Matrices (APM).26 This was a set of 12 nonverbal abstract reasoning problems; the number of problems correctly completed was converted to an age-adjusted scaled score, which was used as a measure of current intellectual ability.
The following tests of executive function were administered:
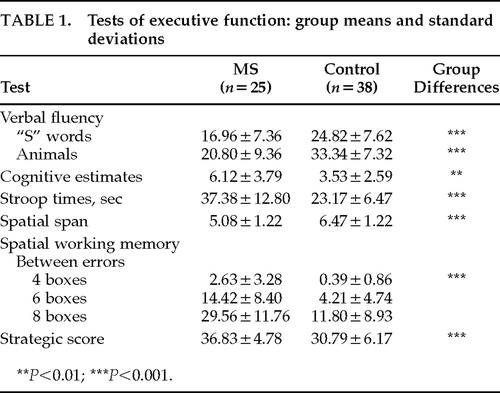
Verbal Fluency Test. The subject was required to generate as many words as possible (excluding proper nouns) beginning with the letter S in 90 seconds and as many animals as possible in 90 seconds. The two scores obtained were the total number of acceptable words generated for each condition.
Cognitive Estimates Test.27 Each subject was required to make estimates in response to 10 questions such as “What is the length of the average man's spine?” or “What is the largest object normally found in a house?” Estimates were scored according to normative data (range 0–3); higher scores reflected worse performance.
Stroop Test.28 A computerized version of this test was used. In the test condition, names of colors printed in different colored ink were presented on the screen. The time taken for the subject to name the color in which each word was printed was recorded.
Three further tests from the CANTAB29 battery were administered on a computer with a touch-sensitive screen:
Spatial Span. This assessed subjects' ability to remember a sequence of boxes lighting up on a computer screen. The spatial span was calculated as the longest sequence that the subject could recall accurately on at least one of three trials.
Spatial Working Memory. The subjects were required to search for blue tokens hidden within a number of boxes on the screen by touching the boxes in turn until the token was located. They were instructed that the blue token would not be hidden in the same box it was located in previously. Two types of error were recorded: returning to a box in which a blue token had previously been located (between error) and returning to a box previously shown to be empty during the same search (within error). The “between errors” is considered to be a more stringent measure. Four trials were presented at each level of difficulty (three-, four-, six- and eight-box problems).
Tower of London. In this planning task, two sets of colored balls in suspended socks were presented on the computer screen. The subject was instructed to rearrange the balls in the lower display to copy the pattern in the upper one. A minimum of two, three, four, and five moves was required to solve each problem, and subjects were instructed to attempt to solve the problem in the minimum number of moves. The times taken to solve each problem were recorded, and the initial and subsequent thinking (planning) times were calculated for each level of difficulty.
Statistical Analysis
The data were analyzed by using the Statistical Package for the Social Sciences (SPSS). Group means were examined by using independent t-tests and analysis of variance (ANOVA). Where appropriate, nonparametric tests (Mann-Whitney, Kruskal-Wallis) were used. Partial correlation analysis was used to examine the relationship between MRS abnormalities and performance on executive tests.
RESULTS
There were no significant differences between MS patients and control subjects with respect to age (mean age 37.52 and 36.16 years, respectively). Sixteen patients had secondary progressive MS, 6 had relapsing/remitting MS, and 3 had primary progressive MS. The mean EDSS score for the patient group was 6.44.
The group mean HAD scores did not reach “caseness” (scores>10) on the depression or anxiety scale in either patients or control subjects.
MRS
The NAA/Cr ratio in the volume of interest for the MS group was significantly reduced compared with the control group (t=–4.19, P<0.001). The ratio for patients (mean±SD) was 1.15±0.25 for patients and 1.39±0.19 for control subjects. Results are illustrated in Figure 1. There was no significant difference in the choline/creatine ratio between MS patients and control subjects (mean ratios 1.16 and 1.06, respectively).
In 16 patients, the VOI contained lesions and NAWM. In 9 patients, the VOI contained NAWM only. We did not find a significant difference in the NAA/Cr ratios between the two groups. The group NAA/Cr ratios (mean±SD) were 1.16±0.29 and 1.18±0.12, respectively. Furthermore, there were no significant differences in the NAA/Cr ratios between the subgroups of primary progressive, secondary progressive, and relapsing/remitting patients.
As previously reported,14 frontal lesion load ranged from 177 to 65,019 mm3 and correlated highly with total lesion load (r=0.96, P<0.001).
Neuropsychological Performance
There were no significant differences between patients and control subjects in premorbid IQ (means 110.28 and 113.21, respectively). Performance on the APM, taken to indicate current intellectual ability, was significantly worse in the MS group (z=–6.36, P<0.001), suggesting a significant intellectual decline in the patient group. MS patients performed significantly worse than control subjects on the verbal fluency, cognitive estimates, Stroop, spatial span, and spatial working memory tests when the APM score was used a covariate in the data analysis. These results indicate that the patients' poor performance on executive tests could not be fully explained as a result of general intellectual impairment. The results are summarized in Table 1.
No significant correlations between the NAA/Cr ratios and executive test scores, after controlling for current intellectual ability, were found in MS patients as a group. There were also no significant correlations between the NAA/Cr ratio and frontal or total lesion load. These results were similar whether the VOI contained lesions or only NAWM. No significant correlations were present between choline/Cr ratios and the executive test scores. Correlations were also not significant between MRS findings and the executive test scores in the control group. No significant correlations were observed between physical disability and MRS findings.
To examine the contribution of frontal MRS abnormalities to executive deficits, we categorized MS patients into three subgroups based on the mean NAA/Cr ratio of the control group: Group A (n=9), NAA/Cr ratio within 1 SD of the mean ratio for control subjects; Group B (n=11), NAA/Cr ratio between 1 and 2 SD; and Group C (n=5), NAA/Cr ratio below 2 SD.
The performance in the executive tests was compared between the three groups. Significant group differences were found in the spatial working memory test scores, as shown in Table 2. Further analysis indicated that Group C performed significantly worse than the other two groups on this test. There were no significant differences in frontal lesion load between the groups. In Group C, the VOI contained lesions in 4 patients and only NAWM in 1 patient. Furthermore, the HAD anxiety and depression scores did not reach caseness in any of the subgroups, indicating that psychiatric symptoms were unlikely to have contributed to neuropsychological performance.
DISCUSSION
In this study we have confirmed that MRS abnormalities are present in MS patients. Although there was no significant correlation between the executive deficits and frontal NAA/Cr ratio in the MS patients as a group, there was some evidence that patients with more severe abnormalities of NAA/Cr ratio performed worse on particular executive tests.
This study has several limitations that should be considered in interpreting these findings. First, the VOI may not accurately reflect the general biochemical profile or neuropathological abnormalities in other frontal areas. The use of NAA/Cr ratios may have also underestimated the magnitude of NAA changes; postmortem studies30 have reported that the absolute concentration of creatine is decreased within the MS lesions, and it is possible that absolute quantification of metabolites31 might have yielded more significant results. Furthermore, the number of patients in this study who were found to have severe abnormalities of NAA/Cr ratio was very small.
For the purpose of our study, we measured MRS abnormalities in a VOI of the frontal lobe that included both lesions and NAWM, since previous studies have detected abnormalities in both.6 However, given the variable location and size of the lesions, in some patients the VOI contained only NAWM. To find no significant difference in NAA/Cr ratio between the two subgroups was unexpected, but perhaps this finding may be explained by the facts that the NAWM surrounding lesions is abnormal and the difference between T2 lesions and NAWM in MRS is not a large one.
An interesting observation in this study was that a small number of patients who had more severe abnormalities in NAA/Cr ratio performed significantly worse than the other patients on the more difficult levels of the spatial working memory test. Given that there were no differences in frontal lesion load among the three groups, the deficit in working memory performance in these patients would suggest that MRS may reflect the presence of more subtle pathological processes that also contribute to cognitive deficits.
Further investigation in a larger sample to confirm our findings is warranted. It is also likely that MRS may be more useful in detecting changes in MS lesions and normal-appearing white matter over time, and these may more accurately reflect longitudinal changes in cognition.
ACKNOWLEDGMENTS
The authors are grateful to Drs. M. Maier, L. Wang, and other members of the NMR group at the Institute of Neurology, London, for their assistance. They also thank all the patients and control subjects who participated in this study. This work was supported by a grant from the MS Society of Great Britain and Northern Ireland. Professor Ron was partly funded by the SCARFE Trust.
 |
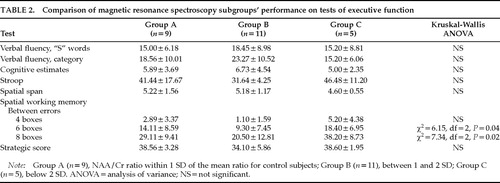 |
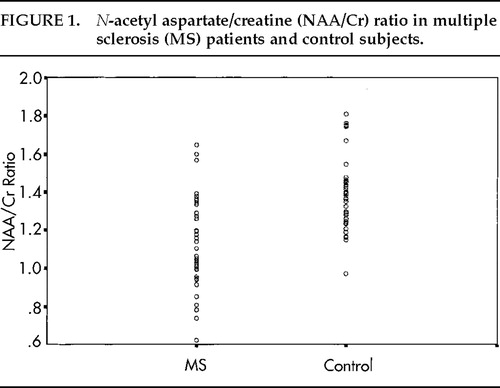
FIGURE 1. N-acetyl aspartate/creatine (NAA/Cr) ratio in multiple sclerosis (MS) patients and control subjects.
1 Arnold DL, Matthews PM, Francis GS, et al: Proton magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann Neurol 1992; 31:235–241Crossref, Medline, Google Scholar
2 Matthews PM, Francis G, Antel J, et al: Proton magnetic resonance spectroscopy for metabolic characterization of plaques in multiple sclerosis. Neurology 1991; 41:1251–1256Crossref, Medline, Google Scholar
3 Miller DH, Austin SJ, Connelly A, et al: Proton magnetic resonance spectroscopy of an acute and chronic lesion in multiple sclerosis (letter). Lancet 1991; 337:8–9Google Scholar
4 Van Hecke P, Marchal G, Johannik K, et al: Human brain proton localized NMR spectroscopy in multiple sclerosis. Magn Reson Med 1991; 18:199–206Crossref, Medline, Google Scholar
5 Simmons ML, Frondoza CG, Coyle JT: Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience 1991; 45:37–45Crossref, Medline, Google Scholar
6 Davie CA, Hawkins CP, Barker GJ, et al: Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain 1994; 117:49–58Crossref, Medline, Google Scholar
7 Allen IV: Pathology of multiple sclerosis, in McAlpine's Multiple Sclerosis, 2nd edition, edited by Matthews WB, Compston A, Allen IV, et al. Edinburgh, Churchill Livingstone, 1991, pp 341–378Google Scholar
8 Beatty WW, Goodkin DE, Beatty PA: Frontal lobe dysfunction and memory impairment in patients with chronic progressive multiple sclerosis. Brain Cogn 1989; 11:73–86Crossref, Medline, Google Scholar
9 Litvan I, Grafton J, Vendrell P, et al: Multiple memory deficits in patients with multiple sclerosis: exploring the working memory system. Arch Neurol 1988; 45:607–610Crossref, Medline, Google Scholar
10 Rao SM, Leo GJ, Bernardin L, et al: Cognitive dysfunction in multiple sclerosis: frequency, patterns and prediction. Neurology 1991; 41:685–691Crossref, Medline, Google Scholar
11 Rao SM, Leo GJ, St. Aubin-Faubert P: On the nature of memory disturbance in multiple sclerosis. J Clin Exp Neuropsychol 1989; 11:699–712Crossref, Medline, Google Scholar
12 Swirsky-Sacchetti T, Mitchell DR, Seward J, et al: Neuropsychological and structural brain lesions in multiple sclerosis: a regional analysis. Neurology 1992; 42:1291–1295Crossref, Medline, Google Scholar
13 Arnett PA, Rao SM, Bernardin L, et al: Relationship between frontal lobe lesions and Wisconsin Card Sorting Test performance in patients with multiple sclerosis. Neurology 1994; 44:420–425Crossref, Medline, Google Scholar
14 Foong J, Rozewicz L, Quaghebeur G, et al: Executive function in multiple sclerosis: the role of frontal lobe pathology. Brain 1997; 120:15–26Crossref, Medline, Google Scholar
15 Incisa-della-Rocchetta A, Gadian DG, Connelly A, et al: Verbal memory impairment after right temporal lobe surgery: role of contralateral damage as revealed by 1H magnetic resonance spectroscopy and T2 relaxometry. Neurology 1995; 45:797–802Crossref, Medline, Google Scholar
16 Buckley PF, Moore C, Long H, et al: 1H-Magnetic resonance spectroscopy of the left temporal and frontal lobes in schizophrenia: clinical, neurodevelopmental and cognitive correlates. Biol Psychiatry 1994; 36:792–800Crossref, Medline, Google Scholar
17 Davie CA, Feinstein A, Kartsounis LD, et al: Proton magnetic resonance spectroscopy of systemic lupus erythematosus involving the central nervous system. J Neurol 1995; 242:522–528Crossref, Medline, Google Scholar
18 Meyerhoff DJ, MacKay S, Poole N, et al: N-acetyl aspartate reductions measured by 1H MRS in cognitively impaired HIV-seropositive individuals. Magn Reson Imaging 1994; 12:653–659Crossref, Medline, Google Scholar
19 Poser CM, Paty DW, Schember L, et al: New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983; 13:227–331Crossref, Medline, Google Scholar
20 Kurtzke JF: Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33:1444–1452Crossref, Medline, Google Scholar
21 Zigmond AS, Snaith RP: The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1984; 67:361–370Google Scholar
22 Frahm J, Bruhn H, Gyngell ML, et al: Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn Reson Med 1989; 9:79–93Crossref, Medline, Google Scholar
23 Behar KL, Ogino T: Assignment of resonance in the 1H spectrum of rat brain by two-dimensional shift correlated and J-resolved NMR spectroscopy. Magn Reson Med 1991; 17:285–303Crossref, Medline, Google Scholar
24 Frahm J, Bruhn H, Gyngell ML, et al: Localized proton NMR spectroscopy in different regions of the human brain in vivo: relaxation times and concentrations of cerebral metabolites. Magn Reson Med 1989; 11:47–63Crossref, Medline, Google Scholar
25 Nelson H, Willison J: The National Adult Reading Test (NART), 2nd edition, Windsor, UK, NFER-Nelson, 1991Google Scholar
26 Raven JC: Advanced Progressive Matrices, Set 1: Manual. London, H K Lewis, 1958Google Scholar
27 Shallice T, Evans ME: The involvement of the frontal lobes in cognitive estimation. Cortex 1978; 14:294–303Crossref, Medline, Google Scholar
28 Stroop JR: Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18:643–662Crossref, Google Scholar
29 Sahakian BJ, Owen AM: Computerised assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med 1992; 85:399–402Medline, Google Scholar
30 Davies SEC, Newcombe J, Williams SR, et al: High resolution proton NMR spectroscopy of multiple sclerosis lesions. J Neurochem 1995; 64:742–748Crossref, Medline, Google Scholar
31 Maier M, Ron MA, Barker GJ, et al: Proton magnetic resonance spectroscopy: an in vivo method of estimating hippocampal neuronal depletion in schizophrenia. Psychol Med 1995; 25:1201–1209Crossref, Medline, Google Scholar



