Neurological Function During Long-Term Therapy With Recombinant Interferon Alpha
Abstract
In 14 patients with myeloproliferative disorders associated with thrombocytosis, neurological and neuropsychological function were monitored prior to therapy with recombinant human interferon alpha (rIFN; dose 25 mU/week; range 10–35 mU/week) and after 3, 6, 9, and 12 months of treatment. No overt neurological side effects were observed except 1 case of cerebral insult, probably not treatment-related. Attention, memory function, and tapping improved significantly. The P2–N3 amplitudes of visually evoked potentials increased during normalization of platelet counts. Muscular strength and the amplitude of the compound muscle action potential of the median and peroneal nerves increased significantly in an inversely dose-related fashion. In patients with myeloproliferative disorders, long-term therapy with low to intermediate doses of rIFN does not seem to impair neurological function, but rather is associated with enhanced muscle power and the level of mental arousal.
For almost two decades now, human interferon alpha has been cloned and produced by recombinant DNA technology.1 Its wide clinical application in the treatment of various neoplastic diseases and viral infections has yielded partial and complete remissions in hematological disorders such as hairy cell leukemia; in chronic myeloid leukemia; in some other chronic myeloproliferative and lymphoproliferative disorders; and in chronic hepatitis.2–6 Partial success could be achieved in malignant melanoma and hypernephroma,7–9 and reports on trials in the treatment of AIDS-related Kaposi's sarcoma with recombinant human interferon alpha (rIFN) are promising.10–12
Interferon therapy is known to induce some undesirable side effects.13,14 A transient flu-like syndrome including fever, chills, fatigue, and muscle pain occurs frequently during the initial treatment phase. In addition, leukopenia, elevated transaminases, hypotension, tachycardia, and neurotoxic symptoms—mainly confusion and somnolence—have been reported. Most of these side effects are strongly dose-dependent and generally prove to be reversible after dose reduction or termination of the treatment.
Only a few studies have specifically dealt with the neurological side effects of rIFN therapy. They either concerned short-term application of high doses15–17 or reported single observations in patients under low-dose rIFN.18,19 High doses induced severe neurological side effects—such as paresthesia, the development of an encephalitis-like syndrome, somnolence, lethargy, impaired concentration, and disorientation—and were associated with marked diffuse disturbances in the EEG.20,21 Treatment with low-dose rIFN resulted in either no neurological side effects at all or rare incidences of lethargy, disorientation, and paresthesia.21
Our investigation pertains to neurological side effects of long-term low-dose rIFN therapy, the most successful rIFN regimen applied in the treatment of hematological neoplasias. We have studied patients with myeloproliferative disorders, afflictions that are known to respond to rIFN therapy22 and that render the patients susceptible to microcirculatory disturbances. The aim of our study was to evaluate the effects of long-term rIFN therapy on selected parameters of neurological and neuropsychological function.
METHODS
Patients
Fourteen patients (12 females, 2 males; median age 64.5 years, range 46–85) suffering from essential thrombocythemia (ET), polycythemia vera (PV), or myelodysplastic syndrome (MDS) received long-term rIFN therapy for 12 months (Table 1). None of them presented with known cerebral disease, accompanying infections, or metabolic diseases, nor did any patient require primarily cerebrally active substances, chemotherapy, hormonal therapy, or radiation therapy during the study period. Informed consent was obtained from all patients to participate in the study.
Recombinant interferon alpha 2C (Boehringer-Ingelheim; Germany) was administered subcutaneously three times a week. The initial median rIFN dose was 25 mU/week; dosage was then individually adjusted according to response and tolerance (Table 1).
Prior to rIFN therapy and every 3 months during the treatment, the following examinations were carried out:
| 1. | Clinical neurological examination. | ||||
| 2. | A battery of four psychometric tests: a) attention load test according to Brickenkamp,23 which examines visual attentional load independent of intelligence (the letters b and d, with one or two bars above or below, were arranged in two lines of 47 characters; patients were asked to mark all letters b—or, alternatively, all letters d—containing two bars); b) recall of 10 two-digit numbers, regardless of the sequence, after all numbers had been recited and immediately repeated by the patient; c) arithmetic: three multiplication tasks, two involving one-digit numbers and one involving two-digit numbers; and d) tapping with a pencil for 30 seconds at individual speed without any procedural instructions about speed and amplitude of tapping. | ||||
| 3. | Measurement of maximal strength in elbow flexion at an angle of 90°. The forearm was to be pulled isometrically with maximal strength against a dynamometer; the mean of three trials was calculated. | ||||
| 4. | A routine EEG (19 electrodes, 16 channels, International 10–20 System, unipolar and bipolar recordings) over a 20-minute period including hyperventilation and stroboscopic stimulation. EEG abnormalities were classified in three grades: marginal abnormal (slight slowing), moderate abnormal (significant slowing, mainly theta activity), and pronounced abnormal (theta and delta activity). | ||||
| 5. | Visually evoked potentials (VEP) stimulated by the projection of a checkerboard pattern at a distance of 1 m (visual angle individual field 50′). EEG was derived from an electrode at the midline, 5 cm above the inion, referenced to a vertex electrode. Analysis period was 500 ms, bandpass 0.5–200 Hz; averages of 100 stimuli per eye were performed by means of a DISA 2000 averager (Dantec, Copenhagen, Denmark). P2 latencies and P2-N3 amplitudes were recorded. Normal values according an own investigation were: P2 latencies <107 ms (95% confidence interval [CI]); P2-N3 amplitudes >6.9 μV (95% CI). | ||||
| 6. | Electroneurographic studies on the maximal motor nerve conduction velocity (NCV), the distal latency, and the amplitude of the compound muscle action potential (CMAP-amplitude) for the median and the peroneal nerve. In addition, the sensory antidromic nerve conduction velocity was determined for the median nerve. | ||||
All examinations listed above are routine clinical procedures with established ranges of normal findings.24 Normal values (“age”=years of age): NCV: median nerve (elbow–wrist), 64.482−(0.23×age) m/s, SD 3.338; peroneal nerve (knee–wrist), 55.941−(0.155×age) m/s, SD 4.055; distal latency: median nerve, 2.994+(0.004×age) ms, SD 0.392; peroneal nerve, 3.519+(0.0013×age) ms, SD 0.549; CMAP-amplitude, median and peroneal nerves, >4,000 μV; sensory antidromic conduction velocity, median nerve (wrist–dig II), 71.99−(0.3×age) m/s, SD 4.86. Partial response of disease was defined by a drop in platelet number that was greater than 25% of the initial value but that did not result in a platelet count less than 440 g/l; complete remission was defined by a normalization of platelet counts below 440 g/l.
Statistical Evaluation
All changes during rIFN therapy were calculated as percentages of the initial values, each patient serving as his or her own control. Evaluations of statistical significance were performed by using Student's t-test for paired observations, and partial correlations on Kendall's tau-b correlation coefficients were calculated with the aid of an IBM computer. The dimension “duration of remission” originated at the individual time of remission; periods before this point in time were represented as negative numbers. Patients who failed to achieve remission throughout the year's observation period were assigned a value of 52 weeks.
RESULTS
rIFN dosage started at a median of 25 mU/week (range 10–35) and was steadily decreased to median values of 20 mU/week (range 10–25), 17.5 mU/week (range 10–25), 15 mU/week (range 6–25), and 12 mU/week (range 6–25) at months 3, 6, 9, and 12, respectively. Normalization of platelet counts was achieved by 12 patients after a median time of 7.5 weeks (range 3–39) of rIFN therapy (Table 1).
The clinical neurological status was normal in all patients before the start of rIFN therapy. Patient #5 had an ischemic cerebral insult after 16 days of treatment, resulting in motor aphasia and a right hemiparesis, accentuated in the arm. Under continued interferon treatment, these symptoms showed an excellent remission tendency and subsided during the following month. None of the other patients manifested overt neurological symptoms. In particular, no signs of polyneuropathy could be detected.
The results of psychometric testing and muscular strength testing are depicted in Figure 1. The indices of attention (pre-IFN: median 1, range 0–14) and memory (pre-IFN: median 3.5, range 0–8) had improved significantly (P<0.01 and P<0.05, respectively) by the third month of treatment. The median speed of spontaneous tapping (pre-IFN: median 69, range 20–120) almost doubled, reaching significance (P<0.05) after 6 months. Although the initial results of the arithmetic test covered the entire range of possible correct answers (median 2.5, range 0–3), they remained almost identical on retesting. Muscular strength (pre-IFN: median 17 kp (kilopond), range 9–22) increased only slightly, but consistently enough to yield significant results (P<0.05) after 6 months of rIFN treatment.
Prior to rIFN therapy, EEG was normal in 5 patients but revealed marginal, moderate, and pronounced signs of abnormality in 5, 3, and 1 patients, respectively (Table 1). After 9 months of treatment, Patient #9 showed moderate diffuse pathological changes without any clinical correlates. None of the other patients' findings changed significantly (data not shown).
All parameters of electroneurography were within the normal range. Figure 2 shows the results of VEP. Although the P2 latencies remained constant, P2-N3 amplitudes—after a slight decrease (not significant) during the first 3 months—increased significantly (P<0.05) after 6 months of rIFN therapy.
The CMAP-amplitude of the peroneal nerve (pre-IFN: median 6,500 μV, range 4,600–15,000) had increased significantly (P<0.05) by the sixth month of treatment; the equivalent increase of the median nerve (pre-IFN: median 12,500 μV, range 6,600–15,000) reached the level of significance only after 12 months of rIFN therapy (P<0.1 for months 6 and 9). Neither motor (pre-IFN: median nerve, median 55.5 m/s, range 51–62; peroneal nerve, median 46 m/s, range 39–54) nor sensory antidromic NCV, nor distal latency (pre-IFN: median nerve, median 3.9 ms, range 3.4–4.8; peroneal nerve, median 4.75 ms, range 3.4–6) changed significantly during the course of treatment (Figure 3). Regarding the observed significant changes, it must be emphasized that practically all improved values still remained within normal limits.
Evaluation of significant correlations (P<0.05) focused on rIFN dosage and duration of remission, eliminating the intercorrelation between these two parameters. Attention, memory, and tapping correlated with remission duration (r=0.620, r=0.607, and r=0.461, respectively). On the other hand, the CMAP-amplitude (median nerve, r=–0.535; peroneal nerve, r=–0.375, P<0.1) and strength (r=–0.721) inversely correlated with the dose of rIFN. No correlation was found with the parameters of visually evoked potentials.
DISCUSSION
Our results basically confirm the rare incidence of toxic neurological side effects of rIFN that has been reported by several authors.13,18,19 The only clinically overt neurological manifestation observed in our patient group (one case of a cerebral insult) occurred during an early stage of induction therapy and under the highest dosage applied in this study. After dosage reduction, the patient recovered and eventually achieved remission. However, at the start of rIFN treatment the patient was 66 years old, suffered from polycythemia vera with excessive thrombocytosis, and presented with pronounced pathological signs in his EEG. The treatment association of this neurological affection during rIFN therapy, therefore, remains open to question.
Although 13 of 14 patients showed no neurological deficits during rIFN therapy, even subclinical impairments of brain functions, such as vigilance, could be hazardous to the patient or detrimental to his or her well-being. Two psychometric studies15,25 revealed slight to moderate deteriorations of various psychological functions, and one group25 reported changes in emotional life and personality within the first week of treatment. Since our investigational design did not include the earliest stages of rIFN therapy, we cannot rule out similar effects in our patient population. During long-term treatment, however, attention and short-term memory improved, whereas a learned higher mental capacity, arithmetic, was not affected. Attention, memory function, and tapping correlated significantly with the duration of remission. An improvement of these higher brain functions may be due to a gain in the level of arousal as an effect of the rIFN treatment, an effect similar to that observed by Mapou et al.26
Enhanced arousal could also explain the observed increases in the P2-N3 amplitude of VEP. Considering the report of pathological VEP under high-dose rIFN,21 our observation of a small, statistically insignificant drop in the amplitude at month 3 could indicate some initial detrimental effect of rIFN.
Electroneurography showed that sensory and maximal motor NCVs remained essentially unchanged. These findings are consistent with reports in the literature.21 CMAP-amplitudes increased significantly, as did muscular strength. The improvement in these electrophysiological parameters might be influenced by several factors, such as decreasing dosages of rIFN during the course of therapy, duration of rIFN exposure, or improvement of platelet counts.
Our patients were afflicted with a disease entity that rendered them prone to disturbances of the microcirculation. Successful treatment, therefore, is likely to have benefited the brain tissue and may even have overcome possible subduing side effects of rIFN. Previous observations of microcirculatory disturbances in the extremities of patients treated for thrombocytosis in myeloproliferative diseases have shown highly significant improvements after achievement of complete remission.22 The timing of this therapeutic effect was comparable to the currently observed gain in muscle power and increase of CMAP-amplitudes.
Our positive findings of an absence of serious cognitive dysfunctions during long-term rIFN are in line with the results of a large randomized quality-of-life study in myeloma patients.27 In that study, IFN-treated patients did not observe a reduction of their cognitive and emotional functions in comparison to the control group. The present findings are at variance with the conventional wisdom that IFN commonly causes cognitive dysfunction. Although all observed changes remain at a subclinical level, the tests performed in the present study may be useful tools in monitoring the effects of rIFN therapy in patients with microcirculatory impairments. They also show that in these patients, long-term therapy with low-dose rIFN does not increase the risk of neurological or neuropsychological side effects, but rather diminishes it.
 |
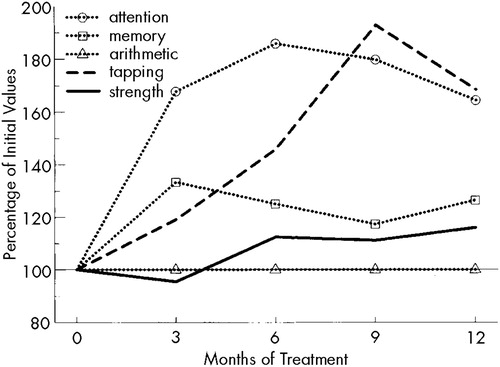
FIGURE 1. Changes in the results of psychometric testing, tapping speed, and muscular strength during rIFN therapy
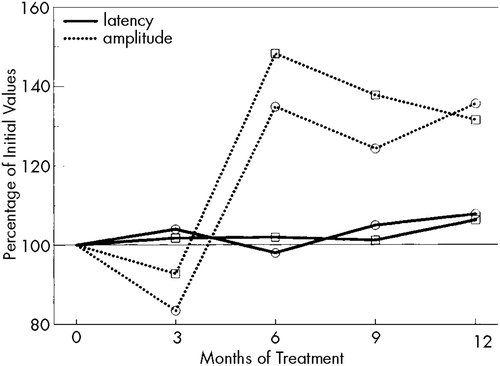
FIGURE 2. Changes in the parameters of visual evoked potentials during rIFN therapyCircles: right eye; squares: left eye.
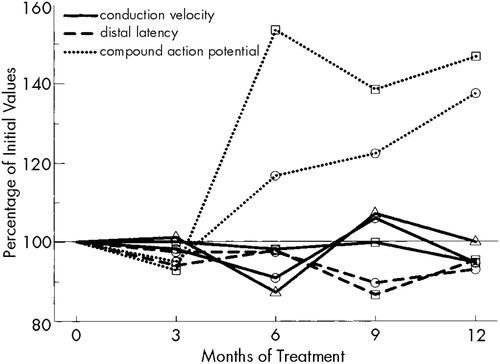
FIGURE 3. Changes in the results of electroneurographic examinations during rIFN therapyCircles: median nerve; squares: peroneal nerve; triangles: sensory antidromic nerve conduction velocity in the median nerve.
1 Goedel DV, Yelverton E, Ullrich A, et al: Human leukocyte interferon produced by E. coli is biologically active. Nature 1980; 287:611–616Google Scholar
2 Smalley RV, Connors J, Tuttle RL, et al: Splenectomy vs. alpha interferon: a randomized study in patients with previously untreated hairy cell leukemia. Am J Hematol 1992; 41:13–18Crossref, Medline, Google Scholar
3 Kumar L, Gulati SC: Alpha-interferon in chronic myelogenous leukemia. Lancet 1995; 346:984–986Crossref, Medline, Google Scholar
4 Taylor PC, Dolan G, Ng JP, et al: Efficacy of recombinant interferon-alpha (rIFN-α) in polycythaemia vera: a study of 17 patients and an analysis of published data. Br J Haematol 1996; 92:55–59Crossref, Medline, Google Scholar
5 Gisslinger H, Chott A, Scheithauer W, et al: Interferon in essential thrombocythemia. Br J Haematol 1991; 79(suppl):42–47Google Scholar
6 Rabinovitz M, Block G, Finkelstein SD: Alpha-interferon retreatment of patients with chronic hepatitis C. Am J Gastroenterol 1996; 91:1523–1526Google Scholar
7 Hofmockel G, Langer W, Theiss M, et al: Immunochemotherapy for metastatic renal cell carcinoma using a regimen of interleukin-2, interferon-alpha and 5-fluorouracil. J Urol 1996; 156:18–21Crossref, Medline, Google Scholar
8 Tsavaris N, Mylonakis N, Bacoyiannis C, et al: Treatment of renal cell carcinoma with escalating doses of alpha-interferon. Chemotherapy 1993; 39:361–366Crossref, Medline, Google Scholar
9 Kirchner HH, Atzpodien J, Poliwoda H: Chemo-/immunotherapy in advanced malignant melanoma: carboplatin and DTIC or cisplatin, DTIC, BCNU and tamoxifen followed by immunotherapy with interleukin-2 and interferon alpha-2a. Med Klin 1996; 91:44–49Google Scholar
10 Skillman DR, Malone JL, Decker CF, et al: Phase I trial of interferon alpha-n3 in early stage human immunodeficiency virus type 1 disease: evidence for drug safety, tolerance and antiviral activity. J Infect Dis 1996; 173:1107–1114Google Scholar
11 Lane HC: Interferons in HIV and related diseases. AIDS 1994; 8:19–23Crossref, Google Scholar
12 Rozenbaum W, Gharakhanian S, Navarette MS, et al: Long-term follow-up of 120 patients with AIDS-related Kaposi's sarcoma treated with interferon alpha-2a. J Invest Dermatol 1990; 95:161S-165SCrossref, Medline, Google Scholar
13 Jones GJ, Itri LM: Safety and tolerance of recombinant interferon alpha-2a (Roferon-A) in cancer patients. Cancer 1986; 57:1709–1715Google Scholar
14 Wandl UB, Nagel-Hiemke M, May D, et al: Lupus-like autoimmune disease induced by interferon therapy for myeloproliferative disorders. Clin Immunol Immunopathol 1992; 65:70–74Crossref, Medline, Google Scholar
15 Mattson K, Niiranen A, Laaksonen R, et al: Psychometric monitoring of interferon neurotoxicity. Lancet 1984; 1:275–276Crossref, Medline, Google Scholar
16 Rohatiner AZS, Prior PF, Burton AC, et al: Neurological effects of recombinant interferon (letter). British Medical Journal 1983; 286:1054Medline, Google Scholar
17 Valentina AD, Meyers CA, Talpaz M: Treatment of neurotoxic side effects of interferon-alpha with naltrexone. Cancer Invest 1995; 13:561–566Crossref, Medline, Google Scholar
18 Crols R, Lowenthal A: Long-term intramuscular recombinant DNA interferon alpha 2 therapy in subacute sclerosing panencephalitis: reduction of serum measles antibodies without clinical improvement. Eur Neurol 1987; 27:72–77Crossref, Medline, Google Scholar
19 Honigsberger L, Fielding JW, Priestmann TJ: Neurological effects of recombinant human interferon. British Medical Journal 1983; 286:719Crossref, Medline, Google Scholar
20 Smedley H, Katrak M, Sikora K, et al: Neurological effects of recombinant human interferon. Briitish Medical Journal 1983; 286:262–264Crossref, Medline, Google Scholar
21 Iivanainen M, Laaksonen R, Niemi M-L, et al: Memory and psychomotor impairment following high-dose interferon treatment in amyotrophic lateral sclerosis. Acta Neurol Scand 1985; 72:475–480Crossref, Medline, Google Scholar
22 Gisslinger H, Ludwig H, Linkesch W, et al: Long-term interferon therapy for thrombocytosis in myeloproliferative diseases. Lancet 1989; 1:634–637Crossref, Medline, Google Scholar
23 Brickenkamp R: Test d2: Aufmerksamkeit-Belastungstest. Göttingen, Verlag für Psychologie Hogrefe, 1962Google Scholar
24 Ludin HP: Praktische Elektromyographie. Stuttgart, Ferdinand Enke Verlag, 1993, pp 145 ffGoogle Scholar
25 Adams F, Quesada JR, Guttermann JU: Neuropsychiatric manifestations of human leukocyte interferon therapy in patients with cancer. JAMA 1984; 252:938–941Crossref, Medline, Google Scholar
26 Mapou RL, Law WA, Wagner K, et al: Neuropsychological effects of interferon alfa-n3 treatment in asymptomatic human immunodeficiency virus-1–infected individuals. J Neuropsychiatry Clin Neurosci 1996; 8:74–81Link, Google Scholar
27 Wisloff F, Hjorth M: Health-related quality of life assessed before and during chemotherapy predicts for survival in multiple myeloma: Nordic Myeloma Study Group. Br J Haematol 1997; 97:29–37Crossref, Medline, Google Scholar



