Interictal 18FDG PET Findings in Temporal Lobe Epilepsy With Déjà vu
Abstract
The authors studied the functional anatomy of the déjà vu (DV) experience in nonlesional temporal lobe epilepsy (TLE), using interictal fluorine-18 fluorodeoxyglucose PET in 14 patients with and 17 patients without DV. Several clinical conditions, such as age at PET study, side of ictal onset zone, and dominance for language, were no different between the two groups. The patients with DV showed significant relative reductions in glucose metabolism in the mesial temporal structures and the parietal cortex. The findings demonstrate that ictal DV is of no lateralizing value. They further suggest that temporal lobe dysfunction is necessary but not sufficient for the generation of DV. Extensive association cortical areas may be involved as part of the network that integrates this distinct experience.
Déjà vu (DV) is defined as any subjectively inappropriate impression of familiarity of a present experience with an undefined past.1 It has been suggested that different mental components such as memory, attention, and perception are associated with this distinct experience.2,3 Although DV is probably a universal experience,4 patients with epilepsy often develop the phenomenon as an ictal symptom in partial seizures. Previous clinical observations have linked DV to temporal lobe epilepsy (TLE),5–10 but the precise origin and cause of the symptom are still controversial. Three possible sites of dysfunction have been proposed on the basis of intracranial electroencephalographic (EEG) monitoring and stimulation studies: the mesial temporal lobe,5,9 especially in the nondominant hemisphere;10 the superior lateral temporal cortex;11 and a neuronal network that engages both medial and lateral aspects of the temporal lobe.12,13 However, it is uncertain whether temporal lobe disturbance is sufficient and necessary for the generation of DV, since the incidence of the symptom is comparatively low in TLE14 and it is difficult to evaluate wider cortical regions in TLE by using intracranial recordings.
Interictal studies using fluorine-18 fluorodeoxyglucose positron emission tomography (18FDG PET) may demonstrate reduced metabolism in the region of the epileptic focus and surrounding functional disturbance; these may be secondary to structural changes or related to interictal and ictal epileptiform discharges,15,16 which in turn give rise to ictal symptoms and signs. Working from the hypothesis that regional hypometabolism could involve symptomatogenic brain areas, we attempted to gain further insight into the functional anatomy of DV by examining the interictal metabolic features of patients with TLE with and without ictal DV experience.
PATIENTS AND METHODS
The subjects of this study were selected from a large series of surgical candidates with medically intractable epilepsy who had interictal 18FDG PET in St. Thomas' Clinical PET Centre between 1992 and 1996 as part of a comprehensive preoperative evaluation. The scheme of preoperative investigation and management that is used in our Epilepsy Surgery Centre17 includes obtaining a clinical history. Along with other information, the early clinical ictal characteristics of the habitual seizures, including the presence of auras and their exact content, are systematically sought by direct questioning of the patients and their relatives by one of the authors (C.E.P.), and the relevant information is entered into a database specifically designed for this purpose.
Fourteen patients with TLE who reported DV as a habitual ictal experience were consecutively selected from this database. Patients who had a past history of DV but lacked the experience during the period prior to the PET scan, as well as those with poorly defined auras of strangeness, were not included in the study. Seventeen patients with TLE who did not report DV were randomly selected from the same series for comparison. The diagnosis of TLE was based on historical evidence and concordant interictal and ictal video-EEG findings. Additional selection criteria were 1) unilateral temporal onset of the habitual seizures, so that a correlation of the occurrence of the symptom and the side of the epileptogenic focus could be established, and 2) absence of a mass lesion on brain magnetic resonance imaging (MRI) to eliminate any significant deafferentation effect that could contaminate the 18FDG PET findings. None of the patients had any neurological condition other than epilepsy, or any acute or chronic medical illness, at the time of the PET study. All patients gave their written informed consent to participate in comprehensive studies for epilepsy surgery.
Positron Emission Tomography
All patients underwent interictal 18FDG PET brain scans using an ECAT 951R scanner (Siemens CTI; Knoxville, TN) while on full antiepileptic medication after a 6-hour fast. The intravenously injected dose of [18F]FDG was 250 MBq. Patients were positioned supine within the field of view of the camera, and images from skull base to vertex were acquired over a period of 30 minutes commencing 30 minutes after tracer injection. Thirty-one slices were produced over a 10.6-cm axial field of view; six sequential static scans of 5 minutes' duration were acquired. Frames were summed, attenuation correction was performed by the method of Bergstrom et al.,18 and the complete set of image planes was reconstructed and smoothed to obtain an image dataset with a spatial resolution of 8.5 mm in all three orthogonal directions. The scans were visually interpreted by two nuclear medicine physicians, blind to patient identity, with any difference resolved by consensus. Semiquantitative analysis of six transaxial slices aligned parallel to the longitudinal axis of the temporal lobe was performed, also blind to the clinical information, by using a standard template of multiple, 7-mm, circular regions of interest (ROIs). ROIs were placed under visual guidance over selected areas chosen by matching PET planes to an anatomical brain atlas.19 Counts (cts) within these regions were averaged to provide nine anatomical paired regions (Figure 1), for which asymmetry indices (AI) were calculated according to the following formula: AI=([cts in left ROI−cts in right ROI] / sum of cts in both ROIs)×200%.
Electroencephalography
Initially, all patients had an interictal scalp EEG in both awake and sleep states. Twenty-seven patients (11 with and 16 without DV) had further ictal recordings obtained with video-EEG telemetry, of which 20 were with foramen ovale, four with subdural strips, one with depth, and two with scalp electrodes. All intracranial recordings were performed after the PET studies. Ictal studies were not pursued in the remaining 4 patients because typical anterior temporal spike-and-wave discharges were strongly lateralized and were concordant with the findings in the other investigations.
Magnetic Resonance Imaging
Standard qualitative brain MRI studies were performed in all patients and included T1- and T2-weighted images. MR images were obtained on a 1.5-T General Electric Signa Advantage scanner (GE Medical Systems, Milwaukee, WI), using a 3-D coronal volumetric spoiled gradient echo sequence (SPGR) with a flip angle of 35 degrees, repetition time of 33, and time-to-echo of 5. One hundred and twenty-four 1.5-mm contiguous slices were obtained, using a 22-cm field of view and a matrix size of 256×192 to cover the whole cerebrum.
Intracarotid Amobarbital Test (IAT)
As a part of a full preoperative neuropsychological battery, hand preference was assessed in all patients, and verbal dominance was assessed by using the IAT in 22 patients (11 with and 11 without DV). The methodology of the IAT used in our institution has been detailed elsewhere.17
Resective Surgery and Histopathology
Thirteen patients so far have undergone standard anterior temporal lobectomy (9 on the right and 4 on the left side). Mesial temporal sclerosis was found in 12 patients, of whom 1 had additional cortical dysplasia and 1 had nonspecific gliotic changes.
Statistical Analysis
Clinical characteristics showing normal distribution were compared by Student's t-test. Nominal data were analyzed by chi-square analysis with Yates' correction or Fisher's exact test. To avoid multiple testing, a factor-analytic approach with principal component solution was used. In this analysis, varimax rotation was used to facilitate the interpretation. Initial unrotated factors were extracted by the principal component methods. The new factors derived from the factor analysis were examined in relation to the effects of déjà vu and lateralization of the EEG focus by two-way analysis of variance (ANOVA). The two-tailed Bonferroni correction for multiple comparisons was used to control for type I error.
RESULTS
Clinical Characteristics
Seventeen of the 31 patients were women. The median age at PET examination was 26 years (range 13–51 years), the median age at seizure onset was 9 years (range 1–33 years), and the median disease duration before PET examination was 18 years (range 3–31 years). All patients suffered complex partial seizures, and 19 of them (6 with DV, 13 without DV) had in addition secondary generalized tonic-clonic seizures, with an estimated frequency ranging from once ever to 10 per month. No significant difference was noted in any of the clinical variables between patients with and those without DV (Table 1).
Electroencephalography
The ictal onset zone was determined by video-EEG telemetry in 27 patients. In the 4 remaining patients, ictal EEG recordings were not deemed necessary in the presence of congruent interictal scalp EEG and neuroimaging findings; 2 underwent standard anterior temporal lobectomy and remain seizure-free for 4 and 2 years, respectively, and the 2 others await surgery. The lateralizations of the epileptogenic zones in the 31 patients are shown in Table 1. There was no significant difference between the groups.
MRI
In 2 patients with DV and 5 without DV, atrophy or abnormal signal or both were found in the left mesial temporal structures; 3 patients with DV and 6 patients without DV had similar abnormalities in the right temporal lobe. No other abnormality was observed in any of the scans. There was no significant difference between the groups (Table 1).
Handedness/IAT
Three patients with DV and 1 patient without DV had left hand preference. The others were right-handed. On the IAT, none showed clear verbal dominance in the right hemisphere. Two of 11 patients with DV and 2 of 11 patients without DV showed mixed cerebral dominance of verbal function. Among 4 left-handers, only 1 demonstrated mixed verbal dominance. Again, no significant difference was observed (Table 1).
18FDG PET
Visual analysis of PET images demonstrated diffuse hypometabolism involving the temporal areas and concordant with the other investigations in 22 patients (71%) and normal metabolism in the remaining 9. Five patients with DV and 7 without DV had left temporal hypometabolism. Right temporal hypometabolism was noted in 5 patients in both groups (Table 1). No evidence of bilateral metabolic depression was observed in any of the patients.
Using a factor-analytic approach, we reduced the raw AI data (Table 2) from nine paired anatomical regions to two orthogonal region profiles. The two factors accounted for 79.8% of the variance of data. Factor 1 (eigenvalue 6.146), for which eight regions had larger loading (inferior lateral temporal lobe, factor loading 0.956; superior lateral temporal lobe, 0.926; mesial temporal lobe, 0.888; tip of the temporal lobe, 0.864; parietal lobe, 0.842; dorsolateral frontal lobe, 0.812; thalamus, 0.808; and occipital lobe, 0.788), was labeled “visual network.” For factor 2 (eigenvalue 1.032), which was labeled “nonvisual region (striatum),” higher loading was noted in the caudate (0.788).
On both of the new derived variables, there were no significant interaction effects of DV and EEG focus by ANOVA. There were both significant DV and EEG focus effects on visual network region. In contrast, nonvisual area showed nonsignificant DV effect and significant EEG focus effect (Table 3). Then, to evaluate DV effect precisely, we returned to the original AI data that represent the “visual network” factor scores. Metabolism in the mesial temporal structures and the parietal cortex was significantly lower in the patients with DV than in those without. In these regions, metabolism was lower on the left. In other regions, the dorsolateral frontal lobe, the inferior and superior lateral temporal lobe, and the occipital cortex, patients with DV again demonstrated lower metabolism on the left, albeit not significantly. These results are shown in Table 3.
DISCUSSION
In this study, patients with DV did not differ from those without DV regarding age, duration of epilepsy, side of the ictal onset zone, or qualitative FDG PET and MRI findings. In contrast with our results, previous investigators have reported a strong association of DV with the nondominant hemisphere,5–8,10 but even in those studies some exceptions were noted.7,8 Gloor et al.20 failed to observe DV responses more often with right than with left temporal stimulation. The side of the seizure onset may not always determine the occurrence of a symptom, since electrical discharges may spread fast and extensively12,13 and both hemispheres can be engaged as the result of interhemispheric propagation of the electrical discharges. Therefore it is possible that the generation of ictal experiential phenomena like déjà vu is dependent more on the involvement of the relevant cerebral areas (or functional neuronal circuits) by the spread of the ictal discharges than on the side of the epileptogenic focus. Palmini and Gloor14 suggested that DV provides no useful lateralizing information, since it has been shown to occur with most of the auras in partial seizures.
The principal finding of the present study is a significant relative reduction of glucose metabolism in the left mesial temporal structures and the parietal cortex in the group of patients with déjà vu, irrespective of the side of the seizure onset. This pattern was not evident in individual patients in whom temporal hypometabolism was always ipsilateral to the EEG findings, but was manifested by the group averages. Patient selection was biased to intractable epilepsy, and by implication to long-standing and diffuse brain damage and dysfunction. The relatively small sample size may have influenced the statistical analysis; however, the incidence of DV in TLE is generally low.14 None of our patients had a mass lesion; therefore, the extent and degree of the hypometabolism were not related to widespread deafferentation. The mesial temporal hypometabolism observed in the DV group could be due to primary neuronal loss in the hippocampus/amygdala complex, and the remote (parietal) metabolic depression could reflect secondary chronic deactivation. In that case, the effect should have been equally divided between the groups, since no difference was observed in the MRI findings. The scans were qualitatively evaluated, raising the possibility that subtle changes in the mesial temporal structures may have been missed, but they were in fact more often abnormal in the group without DV (Table 1). Furthermore, it has been shown that the magnitude of the hippocampal volume asymmetry is not related to the degree of the mesial and lateral temporal hypometabolism.21 Although information on the clinical characteristics of the last seizure before the PET scan was not available for most of our patients, it is rather unlikely for the parietal hypometabolism observed in the DV group to have been due to a chance recent occurrence of secondary generalized seizures22 exclusively in these patients. In contrast with those without DV, most of the DV patients never had secondary generalized seizures; scans were invariably performed under full antiepileptic medication, so rapidly generalized withdrawal seizures can be effectively ruled out. An alternative explanation can be based on the evidence that regional cerebral metabolic depression may reflect propagation pathways of interictal23,24 and ictal discharges and therefore may be potentially related to the symptomatogenic zone. In this context, recent studies showed that different patterns of early ictal motor signs correspond to distinct interictal cerebral metabolic patterns in TLE22 and frontal lobe epilepsy.25
On the basis of the above evidence, the most probable, although admittedly speculative, interpretation of our findings is that extensive cortical areas beyond the temporal lobe, such as the parietal and possibly other association areas, may be involved in the network that integrates the DV experience. Hypometabolic regions overlapped the visual pathways and association cortex, mainly involving the V4 area, the posterior parietal cortex, the inferior temporal area, the dorsolateral prefrontal cortex, and the classic visual cortex (V1, 2, 3).26 Each of the visual association areas has distinct paralimbic connections.27 With activation of the mesial temporal structures, simultaneous spread to these visual association areas may elicit some distortion in imagery—or vice versa. Bancaud et al.12 suggested that when the nonspecific activation arising from either stimulation or spontaneous epileptic discharges meets and is sculpted by activity arising from perception and moving in the other direction from the specific sensory cortices, DV generation could result—the feeling of memory attached to a current sensory experience. The mesial temporal structures are deemed to integrate memory and emotion; thus, abnormal electrical interchange within this network could result in distortion of visual cognition and memory as a cue to DV, and could decrease regional cerebral metabolism through enhanced and sustained inhibition.28 Given that DV may occur as a nonepileptic phenomenon, subsequent ictal propagation through pathways in less hypometabolic or eumetabolic brain areas may also be required for the integration of the actual DV perception. Involvement of the left side, irrespective of the side of ictal onset, seems to be of particular importance for the generation of DV experience. Further prospective studies, controlled for the symptomatology of the last seizure and DV occurrence before the PET scanning, may confirm and expand our findings.
ACKNOWLEDGMENTS
This study was supported by the Japan Foundation for Aging and Health and the Fund for Epilepsy.
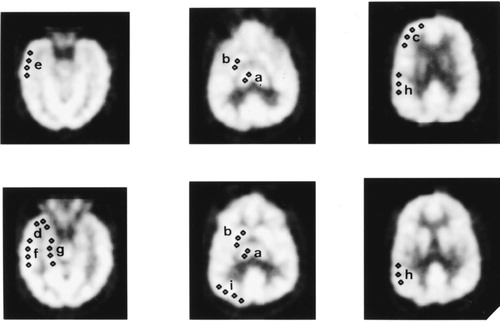
FIGURE 1. Illustration of region of interest location in six transaxial planes aligned parallel to the longitudinal axis of the temporal lobeThe nine anatomical paired regions are: a) thalamus, b) basal ganglia, c) dorsolateral frontal area, d) tip of the temporal lobe, e) inferior lateral temporal area, f) superior lateral temporal area, g) mesial temporal area, h) parietal area , and i) occipital area.
 |
 |
 |
1 Neppe VM: The concept of déjà vu. Parapsychology Journal of South Africa 1983; 4:1–10Google Scholar
2 Sno HN, Linszen DH: The déjà vu experience: remembrance of things past? Am J Psychiatry 1990; 147:1587–1595Google Scholar
3 Berrios GE: Déjà vu in France during the 19th century: a conceptual history. Compr Psychiatry 1995; 36:123–129Crossref, Medline, Google Scholar
4 Harper MA: Déjà vu and depersonalization in normal subjects. Aust NZ J Psychiatry 1969; 3:67–74Crossref, Google Scholar
5 Jackson JH: On a particular variety of epilepsy (“intellectual aura”): one case with symptoms of organic disease. Brain 1888; 11:179–207Crossref, Google Scholar
6 Mullan S, Penfield W: Illusions of comparative interpretation and emotion. Arch Neurol Psychiatry 1959; 81:269–284Crossref, Google Scholar
7 Cole M, Zangwill OL: Déjà vu in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 1963; 26:37–38Crossref, Medline, Google Scholar
8 Gupta AK, Jeavons PM, Hughes RC, et al: Aura in temporal lobe epilepsy: clinical and electroencephalographic correlation. J Neurol Neurosurg Psychiatry 1983; 46:1079–1083Google Scholar
9 Halgren E, Walter RD, Cherlow DG, et al: Mental phenomena evoked by electrical stimulation of the human hippocampal formation and amygdala. Brain 1978; 101:83–117Crossref, Medline, Google Scholar
10 Weinand ME, Hermann B, Wyler AR, et al: Long-term subdural strip electrocorticographic monitoring of ictal déjà vu. Epilepsia 1994; 35:1054–1059Google Scholar
11 Penfield W, Perot P: The brain's record of auditory and visual experience. Brain 1963; 86:596–695Crossref, Google Scholar
12 Bancaud J, Brunet-Bourgin F, Chauvel P, et al: Anatomical origin of déjà vu and vivid “memories” in human temporal lobe epilepsy. Brain 1994; 117:71–90Crossref, Medline, Google Scholar
13 Gloor P: Experimental phenomena of temporal lobe epilepsy: facts and hypotheses (review). Brain 1990; 113:1673–1694Google Scholar
14 Palmini A, Gloor P: The localizing value of auras in partial seizures: a prospective and retrospective study. Neurology 1992;42:801–808Google Scholar
15 Henry TR, Mazziotta JC, Engel J Jr: Interictal metabolic anatomy of medial temporal lobe epilepsy. Arch Neurol 1993; 50:582–589Crossref, Medline, Google Scholar
16 Spencer SS: The relative contributions of MRI, SPECT, and PET imaging in epilepsy. Epilepsia 1994; 35(suppl 6):S72–S89Google Scholar
17 Polkey CE, Binnie CD: Neurosurgical treatment of epilepsy, in A Textbook of Epilepsy, 4th edition, edited by Laidlow J, Richens A, Chadwick D. Edinburgh, Churchill Livingstone, 1993, pp 561–611Google Scholar
18 Bergstrom M, Litton J, Eliksson L, et al: Determination of object contour from projections for attenuation correction in cranial positron emission tomography. J Comput Assist Tomogr 1982; 6:365–372Crossref, Medline, Google Scholar
19 Matsui T, Hirano A: An Atlas of the Human Brain for Computerized Tomography. Tokyo, Igaku-Shoin, 1978Google Scholar
20 Gloor P, Olivier A, Quesney LF, et al: The role of limbic system in experimental phenomena of temporal lobe epilepsy. Ann Neurol 1982; 12:129–144Crossref, Medline, Google Scholar
21 O' Brien TJ, Newton MR, Cook MJ, et al: Hippocampal atrophy is not a major determinant of regional hypometabolism in temporal lobe epilepsy. Epilepsia 1997; 38:74–80Crossref, Medline, Google Scholar
22 Savic I, Altshuler L, Baxter L, et al: Pattern of interictal hypometabolism in PET scans with fludeoxyglucose F 18 reflects prior seizure types in patients with mesial temporal lobe seizures. Arch Neurol 1997; 54:129–136Crossref, Medline, Google Scholar
23 Alarcon G, Koutroumanidis M, Martin-Miguel MC, et al: Topographic congruence of FDG PET and electrocorticographic abnormalities in temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol (in press)Google Scholar
24 Koutroumanidis M, Binnie CD, Elwes RDC, et al: Interictal regional slow activity correlates with lateral temporal hypometabolism as imaged with 18FDG PET: neurophysiologic and metabolic implications. J Neurol Neurosurg Psychiatry 1998 (in press)Google Scholar
25 Schlaug G, Antke C, Holthousen H, et al: Ictal motor signs and interictal regional cerebral hypometabolism. Neurology 1997; 49:341–350Crossref, Medline, Google Scholar
26 LaBerge D: Computational and anatomical models of selective attention in object identification, in The Cognitive Neurosciences, edited by Gazzaniga MS. Cambridge, MA, MIT Press, 1995, pp 649–663Google Scholar
27 Pandya DN, Yeterian EH: Architecture and connections of cortical association areas, in Cerebral Cortex, vol 4, Association and Auditory Cortices, edited by Peters A, Jones EG. New York, Plenum, 1985, pp 3–61Google Scholar
28 Ackermann RF, Finch DM, Babb TM, et al: Increased glucose metabolism during long duration recurrent inhibition of hippocampal pyramidal cells. J Neurosci 1984; 4:251–264Crossref, Medline, Google Scholar



