Estimating the Prevalence of Agitation in Community-Dwelling Persons With Alzheimer's Disease
Abstract
To estimate the prevalence of, and develop norms for, significant agitation in community-dwelling persons with Alzheimer's disease (AD), the authors applied three different criteria to persons with AD (n=235) and normal elderly control subjects (NEC; n=64). The criteria were used to identify the minimum total score on the Cohen-Mansfield Agitation Inventory (CMAI) that represents significant or “excessive” agitation and to estimate its prevalence. The “ultraliberal” criterion resulted in 99.1% of persons with AD and 56.6% of NEC being classified as “excessively” disturbed. The “liberal” and “conservative” criteria classified 66.7% and 68.2% of persons with AD, and no NEC, as “excessively” disturbed. The authors conclude that the best estimate of prevalence of excessive agitation in this population is 67.5%, and that individuals with CMAI scores of 0 to 14 probably should not be considered to have excessive agitation.
Although researchers and clinicians are in agreement that agitation is a serious problem in persons with Alzheimer's disease (AD), estimates of the prevalence of agitation, especially in AD, differ greatly. Recent estimates include those of Reisberg et al.,1 who found symptoms of agitation in 48% of the medical charts they reviewed from outpatients with probable AD. Devanand et al.2 reported that 55% of the 106 outpatients with probable AD they studied exhibited motor agitation. Levy et al.3 found agitation in 28% to 32% of 181 outpatients with probable AD; Mega et al.4 reported that symptoms of agitation were observed in 60% of the 50 persons with probable AD in their study, and Devanand et al.5 found “behavioral disturbance” in 51.9%, but “wandering or agitation” in 38.7% of their sample of 235 subjects with probable AD. Most recently, Lyketsos et al.6 reported that 22% of 214 persons from the community with probable AD exhibited symptoms of agitation, whereas Haupt et al.7 found agitation in 88% of outpatients with probable AD seen in their clinic.
Among the issues underlying the diversity of these prevalence estimates are the range of symptoms included under the different definitions of agitation and the variety of instruments employed in the assessment of those symptoms. Many types of behavioral disturbances are termed “agitation.” Although Cohen-Mansfield and Billig8 provided a concrete definition of agitation, the collection of symptoms included may be more appropriate for elderly nursing home residents than community-dwelling persons with diagnoses of AD, whose behavioral disturbance may differ in specific ways from the agitation observed in other populations.9 In fact, each of the above-cited studies defined “agitation” as that set of symptoms represented on the assessment instrument employed.
Although agitation is only one type of behavioral disturbance, the symptoms typically associated with agitation by one author or instrument may be classified more or less specifically by another. An example is provided by the comparison of the Cohen-Mansfield Agitation Inventory (CMAI)10 and the Behavior Rating Scale for Dementia (BRSD),11 where restlessness, uncooperativeness, and verbal aggression are all considered symptoms of agitation on the CMAI but are elements of subscores representing irritability/aggression, behavioral dysregulation, and inertia, respectively, on the BRSD. Although we use the terms “agitation” and “behavioral disturbance” somewhat interchangeably, our analyses are focused on clinically important behavioral disturbance that excludes, to the extent possible, significant psychopathology (e.g., delusions, depression, hallucinations) and which may comprise disruptive symptoms that are essentially unrelated to each other.
In spite of sometimes dramatic prevalence rates, the rate of medication prescriptions for the treatment of agitation or behavioral symptoms is quite low—less than 20% in all of the studies cited above in which this information was provided. It may therefore be the case that prevalence of agitation has been determined in a way that overstates (or understates) its true clinical impact. In this study we sought to define “normal” levels of behavioral symptomatology, in order to evaluate various estimates of the prevalence of agitation in persons with probable AD. By establishing a normal range for these behavioral symptoms, we hoped to 1) derive an estimate of their prevalence in AD that closely represents the subpopulation most likely to be treated or to seek intervention; and 2) establish criteria such that persons with AD who exhibit behavioral symptoms below this threshold will not be considered “agitated.”
METHODS
We evaluated the prevalence of agitated behaviors at the initial assessment of a population of persons with probable AD residing in the community and a cohort of normal elderly control subjects (NEC), who were all participants in a clinical trial designed to evaluate assessment instruments for clinical studies of AD.12
Subjects
These subjects and the clinical trial in which they participated have been described elsewhere.12–14 Subjects were recruited to participate in a 12-month, multicenter clinical study carried out by the Alzheimer's Disease Cooperative Study to evaluate cognitive and behavioral instruments for use in studies of persons with AD. All were participants in ongoing clinical studies at the centers from which they were recruited. Informed consent was obtained for all participants (from the caregivers for AD patients).
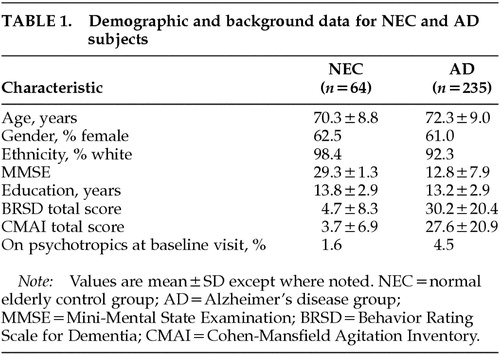
Normal elderly control subjects (NEC) were 64 individuals, and patients (AD) were 235 community-dwelling individuals with National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA)–based diagnosis of probable AD.15 All participants either lived with an informant or had contact with a knowledgeable informant of at least 6 to 8 hours per week, not all in one day. Demographic and background data for the two groups are presented in Table 1.
Patients were required to discontinue all psychoactive medication for 4 weeks prior to the baseline visit, except for patients with Mini-Mental State Examination (MMSE)16 scores of less than 10, for whom the protocol specified that continuation on drug was permitted (n=11). With the exception of this small portion (4.5%) of the AD group, the medications described here were reported by the informant as having been prescribed within the 3 months prior to the baseline visit (i.e., were being taken at baseline) at which the behavioral symptoms (described below and included in Table 4) were assessed. Individuals on nonpsychoactive medication were required to have stable doses for 4 weeks prior to baseline. Participants in clinical trials for behavioral interventions were not eligible.
Materials
Agitation was assessed with the Cohen-Mansfield Agitation Inventory (CMAI),10 an instrument that has been found to be valid, reliable, and very specific to agitated behaviors as opposed to other domains of psychopathology.17 The version of the CMAI used in this study comprises 38 items, 36 of which represent observable symptoms of agitation (items 37 and 38 inquire if there is any behavior not covered by the questionnaire, and if there is a time of day when behaviors are most disturbed). This version is identical to the CMAI-Community version but includes one additional item, which assesses the frequency of “strange movements” (item 23). The instrument is administered by a technician (not a physician) to a caregiver, who rates each symptom for its frequency in the previous 2-week period on a 7-point scale. The ratings are “never occurred” (rated 0), “less than once a week” (rated 1), “once or twice a week” (rated 2), “several times a week”(rated 3), “once or twice a day” (rated 4), “several times a day” (rated 5), or “several times an hour” (rated 6).18 We used the total CMAI score in our prevalence estimates (although see Tractenberg et al.,9 who suggest item analyses rather than total score analysis). In addition, we used an independent measure of agitation taken from another instrument for measuring psychopathology, the CERAD Behavior Rating Scale for Dementia (BRSD).11,19
Statistical Methods
We derived three estimates of the prevalence of agitation in these AD outpatients, “ultraliberal,” “liberal,” and “conservative.” The “ultraliberal” estimate was based on previous prevalence reports: any person who was reported to have at least one symptom (i.e., a CMAI total score>0) was termed “agitated.”
For the second estimate, we identified the highest score achieved by the NEC group and used this to establish a “liberal” estimate, such that any person who scored above this level (i.e., a CMAI total score above the maximum achieved by any NEC) was termed “agitated.” Previous studies of the distribution of behavioral scores achieved by NEC14 suggested that NEC would score somewhere above zero, and so this estimate was considered to be less liberal than the “ultraliberal” one based on a total score of zero.
Finally, we used endorsement of the BRSD item “agitation” (“observable signs of emotional distress,” item 19) to estimate the prevalence of agitation in the group. Thus, if 76% of the AD group had endorsed item 19 on the BRSD, for example, then our “conservative” estimate of the prevalence of agitation would be 76%. Then we would find the corresponding CMAI total score representing the 24th percentile (where 76% of the group had higher total CMAI scores), and that CMAI total score would be the cutoff under the “conservative” estimate of prevalence.
A second BRSD item assesses physical agitation and restlessness, without emotional component (item 24), and was also considered for this estimation of “agitation.” It was not included because there are several items on the CMAI that assess restlessness, pacing, wandering, and other elements of physical agitation (BRSD item 24), but there is no item on the CMAI that directly assesses “observable signs of emotional distress” (BRSD item 19). (See Weiner et al.20 for a discussion of the comparability of these two instruments.) For the greatest degree of validity, we selected BRSD item 19, which does not overlap with the CMAI but which still assesses agitation.
The end results were CMAI total score cutoffs derived under each of the three criteria and the estimates of the prevalence of agitation in the AD group derived under each. We calculated the proportion of the group designated “agitated” under each definition (ultraliberal, liberal, and conservative) to whom psychotropic medications were prescribed for behavioral symptoms. Additionally, we calculated the estimates of prevalence of behavioral disturbance in the NEC group based on each definition.
We hypothesized that one of our estimates would result in the fewest NEC being classified as “agitated” and the greatest proportion of AD classified as “agitated” having been prescribed psychotropics for their symptoms. “Psychotropic medications” were those medications reported by a knowledgeable informant to be currently prescribed at the screening visit, namely: benzodiazepines, anticonvulsants, antidepressants, tranquilizers, barbiturates, anxiolytics, sedatives and hypnotics, and antimanic agents.
Once we identified the best estimate of the highest CMAI score an individual can achieve without being considered to be behaviorally disturbed, we then compared, via chi-square tests, the NEC and AD groups who fell below this cutoff (i.e., were considered not to be agitated) on their respective endorsement rates of each of the 36 CMAI items that represent observable symptoms. CMAI items are considered “endorsed” if the frequency rating is at least once or twice a week in the previous 2 weeks (i.e., a rating of at least 2 on a scale from 0 to 6).9 BRSD frequency ratings are assigned differently (see ref.19), but “endorsed” for an item on the BRSD is also represented by a rating of at least 2.
Mann-Whitney tests were then used to compare the frequency ratings of each CMAI item across the groups (NEC and AD), over all patients (regardless of whether total CMAI score was above the cutoff), in order to describe the relative prevalence and frequency of each symptom in each cohort overall. Holm adjustment21 was employed to account for the multiple comparisons when the endorsement of each item or the mean frequency rating of each item for AD was compared with that for NEC.
RESULTS
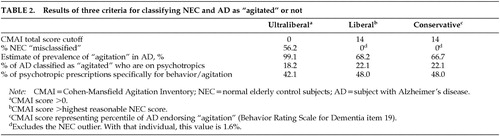
Under the ultraliberal criterion (any person with a CMAI score>0 was considered “agitated”), 56.2% of NEC were classified as “agitated,” compared with 99.1% of AD (all but 2 patients). One NEC had been prescribed a psychotropic at screening (imipramine, for bladder control); 18.2% of patients with AD having CMAI scores>0 had been prescribed psychotropics at screening.
The highest CMAI total score at baseline for a person in the NEC group was 48; however, the rest (98.4% of this group) scored at or below 14. Because of the wide difference between this NEC subject and the rest of the NEC cohort, the individual with the score of 48 was considered an outlier and was excluded from group comparisons. Therefore, we took CMAI=14 to be the cutoff score under the liberal criterion, so that any person with a CMAI total score ≥15 was considered “agitated.” Only 1 NEC (1.6% of this group), with the outlying CMAI score, would have qualified as “agitated” under this liberal criterion.
Under the liberal criterion, 68.2% of AD qualified as “agitated”; of these, 22.1% had been prescribed psychotropics at screening. Of the individuals with AD whose CMAI total scores were below the liberal cutoff (i.e., CMAI≤14), 9.6% (7 individuals with a total of 8 prescriptions) had also been prescribed psychotropics.
The conservative criterion, endorsement (frequency rating of 1 or more days in past month) of the single item “agitation” from the BRSD, resulted in 66.7% of AD patients being classified as “agitated.” This percentile was applied to the frequency listing of CMAI total scores for the AD patients and corresponded to a CMAI cutoff of 14, the same score as was identified by the “liberal” criterion. Table 2 presents the results of the three criteria in terms of CMAI total score cutoff, prevalence estimate, specificity (with respect to NEC), and psychotropic prescription rates.
When we examined the proportion of NEC who endorsed item 19 from the BRSD, we found that 29.7% of NEC would have been classified as “agitated” if we had used item 19 by itself to estimate prevalence of agitation in NEC subjects (rather than using its endorsement to generate a prevalence estimate–derived CMAI score cutoff). When we applied the 66.7 percentile derived from the BRSD item-19 endorsement to the CMAI total scores, we found that one NEC subject (1.6%) would have been classified as “agitated,” the single NEC subject with the CMAI score of 48.
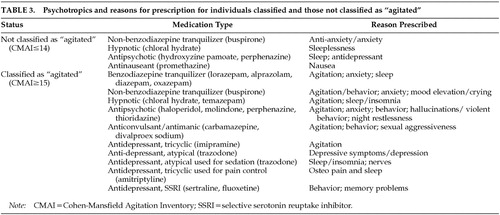
Among the 160 persons with AD whose CMAI total scores were characterized as representing excessive disturbance under the “liberal” and “conservative” definitions (i.e., ≥15), 48% of 50 psychotropic prescriptions were for behavioral disturbance, 28% were for sleeplessness, insomnia, or night restlessness; 22% were for anxiety or depressive symptoms; and 2% (n=1) were for other reasons (memory problems). Among persons with AD not classified as behaviorally disturbed (i.e., CMAI≤14), 6 of 7 psychotropic prescriptions were for depressive symptoms and sleep-related disturbances (3 prescriptions for each symptom); the remaining one was for nausea.
Psychotropic medications and their indications, reported at baseline by knowledgeable informants, for disturbed/nondisturbed persons with AD under the liberal and conservative criteria are listed in Table 3.
Under the ultraliberal criterion, 42% of the 57 psychotropic prescriptions were specifically for behavioral disturbance; the seven prescriptions described above for individuals with CMAI scores ≤14 apply to individuals classified as “agitated” under this criterion.
Chi-square tests showed that none of the three criteria resulted in differential rates of classification of men and women with AD as “agitated” (conservative: men, 60.9%; women, 70.4%; χ2=2.3, not significant; liberal: men, 97.8%; women, 100%; χ2=3.1, not significant; ultraliberal: men, 64.1%; women, 70.9%; χ2=1.9, not significant). Although Mann-Whitney testing indicated that individuals classified as agitated by the “liberal” and “conservative” criteria had significantly lower MMSE scores (Z=–5.3, P<0.001), Figure 1 suggests that individuals with MMSE scores across its range (0–30) fall into both agitated and nonagitated categories.
To compare the symptomatology exhibited by all NEC (except the outlier) and those persons with AD whose CMAI scores were 14 or less (i.e., not disturbed), we compared the rates of endorsement, as defined above, on the CMAI items. The comparisons of endorsement rates on only 8 of the 36 items had pre–Holm adjustment P-valued less than 0.05. More persons with AD than NEC endorsed 5 of these 8 items: repetitiveness, restlessness, pacing, handling things inappropriately, and temper outbursts (items 1, 14, 15, 19, and 24). The other 3 of these 8 items were endorsed by more NEC than persons with AD: verbally threatening or insulting, verbally bossy or pushy, and physical sexual advances (items 9, 11, 13). After Holm adjustment of the P-values, only one difference in endorsement rates, AD>NEC on repetitiveness (item 1), remained significant (data not shown).
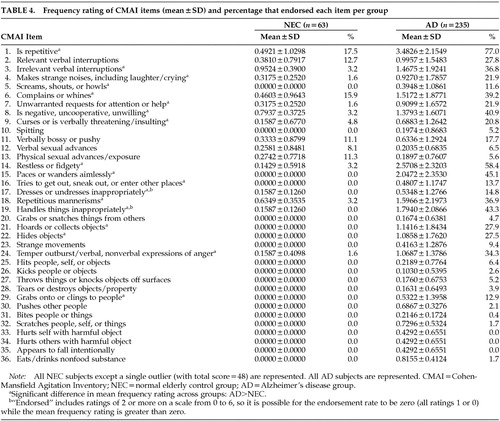
We then compared the mean frequency ratings for each item across the study groups (all AD vs. NEC) and calculated the endorsement rates in each group, for descriptive purposes. Table 4 contains the mean frequency ratings, as well as the endorsement rates, per item for the NEC (except the single high-scoring outlier subject) and the entire AD group.
When we considered the entire sample of community-dwelling persons with AD, roughly 67.5% of whom we could classify as “behaviorally disturbed,” we found that frequency ratings were significantly higher for AD than for NEC for half of the 36 items: repetitiveness; irrelevant verbal interruptions; strange noises; screaming, shouting or howling; complaining; unwarranted requests for attention; uncooperativeness; verbal threatening/insulting; restlessness; pacing; trying to get outside; inappropriate dressing/undressing; repetitious mannerisms; inappropriate handling of objects; hoarding; hiding; temper outbursts; and grabbing/clinging to people (items 1, 3, 4, 5, 6, 7, 8, 9, 14, 15, 16, 17, 18, 19, 21, 22, 24, and 29; all Holm-adjusted P-values<0.05).
One of the 36 symptoms of agitation (repetitiveness) was rated by 17.5% of these NEC as occurring at least once or twice per week; more than 5% of this sample endorsed 6 of 36 items: repetitiveness, relevant verbal interruptions, complaining, verbal bossiness, and verbal and physical sexual advances (items 1, 2, 6, 11, 12, and 13).
DISCUSSION
Limitations of this study include the relatively high level of education of the participants and the relatively low percentage of minority representation. The very low level of behavioral disturbance, in general, in the patient population made this group ideal for investigating the lower limit of the range of agitated behaviors in AD.
Two independent criteria for classifying individuals as behaviorally disturbed were found to correspond to a CMAI total score of 15 or higher; we therefore conclude that for community-dwelling individuals with AD, CMAI total scores of 14 or lower should not be considered to fall into the behaviorally disturbed range.
Practically speaking, individuals with CMAI scores of 14 or less would not be good candidates for clinical studies of behavioral symptoms (and/or their treatment), although such individuals would be ideal subjects in clinical studies that monitor the emergence of new symptoms. Both a “conservative” and a “liberal” approach to defining “excessive” agitation resulted in the same CMAI total score cutoff, 14 points. When we compared persons with AD classified as “not disturbed” under this criterion against NEC in terms of the presence of symptoms of agitation that were endorsed, we found that a significantly greater proportion of persons with AD than NEC endorsed only 1 of 36 items. This suggests that persons with AD classified under this criterion as “not disturbed” are not different from normal control subjects in their behavioral symptomatology, and that excessive agitation is not present in this subsample of persons with AD.
Based on the results of these two approaches to classifying individuals as “behaviorally disturbed” or “agitated” (using the highest reasonable NEC score and using endorsement of BRSD item 19), we conclude that the prevalence rate of agitation in community-dwelling persons with AD is approximately 67.5%. This value is most consistent with the findings of Mega et al.,4 is least consistent with the most recent estimates (22%;6 88%7), and is considerably higher than the prevalence estimate of 44% for global agitation22 that represented the median across earlier studies.
Although the ultraliberal definition of agitation may have been highly sensitive, labeling all but two AD patients as “agitated” (99%), it had an unacceptable level of specificity, resulting in more than 56% of normal control subjects being classified as “agitated”; the ultraliberal prevalence figure may be unacceptably high given earlier published estimates. By contrast, the conservative and liberal criteria resulted in estimates of 66.7% and 68.2% (average: 67.5%) for the prevalence of behavioral disturbance in these AD patients, with very high specificity; only the NEC outlier would have been classified as “agitated.”
Psychotropics were prescribed in 18.2% of those individuals with AD who were classified by the ultraliberal criterion as having “excessive” levels of behavioral symptoms; this was slightly less than the rate in those classified as excessively disturbed under the liberal definition (22.1%). Additionally, 42.1% of psychotropic prescriptions in those classified as agitated under the ultraliberal criterion were specifically for behavioral symptoms, whereas under the liberal criterion, 48.0% of those psychotropics prescribed to AD patients who had agitation were specifically for behavioral symptoms.
The prescription rates (18% and 22%) are roughly comparable to those reported previously for populations of community-dwelling persons with AD. Much higher psychotropic prescription rates were reported by Reisberg et al.,1 who found that 47.4% of 57 AD patients were being treated with thioridazine in their cross-sectional chart study, and by Haupt et al.,7 who found 42% of 77 AD outpatients had neuroleptic prescriptions for motor disturbances at any time over 2 years. A much lower rate was observed by Levy et al.3: only 9.4% of 181 AD patients initiated psychotropic medication during a 1-year period. Devanand et al.2 reported that 27.6% of 106 AD patients were on psychotropic medications at first evaluation, and later, Devanand and co-workers5 reported that 14.0% of their 235 subjects had prescriptions for psychotropic medications at the baseline visit.
We also found that 18 of the 36 symptoms on the CMAI may be the most descriptive for community-dwelling persons with AD in general, as compared with NEC: repetitiveness; irrelevant verbal interruptions; strange noises; screaming, shouting or howling; complaining; unwarranted requests for attention; uncooperativeness; verbal threatening/insulting; restlessness; pacing; trying to get outside; inappropriate dressing/undressing; repetitious mannerisms; inappropriate handling of objects; hoarding; hiding; temper outbursts; and grabbing/clinging to people. In an earlier study, we found that 10 of 36 CMAI items distinguish between persons with AD who had greater and lesser degrees of agitation;9 all but one of these 10 items (verbally bossy or pushy) were among the 18 items that distinguish persons with AD from NEC. Thus, it may be possible to monitor the agitation of community-dwelling persons with AD using a subset of CMAI items that is most descriptive of this population in particular, as opposed to nondemented elders or persons in nursing homes.
Toward this end, we are currently examining the emergence of the behavioral symptoms included in the CMAI. We plan to use the CMAI total score cutoff of 14 to determine if there are items that are more likely to emerge given higher initial CMAI scores, or if more emergence can be expected in community-dwelling persons with AD who have higher or lower initial total scores.
ACKNOWLEDGMENTS
This study was supported by Grant AG10483 from the National Institute on Aging.
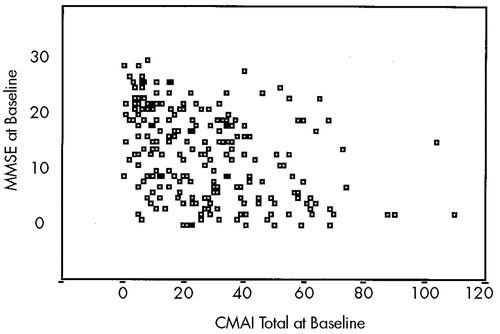
FIGURE 1. MMSE scores by CMAI total scores at baseline visit, AD patients onlyMMSE=Mini-Mental State Examination; CMAI=Cohen-Mansfield Agitation Inventory.
 |
 |
 |
 |
1 Reisberg B, Borenstein J, Salob SP, et al: Behavioral symptoms in Alzheimer's disease: phenomenology and treatment. J Clin Psychiatry 1987; 49(5, suppl):9-15Google Scholar
2 Devanand DP, Brockington CD, Moody BJ, et al: Behavioral syndromes in Alzheimer's disease. Int Psychogeriatr 1992; 4(2, suppl):161-184Google Scholar
3 Levy ML, Cummings JL, Fairbanks LA, et al: Longitudinal assessment of symptoms of depression, agitation, and psychosis in 181 patients with Alzheimer's disease. Am J Psychiatry 1996; 153:1438-1443Crossref, Medline, Google Scholar
4 Mega MS, Cummings JL, Fiorello T, et al: The spectrum of behavioral changes in Alzheimer's disease. Neurology 1996; 46:130-135Crossref, Medline, Google Scholar
5 Devanand DP, Jacobs DM, Tan MX, et al: The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatry 1997; 54:257-263Crossref, Medline, Google Scholar
6 Lyketsos CG, Steinberg M, Tschanz JT, et al: Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry 2000; 157:708-714Crossref, Medline, Google Scholar
7 Haupt M, Kurz A, Jänner M: A two-year follow-up of behavioural and psychological symptoms in Alzheimer's disease. Dement Geriatr Cogn Disord 2000; 11:147-152Crossref, Medline, Google Scholar
8 Cohen-Mansfield J, Billig N: Agitated behaviors in the elderly, I: a conceptual review. J Am Geriatr Soc 1986; 34:711-721Crossref, Medline, Google Scholar
9 Tractenberg RE, Gamst A, Weiner MF, et al: Greater frequency, not diversity, of behavioral symptoms characterizes agitated Alzheimer's disease patients. Int J Geriatr Psychiatry (in press)Google Scholar
10 Cohen-Mansfield J: Instruction Manual for the Cohen-Mansfield Agitation Inventory (CMAI). Rockville, MD, The Research Institute of the Hebrew Home of Greater Washington, 1991Google Scholar
11 Tariot PN, Mack JL, Patterson MB, et al: The CERAD Behavior Rating Scale for Dementia. Am J Psychiatry 1995; 152:1349-1357Crossref, Medline, Google Scholar
12 Ferris SH, Mackell JA, Mohs R, et al: A multicenter evaluation of new treatment efficacy instruments for Alzheimer's disease clinical trials: overview and general results. Alz Dis Assoc Disord 1997; 11(suppl 2):S1-S12Google Scholar
13 Tractenberg RE, Gamst A, Thomas, RG, et al: Investigating emergent symptomatology as an outcome measure in a behavioral study of Alzheimer's disease. J Neuropsychiatry Clin Neurosci (in press)Google Scholar
14 Tractenberg RE, Patterson M, Weiner MF, et al: Prevalence of symptoms on the CERAD Behavior Rating Scale for Dementia in normal elderly subjects and Alzheimer's disease patients. J Neuropsychiatry Clin Neurosci 2000; 12(4):472-479; correction, 2001; 13(1):95Google Scholar
15 McKhann G, Drachman D, Folstein M, et al: Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34:939-944Crossref, Medline, Google Scholar
16 Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar
17 Weiner MF, Koss E, Wild KV, et al: Measures of psychiatric symptoms in Alzheimer's patients: a review. Alzheimer Dis Assoc Disord 1996; 10:20-30Medline, Google Scholar
18 Koss E, Weiner M, Ernesto C, et al: Assessing patterns of agitation in Alzheimer's disease patients with the Cohen-Mansfield Agitation Inventory. Alzheimer Dis Assoc Disord 1997; 11(suppl 2):S45-S50Google Scholar
19 Mack JL, Patterson MB: Manual: CERAD Behavior Rating Scale for Dementia, 2nd edition. Durham, NC, Consortium to Establish a Registry for Alzheimer's Disease, 1996Google Scholar
20 Weiner MF, Williams B, Risser RC: Assessment of behavioral symptoms in community-dwelling dementia patients. Am J Geriatr Psychiatry 1997; 5:26-30Crossref, Medline, Google Scholar
21 Holm S: A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 1979; 6:65-70Google Scholar
22 Wragg R, Jeste D: Overview of depression and psychosis in Alzheimer's disease. Am J Psychiatry 1989; 146:577-587Crossref, Medline, Google Scholar



