Age and Regional Cerebral Blood Flow in Schizophrenia
Abstract
Positron emission tomography ([15O] water PET) was used to examine the relationship between age and regional cerebral blood flow (rCBF) in schizophrenia. Forty-nine unmedicated male patients, ages 20–51, underwent imaging during an eyes-closed resting condition. Negative correlations were observed between age and rCBF in the anterior cingulate, as well as in frontal (Brodmann area 8) and parietal cortex (area 40) bilaterally. The observation of reduced rCBF in the anterior cingulate with increased age is consistent with previous findings in healthy subjects. In contrast, the reduced flow observed in the frontal and parietal regions may be unique to schizophrenia.
The study of aging in the context of lifelong psychiatric disorders represents a special challenge for researchers. This challenge may particularly exist in disorders where neurodevelopmental abnormalities may influence the clinical appearance of neurodegenerative changes in late life. For example, if frontal lobe abnormalities are present neurodevelopmentally in schizophrenia, then age-related changes resulting in frontal hypoperfusion may represent a relatively smaller burden if the individual has functionally adapted to the hypofrontal state. The possibility of attenuated clinical deterioration, or the more likely case of enhanced vulnerability, both await further clarification through research.
Some studies have suggested that late-life cognitive deterioration occurs in schizophrenia, even among noninstitutionalized patients.1 In contrast, other studies suggest that cognitive status may be relatively stable in the chronic phase of the illness, which is supported by the absence of degenerative changes in postmortem histopathologic evaluations.2,3 Along these lines, studies of symptom severity across the life span have also suggested a more static than deteriorative course, with the potential for improvement in later life, particularly in positive symptoms.4–7 This late improvement may be due to relative decrements in dopaminergic tone in frontal and temporal regions,8 but these notions remain speculative, as the neural mechanisms underlying long-term outcome in schizophrenia have not been identified.
The complexity of the multiple mechanisms at work in the aging patient with schizophrenia cannot be overstated. As just one example, the observed presence of cytoarchitectural abnormalities in the hippocampal formation of patients with schizophrenia may incur a greater likelihood of cognitive decline, given that these regions have also been identified as vulnerable to age-related changes,9,10 To add to the complexity of the situation, exposure to neuroleptic medication has been associated with changes in the basal ganglia in functional neuroimaging studies, highlighting the importance of additionally accounting for treatment effects.11
This complexity, along with cohort differences between age groups, likely mediates the variability in neuroimaging studies of young-onset schizophrenia across various age ranges. For example, magnetic resonance imaging studies in younger adults have previously demonstrated decreased tissue volumes in a variety of regions, including frontal and temporal cortex, the amygdala, thalamus, and hippocampus.12 In contrast, studies of schizophrenia in later life have reported nonspecific structural changes, such as increased deep white matter hyperintensities.13 Other work has suggested that enlarged ventricles are present early in the illness, accompanied by progressive cortical loss with age.14 Taken together, age-related structural findings to date have been mixed, and no single degenerative process has been implicated.
Functional neuroimaging studies have demonstrated both early-onset abnormalities and probable later-life vulnerabilities to change among patients with schizophrenia. For example, reduced frontal cerebral blood flow (CBF) and reduced frontal cerebral glucose metabolism have been demonstrated at illness onset, associated with impaired performance on tasks of frontal lobe function.15,16 In addition to these abnormalities early in the course of illness, aging appears associated with further attenuation in frontal blood flow. For example, Goldstein et al.,17 using SPECT imaging, reported a negative correlation between age and mean frontal CBF (r=–0.36) among patients with schizophrenia in mid-adulthood. It is of note, however, that a negative correlation with age was also observed in the nonschizophrenic comparison subjects in this study. Therefore decreased frontal cerebral blood flow appears to be a consequence of both aging and the presence of schizophrenia. However, it may be surmised that individuals with schizophrenia are vulnerable to greater age-related decrements. Along these lines, Dupont et al.18 reported decreased left posterior frontal and bilateral inferior temporal blood flow in older patients relative to age-matched control subjects. In contrast, one longitudinal study encompassing nearly 20 years of follow-up of patients with schizophrenia, between mean ages of 25 and 41, showed no significant change in cerebral blood flow over time.19
These mixed findings imply that the issue of whether schizophrenia imparts a special vulnerability to neurodegeneration remains unresolved. Previous work has suggested that age-related changes in schizophrenia are not substantially different from normal aging, yet there is also some evidence for unique differences in schizophrenia. For example, Buchsbaum et al.20 conducted a series of positron emission tomography studies of cerebral metabolism and demonstrated decreased anterior cingulate activity associated with increased age. This association was present in both patients with schizophrenia and healthy comparison subjects. However, the patients with schizophrenia also had additional areas of reduced metabolism with age in lateral and medial superior frontal cortex and in the anterior temporal regions. On the basis of these findings, we have examined whether similar age-related differences in schizophrenia can be identified by using oxygen-15 water PET imaging, with particular attention to the frontal and temporal regions.
METHODS
The sample consisted of 49 unmedicated male patients with DSM-IV schizophrenia, ages 20 to 51 years. All subjects were assessed with the Comprehensive Assessment of Symptoms and History (CASH) to systematically determine the presence of schizophrenia and to exclude concomitant severe substance abuse, medical or neurologic illness, head trauma, and mental retardation. First-episode and schizophreniform subjects were not included in this study. All subjects were patients with chronic schizophrenia who had discontinued medications for a 3-week period on an inpatient basis for the purpose of participating in neuroimaging studies with the Iowa Mental Health Clinical Research Center. The protocol was approved by the University of Iowa Human Subjects Committee, and all subjects provided written informed consent after a verbal explanation of the imaging procedures.
PET Data Acquisition
Regional cerebral blood flow (rCBF) was measured by using the bolus [15O] water method with a GE4096PLUS scanner. Fifteen slices (6.5 mm center-to-center) were acquired with a 10-cm axial field of view. Images were acquired over a 100-second interval following venous injection of 50–75 millicuries of [15O] water. Images were reconstructed for a 40-second interval following bolus transit, determined by time-activity curves from a region of interest over a cerebral artery.21 Arterial blood sampling allowed calculation of tissue perfusion in ml–1·min–1·100 g tissue, using the autoradiographic method.22 During image acquisition, subjects reclined quietly with eyes closed. No other instructions were given during the condition used in the analysis presented here. Quantitative flow images were transferred to the Image Processing Laboratory (IPL) of the Iowa Mental Health Clinical Research Center for analysis.
Magnetic Resonance Image Acquisition
The MR images are contiguous 1.5-mm thick coronal slices from a 1.5-tesla General Electric Signa scanner using an SPGR sequence, flip angle of 40 degrees, TE of 5 ms, TR of 24 ms, and 2 NEX. Data were processed by the IPL, using locally developed software.23 The initial step involved an automated neural-network procedure followed by hand-editing to separate brain from CSF. All brains were realigned parallel to the anterior commissure–posterior commissure (AC-PC) line and the interhemispheric fissure for comparability across subjects. Finally, images were resliced in three orthogonal planes to produce a three-dimensional data set.
PET and MRI Processing
The outlines of the PET images were automatically identified with an edge detection algorithm. Subjects' PET images were co-registered with their MR images, using a least-squares minimization procedure to fit the surface of the PET and MR images.24,25 The output parameters of this surface registration were then used as input parameters for a variance minimization program.26 The brains were then aligned in a standardized coordinate (Talairach) space using landmarks identified on the coregistered MR.27 An 18-mm Hanning filter was applied to the PET images for each condition to eliminate residual anatomical variability. Blood flow (ml–1·min–1·100 g tissue) was computed from PET count measures, utilizing the arterial blood flow curves.
Edge detection was performed on the PET image by using a series of filtering and thresholding steps. The image was thresholded to 150% of the mean of all pixels in the image, values below the threshold were set to zero, and values over 150% were not changed. This distinguished voxels containing tissue from those containing CSF. Subsequently a 3×3 mean filtering kernel was applied to the image, and the filtering process was repeated 10 times; that is, the remaining voxels were used to generate a mean flow value and the 150% threshold was reapplied. The filtering process generated increasingly sensitive edge detection such that the edge of the brain was clearly delineated from surrounding CSF. In this manner the total brain blood flow rate was adjusted for any atrophic changes. After thresholding, a smoothing technique was employed to enhance visual resolution prior to the regional flow analyses.
Data Analysis
Locally developed software was used to calculate a correlation analysis of age and regional cerebral blood flow (ml–1·min–1·100 g). Resolution was reduced from a voxel size of 1.08 mm×1.35×1.48 to 3.0 mm×2.7 mm×2 mm. In assessing the correlation matrix, the probability levels were adjusted to reflect the number of independent resolution elements or “resels.”28 The number of resels is calculated for each analysis of averaged PET images, and it is the experience of our imaging laboratory that the total PET brain volume typically comprises approximately 300 independent resels. Therefore, for the purpose of conducting the correlation analysis described below, we specified r=0.5 as significant, which corresponds to P=0.00025 given 49 subjects (df=47). To conduct the correlation analysis, resel data were treated as a collection of data vectors, the length being defined by the number of subjects. The blood flow values were used to calculate a Spearman's correlation of that value with age. A three-dimensional correlation matrix displaying correlation coefficients was generated. This correlation image represents an overall pattern of the relationship between blood flow and age across brain regions. A threshold was then applied, such that only regions with Spearman's correlation coefficients greater than r=0.50 in 50 or more contiguous voxels were displayed. Talairach coordinates were used to identify the regions of significance. Lastly, a simple regression analysis was performed to examine the relationship between global CBF and age.
RESULTS
The mean age of the sample was 32.6±8.1 years (mean±SD reported throughout); mean duration of illness was 10.25±7.5 years, mean age at onset was 22.8±6.1 years, and mean amount of education was 13.5±2.2 years. The mean global rating of positive psychotic symptoms (i.e., hallucinations and delusions) as measured by the Scale for the Assessment of Positive Symptoms29 was 6.1±2.8. The mean global negative symptom score from the Scale for the Assessment of Negative Symptoms30 was 12.0±3.4, comprising the sum of alogia, avolition, anhedonia, and affective flattening scores. The mean rating from the SAPS for disorganized symptoms (formal thought disorder, inappropriate affect, bizarre behavior) was 4.8±3.4.
Figure 1 depicts the regional distribution of the reduced rCBF in relation to age. Table 1 displays the Talairach coordinates and Spearman correlations for the regions in which significant correlations between age and rCBF were observed. A significant negative correlation between age and rCBF was observed in mesial frontal cortex, specifically the anterior cingulate cortex (r=–0.54). The Talairach coordinates identifying the location of this region were as follows: x=1 (right/left); y=38 (anterior/posterior); z=20 (superior/inferior). A negative correlation was also observed bilaterally in the frontal cortex, Brodmann area (BA) 8, and a negative correlation between rCBF and age was observed bilaterally in the parietal lobe, BA 40 (see Table 1). As noted above, a regression analysis was also performed for global CBF using age as the independent variable. This analysis revealed a slope of –0.60, R2=0.37, F= 4.9, df=45, P=0.032. These results reflect age-associated decrements in total brain blood flow as well as regional decrements in anterior cingulate, frontal, and parietal regions.
DISCUSSION
This study examined the association between age and regional cerebral blood flow in adult patients with schizophrenia. We found regionally reduced blood flow with increased age in the anterior cingulate as well as bilateral frontal and parietal regions. Although this age group would not be considered “late-life” by any means, a trajectory of age-related changes could conceivably begin at any point in the life span given the probable neurodevelopmental brain abnormalities associated with schizophrenia. Thus, tracking the illness across adulthood may yield insights into the interesting and elusive prognostic indicators of late-life outcome.
One of the key challenges in the study of aging and schizophrenia is distinguishing how typical age-related brain changes interact with changes that may be secondary to the illness. In this study, the anterior cingulate findings are consistent with findings in normal aging by our own group and others.20,31 However, the flow differences in frontal (BA 8) and parietal (BA 40) regions may represent age-related changes that are unique to schizophrenia. Certainly these findings must be interpreted in light of their limitations given the retrospective and cross-sectional nature of the analysis; however, these observations are consistent with some of the current models of the neuropathology of schizophrenia and may help to stimulate further research.
One current influential model proposes that dorsolateral prefrontal cortex and the working memory functions mediated by this brain region are impaired in schizophrenia.32 However, the regional decrements in rCBF in premotor cortex (BA 8) observed here might also be relevant to schizophrenia, considering the involvement of the premotor cortex in the preparation of movement. Premotor cortex receives input from the posterior association cortex directly (e.g., parietal areas including BA 40, as seen in this analysis) as well as input from the prefrontal association cortex.33–35 This cortical network appears to be involved in planning future actions, and loss of function in these regions may therefore contribute to the expression of negative symptoms such as avolition. This notion is consistent with clinical descriptions of schizophrenia in later life; that is, negative symptoms may persist or worsen with age, in contrast to psychotic symptoms, which may improve.36 Negative symptoms have also been associated with both cognitive and adaptive impairment among patients with schizophrenia in late life.37
In addition to the premotor cortex, the reduced rCBF observed in posterior association cortex (BA 40) may also be viewed as related to negative symptom expression in late life. One function of this region is the integration of multimodal sensory information from higher-order sensory cortices, as well as interaction with anterior association cortices and anterior cingulate regions involved in planning and preparation of future movement.38,39 Therefore, dysfunction of parietal cortex (BA 40), much like premotor cortex (BA 8), ties intuitively into the avolitional aspects of negative symptoms. Additionally, there is evidence that posterior parietal cortex (BA 40) may interact with premotor cortex and anterior association cortices in spatial attention and working memory functions, which are known to be impaired in schizophrenia.32,40 Lastly, both normal aging and schizophrenia have been associated with abnormalities in the anterior cingulate region.20,41,42 It is possible that decreased function in this region may have greater implications for patients with schizophrenia because of its role in attention, arousal, vigilance, and selective attention. Recent work has demonstrated abnormalities in anterior cingulate activation among patients with schizophrenia during tasks measuring selective attention performance.43
It appears, then, that patients with schizophrenia may experience unique impairments with aging that manifest in concert with changes in symptom characteristics. This scenario sharply contrasts with the more global impairments associated with conditions such as Alzheimer's disease. The more circumscribed deficits with aging in schizophrenia are likely tied to greater negative symptom severity such as increased avolition. In addition to these symptom changes, current research is also seeking to better characterize discrete cognitive deficits associated with aging in schizophrenia. For example, Harvey et al.44 recently demonstrated a relative preservation of reading skills in late-life schizophrenia even in the face of other more marked cognitive impairments. A combination of studies examining symptoms, cognition, and neural function may be most successful in characterizing the complex nature of age effects in schizophrenia.
ACKNOWLEDGMENTS
This research was supported in part by the National Institute of Mental Health Grants MH31593, MH40856, and MHCRC43271.
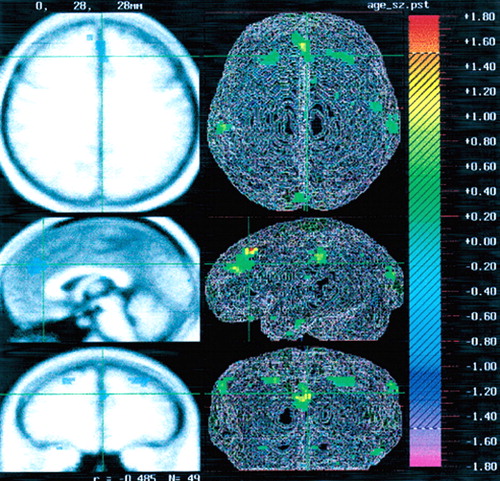
FIGURE 1. Positron emission tomography ([15O] water PET) image demonstrating the relationship between age and regional cerebral blood flow in schizophreniaNegative correlations are present in the anterior cingulate and in frontal and parietal cortex bilaterally.
 |
1 Friedman JI, Harvey PD, Kemether E, et al: Cognitive and functional changes with aging in schizophrenia. Biol Psychiatry 1999; 46:921-928Crossref, Medline, Google Scholar
2 Heaton R, Paulsen JS, McAdams LA, et al: Neuropsychological deficits in schizophrenics: relationship to age, chronicity, and dementia. Arch Gen Psychiatry 1994; 51:469-476Crossref, Medline, Google Scholar
3 Arnold SE, Trojanowski JQ: Cognitive impairment in elderly schizophrenia: a dementia (still) lacking a distinctive histopathology. Schizophr Bull 1996; 22:5-9Crossref, Medline, Google Scholar
4 Ciompi L: Catamnestic long-term study on the course of life and aging of schizophrenics. Schizophr Bull 1980; 6:606-618Crossref, Medline, Google Scholar
5 Harding C, Brooks G, Ashikaga T, et al: The Vermont longitudinal study of persons with severe mental illness. Am J Psychiatry 1987; 144:727-735Crossref, Medline, Google Scholar
6 McGlashan T: A selective review of recent North American long-term follow-up studies of schizophrenia. Schizophr Bull 1988; 14:515-542Crossref, Medline, Google Scholar
7 Huber G, Gross G, Schuttler R, et al: Longitudinal studies of schizophrenia patients. Schizophr Bull 1980; 6592-6605Google Scholar
8 Volkow ND, Logan J, Fowler JS, et al: Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry 2000; 157:75-80Crossref, Medline, Google Scholar
9 Arnold SE: Hippocampal pathology, in The Neuropathology of Schizophrenia, edited by Harrison PJ, Roberts GW. New York, Oxford University Press, 2000, pp 57-80Google Scholar
10 Powers RE: Neurobiology of aging, in Textbook of Geriatric Neuropsychiatry, 2nd edition, edited by Coffey CE, Cummings JL. Washington, DC, American Psychiatric Press, 2000, pp 33-79Google Scholar
11 Miller DD, Andreasen NC, O'Leary DS, et al: Effect of antipsychotics on regional cerebral blood flow measured with positron emission tomography. Neuropsychopharmacology 1997; 17:230-240Crossref, Medline, Google Scholar
12 Pearlson GD, Marsh L: Structural brain imaging in schizophrenia: a selective review. Biol Psychiatry 1999; 46:627-649Crossref, Medline, Google Scholar
13 Keshavan MS, Mulsant BH, Sweet RA, et al: MRI changes in schizophrenia in late life: a preliminary controlled study. Psychiatry Res 1996; 60:117-123Crossref, Medline, Google Scholar
14 Waddington JL, O'Callaghan E, Buckley P, et al: The age dependencies of MRI abnormalities in schizophrenia suggest early ventricular enlargement but later prominence of cortical atrophy. Schizophr Res 1991; 5:188-189Crossref, Medline, Google Scholar
15 Buchsbaum MS: The frontal lobes, basal ganglia and temporal lobes as sites for schizophrenia. Schizophr Bull 1990; 16:379-389Crossref, Medline, Google Scholar
16 Andreasen NC, Rezai K, Alliger R, et al: Hypofrontality in neuroleptic naïve patients and in patients with chronic schizophrenia: assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49:943-958Crossref, Medline, Google Scholar
17 Goldstein PC, Brown GC, Marcus A, et al: Effects of age, neuropsychological impairment and medication on regional cerebral blood flow in schizophrenia and major affective disorder. Henry Ford Hosp Med J 1990; 38:202-206Medline, Google Scholar
18 Dupont R, Lehr P, Lamoureaux G, et al: Preliminary report: cerebral blood flow abnormalities in older schizophrenic patients. Psychiatry Res 1994; 55:121-130Crossref, Medline, Google Scholar
19 Cantor-Graae E, Warkentin S, Franzen G, et al: Aspects of stability of regional cerebral blood flow in chronic schizophrenia: an 18-year follow-up study. Psychiatry Res 1991; 40:253-266Crossref, Medline, Google Scholar
20 Buchsbaum MS, Hazlett EA: Functional brain imaging and aging in schizophrenia. Schizophr Res 1997; 27(2-3):129-141Google Scholar
21 Herscovitch P, Marckham J, Raichle ME: Brain blood flow measured with [15-O] water, I: theory and error analysis. J Nucl Med 1983; 24:782-789Medline, Google Scholar
22 Hichwa RD, Ponto LLB, Watkins GL (eds): Clinical blood flow measurements with [15-O] water and positron emission tomography (PET). Symposium Proceedings of the International Symposium of Chemists: Views of Imaging Centers, edited by Emran AM. New York, Plenum, 1995, pp 804-806Google Scholar
23 Cizadlo T, Andreasen NC, Zeien, G, et al: Image registration in the analysis of multiple-injection 15-O H2O PET studies: BRAINFIT. Proceeding from SPIE, The International Society for Optical Engineering 1994; 2168:423-430Google Scholar
24 Levin DN, Pelizzari CA, Chen GT, et al: Retrospective geometric correlations of MR, CT, and PET images. Radiology 1988; 169:817-823Crossref, Medline, Google Scholar
25 Woods RP, Cherry SR, Mazziotta JC: Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 1992; 16:620-633Crossref, Medline, Google Scholar
26 Arndt S, Cizadlo T, Andreasen NC, et al: A comparison of approaches to the statistical analysis of [15O]H2O PET cognitive activation studies. J Neuropsychiatry Clin Neurosci 1995; 7:155-168Link, Google Scholar
27 Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme, 1992Google Scholar
28 Worsley KJ, Evans AC, Marrett S, et al: P A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 1992; 12:900-918Crossref, Medline, Google Scholar
29 Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA, The University of Iowa, 1983Google Scholar
30 Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA, The University of Iowa, 1984Google Scholar
31 Schultz SK, O'Leary DS, Boles Ponto LL, et al: A positron emission tomography study of age-related changes in regional cerebral blood flow among young to mid-life adults. Neuroreport 1999; 10:2493-2496Crossref, Medline, Google Scholar
32 Goldman-Rakic PS, Selemon LD: Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 1997; 23:437-458Crossref, Medline, Google Scholar
33 Lepage M, Beaudoin G, Boulet C, et al: Frontal cortex and the programming of repetitive tapping movements in man: lesion effects and functional neuroimaging. Brain Res Cogn Brain Res 1999; 8:17-25Crossref, Medline, Google Scholar
34 Toni I, Schluter ND, Josephs O, et al: Signal-, set-, and movement-related activity in the human brain: an event-related fMRI study. Cereb Cortex 1999; 9:35-49Crossref, Medline, Google Scholar
35 Griffiths TD, Green G, Rees G, et al: Human brain areas involved in the analysis of auditory movement. Hum Brain Mapp 2000; 9:72-80Crossref, Medline, Google Scholar
36 Harvey PD, Lombardi J, Leibman M, et al: Cognitive impairment and negative symptoms in geriatric chronic schizophrenia patients: a follow-up study. Schizophr Res 1996; 22:223-31Crossref, Medline, Google Scholar
37 McGurk SR, Moriarty PJ, Harvey PD, et al: The longitudinal relationship of clinical symptoms, cognitive functioning and adaptive life in geriatric schizophrenia. Schizophr Res 2000; 2:47-55Crossref, Google Scholar
38 Senior C, Barnes J, Giampietro V, et al: The functional neuroanatomy of implicit-motion perception or representational momentum. Curr Biol 2000; 10:16-22Crossref, Medline, Google Scholar
39 Corbetta M, Kincade JM, Ollinger JM, et al: Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 2000; 3:292-297Crossref, Medline, Google Scholar
40 Sirigu A, Daprati E, Pradat-Diehl P, et al: Perception of self-generated movement following left parietal lesion. Brain 1999; 10:1867-1874Crossref, Google Scholar
41 Carter CS, Mintun M, Nichols T, et al: Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15-O] H2O PET study during single trial Stroop task performance. Am J Psychiatry 1999; 154:1670-1675Crossref, Google Scholar
42 Mathew RJ, Wilson WH, Tant SR, et al: Abnormal resting regional cerebral blood flow patterns and their correlates in schizophrenia. Arch Gen Psychiatry 1988; 45:542-549Crossref, Medline, Google Scholar
43 Mathew RJ, Wilson WH: Chronicity and a low anteroposterior gradient of cerebral blood flow in schizophrenia. Am J Psychiatry 1990; 147:211-213Crossref, Medline, Google Scholar
44 Harvey PD, Moriarty PJ, Friedman JI, et al: Differential preservation of cognitive functions in geriatric patients with lifelong chronic schizophrenia: less impairment in reading compared with other skill areas. Biol Psychiatry 2000; 47:962-968Crossref, Medline, Google Scholar



