Tardive Dyskinesia Predicts Prolactin Response to Buspirone Challenge in People With Schizophrenia
Abstract
Prolactin response to buspirone was evaluated in patients with schizophrenia, with and without tardive dyskinesia (TD). Prolactin response in patients with schizophrenia without TD was significantly decreased, compared to healthy comparison subjects (F=6.36, df=5, p<0.0001). Furthermore, prolactin levels after administration of buspirone were not significantly increased from baseline. In contrast, there was no prolactin response difference between patients with schizophrenia and TD and healthy subjects. This finding suggests that decreased dopamine (D2) receptor sensitivity may result in lower risk of developing TD and may lead to a fuller understanding of the variable expression of D2-receptor mediated side effects.
Tardive dyskinesia (TD) is a movement disorder that affects approximately 20%–60% of people continually treated with conventional antipsychotics.1,2 It has an unknown pathophysiological basis. Dopamine (D2) receptor supersensitivity, gamma aminobutyric acid (GABA)-ergic hypofunction, excitotoxicity, and oxidative stress have all been implicated in the pathophysiology of TD.3–5 However, the D2 hypothesis has been widely accepted as a major contributing factor to this movement disorder. Long-term administration of conventional antipsychotics increases D2 receptor sensitivity and D2 turnover. These changes are believed to be associated with the development of TD.6 However, this is not consistently reported, and some investigators have found no significant association between the D2 system and TD.7–11 Methodological issues have contributed to these reported differences. First, medication effects relating to the evaluation of TD are a primary consideration. Because most people with schizophrenia need to take antipsychotics throughout their entire life, one must consider the influence of antipsychotics on the severity and clinical course of TD, including covert or withdrawal TD. Second, TD is a dynamic disorder associated with changes in both severity and pattern over time, possibly unrelated to medication treatment. To have confidence in the TD diagnosis, the evaluation should be conducted consecutively.12 Third, because antipsychotics may induce other motor side effects and other neurological disorders are frequently combined with TD, false positive diagnoses are common.
Buspirone is an azaspirodecanedione derivative that has affinity for both 5-HT1A as a partial agonist and D2 receptors as an antagonist.13 Buspirone evokes the release of prolactin through both mechanisms.14–17 Prolactin provocation to buspirone has been considered useful in evaluating serotonin and D2 receptor function. We evaluated D2 and 5HT1A receptor sensitivity in people with stringently evaluated TD, using prolactin responses to buspirone in order to assess the underlying physiology of vulnerability to TD. Prolactin response to buspirone varies in many psychiatric disorders, and buspirone challenge studies are uncomplicated. Differences in response to buspirone, if present, may also lead to a useful predictive test of vulnerability to dyskinesia. Second generation antipsychotics have lowered but not eliminated the risk of dyskinesia, particularly in sensitive populations such as the elderly.18 Therefore, accurate assessments of risk and greater understanding of pathophysiology in this area are still critically needed.
METHOD
Subjects
In this study, 87 Korean male subjects participated: 22 subjects with schizophrenia and TD; 28 subjects with schizophrenia but without TD; and 37 healthy comparison subjects. All patients were recruited through Dongsuh Hospital Inpatient Unit. Women were not included in this study, and we wanted to avoid the confounding effect of the difference of baseline prolactin level on the prolactin response to buspirone because prolactin levels in women can be influenced by their menstrual cycle. All patients met DSM-IV criteria for a schizophrenia diagnosis, and all were considered clinically stable. No subjects met DSM-IV criteria for any other axis I or II disorder, as determined by means of psychiatric interview and the Structured Clinical Interview for DSM-IV, which were conducted by a psychiatrist. Subjects who had a history of neurological and endocrine disorders, recent dental problems or artificial teeth were excluded. Comparison subjects were healthy volunteers, with no history of major psychiatric disorder and no family history of major psychiatric disorder in first-degree relatives.
Clinical Ratings
To avoid the compounding effect of extrapyramidal symptoms, subjects who showed overt parkinsonian symptoms (rated more than two points on Simpson-Angus Rating Scale26) were excluded. Diagnosis for TD was based on DSM-IV and Schooler and Kane criteria for TD.19 Two experienced psychiatrists performed the research ratings independently, and interrater reliability was greater than r=0.90 (p<0.001), with standard videotape ratings. To confirm the presence of TD, three consecutive evaluations were performed for each subject: at baseline, 3-month follow-up, and after the drug washout period. Subjects who met the criteria for TD on all three occasions were classified as having TD. Stringent characterization of the non-TD subjects included a total score of less than 2 points on the Abnormal Involuntary Movement Scale (AIMS) 27and less than 1-point on each individual AIMS item on three consecutive evaluations. The physical health of each subject was confirmed by medical examination, standard laboratory tests, a chest X-ray, electrocardiogram (ECG), and electroencephalogram (EEG) prior to participation. The Institutional Review Board approved the study, and all subjects gave informed consent prior to study participation.
Neuroendocrine Testing
All subjects had been taking conventional antipsychotics (haloperidol, chlorpromazine, thioridazine, trifluoperazine, fluphenazine) and were withdrawn from their medication 1 week prior to study. Five people without TD were withdrawn from the study after drug washout because of newly onset dyskinetic movements and refusal of cannular insertion for blood drawing. No subjects showed aggravated psychotic symptoms during the washout. Thus, data were analyzed for 82 Korean, male subjects: 22 with schizophrenia and TD; 25 with schizophrenia but without TD; and 37 healthy comparison subjects. At baseline, subjects were required to attend the laboratory at 7:00 a.m. after an overnight fast. Between 7:30 a.m. and 8:00 a.m., an indwelling venous cannular was inserted into the superficial forearm vein. For the duration of the test, subjects rested and remained fasting except for water. To measure serum prolactin concentrations, blood was drawn at 0, 30, 60, 90, 120 and 150 minutes after oral administration of 30 mg buspirone hydrochloride, which was administered at 9:00 a.m. Serum was stored at −20°C until assayed. Serum concentrations of prolactin were determined by Electrochemiluminoscence assay, using a commercial kit (Boehringer Mannheim). Inter- and intraassay coefficients of variation were 4.8% and 2.8%, respectively.
Statistical Analysis
Two-tailed student’s t test and analysis of variance (ANOVA) were used to characterize differences between demographic and clinical variables. Prolactin levels in healthy comparison subjects were compared with subjects with and without TD separately. Repeated measures ANOVA was used to evaluate the effect of time and time-by-group interaction between groups. Analysis of covariance (ANCOVA) was used to determine the significance of difference between groups in prolactin levels at each time point following buspirone administration. Analysis of variance was used to compare the peak levels among the groups. An alpha level of 0.05 was considered statistically significant, and all tests were two-tailed.
RESULTS
Subjects’ Characteristics
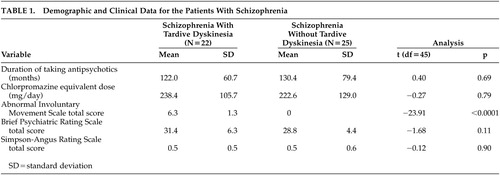
The mean age was not significantly different among schizophrenia subjects with and without TD and the healthy comparison subjects (mean=41.1 years, SD=4.7; mean=39.8 years, SD=6.4; mean=42.4 years, SD=5.4) (F=1.72, df=2, p=0.19). There were no significant differences in clinical characteristics, except in the AIMS total score (t=22.4, df=21, p<0.0001), between schizophrenia subjects with and without TD at baseline (Table 1).
Prolactin Response to Buspirone
Baseline prolactin levels were not significantly different among all three groups (F=0.11, df=2, p=0.89). Prolactin levels in subjects with TD, as compared to healthy comparison subjects, demonstrated a significant effect over time on repeated measures ANOVA (F=28.38, df=5, p<0.0001) but not a significant effect of time-by-group interaction (F=0.34, df=5, p=0.74). Analysis of covariance showed no significant differences at each time point between two groups, controlling for baseline group difference. Prolactin levels in the schizophrenia subjects without TD, as compared to healthy comparison subjects, revealed both a significant time effect (F=16.45, df=5, p<0.0001) and time-by-group interaction on repeated measures ANOVA (F=6.36, df=5, p<0.0001), indicating that two groups have different response profiles. When controlling for baseline differences, ANCOVA showed significant differences in prolactin levels from 60 minutes through 150 minutes between schizophrenia subjects without TD and comparison subjects (60 minutes: F=8.10, df=1, p=0.0006, 90 minutes: F=13.24, df=1, p=0.0006, 120 minutes: F=10.58, df=1, p=0.0020, 150 minutes: 10.32, df=1, p=0.0022) (Figure 1). The peak levels of prolactin in subjects without TD were significantly lower than prolactin levels for subjects with TD and healthy comparison subjects (F=6.76, df=3, p=0.0004) (Figure 2).
DISCUSSION
The principal findings of this study indicate that schizophrenia subjects without TD showed a significantly decreased prolactin response, reflecting D2 receptor down-regulation and/or serotonergic system insensitivity. However, schizophrenia subjects with TD showed no significant difference in prolactin responses, when matched up against healthy comparison subjects.
Although D2 receptor sensitivity is a leading hypothesis of the pathogenesis of TD, our results do not match the D2 receptor supersensitivity theory. Although the D2 supersensitivity hypothesis has been widely accepted as the leading theory of TD, it still has not been confirmed. Moreover, it should be noted that D2 supersensitivity does not always follow long-term administration of antipsychotic drugs.7–11,20
The effects of previous antipsychotic drug treatment in subjects with schizophrenia may be a confounding factor and limitation to the study. Although all drugs were withdrawn at least 1 week prior to the study, this may have not been enough time to exclude persisting effects of antipsychotics on the brain. However, if effects of previous drug treatment were markedly persistent, baseline prolactin levels in the schizophrenia group should have been different from the healthy comparison subjects. This was not the case, however. Additionally, differences in the clinical characteristics of our subjects and those of patients in previous studies might produce different results, as dopaminergic transmission, D2 synaptic concentration, and D2 receptor occupancy by D2 may differ, according to the severity and course of schizophrenic illness.21 However, illness characteristics did not vary between our TD and non-TD group, which is probably an unlikely explanation of our findings. It is difficult to explain explicitly why schizophrenia subjects with TD showed no significant difference in prolactin responses, compared with healthy subjects.
It is interesting to note that subjects without TD in our study demonstrated blunted prolactin responses to buspirone, which may mean there was decreased D2 receptor sensitivity in these subjects. It is well known that the prevalence of TD occurs in approximately 20%–60% of subjects treated continually with antipsychotic medication. This means that 40%–80% are relatively resistant to developing TD. There are few explanations as to why some patients remain free from TD, even though they have been taking antipsychotics for long periods. Previous studies indicate that decreased or absent D2 receptor supersensitivity could be related to a reduction of TD risk. In animal studies, acute administration of sulpiride and clozapine, known not to produce TD, appear to act at D2 receptor sites, but continuous chronic administration of these compounds does not result in the development of striatal D2 receptor hypersensitivity.22 Chronic administration of haloperidol increases striatal D2 receptor hypersensitivity. For example, it enhances 3-hour spiroperidol binding in the striatum and in mesolimbic loci.23 Other evidence demonstrates that certain drugs, such as carbamazepine, may improve haloperidol induced dyskinetic movement by reducing D2 supersensitivity24 or decreasing blood levels25 and, as a result, brain exposure to haloperidol. Although these results cannot provide direct evidence to show that decreased D2 receptor sensitivity is related to the low incidence of TD, they suggest that decreased D2 receptor sensitivity may play an important role in reducing the development of TD. Therefore, we can propose that decreased D2 receptor sensitivity may play a role in reducing the development of TD in some patients.
We do not know the mechanism by which people without TD in our study showed a decrease in D2 sensitivity. This may have been a result of individual variable compensation to the chronic administration of antipsychotics, reactions which could be influenced by genetic variability.
Our results, however, should be interpreted with caution due to other limitations. First, the effect of buspirone on prolactin response may not directly reflect the changes in central dopaminergic or serotonergic function but some combination of both. This nonspecificity challenges our result regarding the D2 receptor sensitivity-TD relationship. This work should be followed by a study of buspirone challenge with and without pindolol, a 5-HT1A agonist, which would help differentiate the dopaminergic versus serotonergic components of the response. Second, long-term administration of antipsychotics may change the D2 receptor sensitivity. However, it is not yet clear how well prolactin response to buspirone can reflect the functional state of D2 receptors. Third, medication-related effects, such as differences in the past history of antipsychotic medication and the potential role of drug metabolism, should be considered in interpreting our results, although there were no obvious differences between the TD and non-TD groups. All subjects were men and fairly young, limiting the generalizability of our results. Additionally, there was no placebo-control, although a stringent criterion was utilized for diagnosing TD. However, the inclusion of a healthy comparison group partially mitigates such a problem. These findings may yield more needed research in this area. This result may lead to a fuller understanding of the variable expression of D2-receptor mediated side effects in patients treated with antipsychotics.
ACKNOWLEDGMENTS
This study was supported by a grant to Drs. Kim and Shim from the Department of Psychiatry, Inje University, Korea and an Intervention Research Center grant (MH-40279).
The authors thank Jeong-Ik Kim, M.D., Seong-Hwan Yoon, M.D., and Tae-Min Kang, M.D. for referring patients to this study and Yang Yu, M.S. for assistance with data analysis.
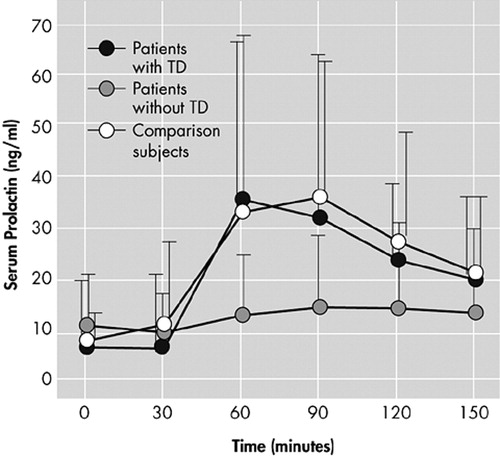
Figure 1. Prolactin Responses to Buspirone in Schizophrenia Patients With (N=22) and Without TD (N=25), and Normal Comparison Subjects (N=37)a
aThe prolactin concentrations are shown as mean and standard deviation.
TD=tardive dyskinesia
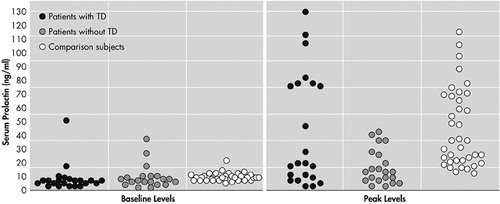
Figure 2. Scattergram of Baseline and Peak Levels of Prolactin After Administration of Buspirone in Schizophrenia Patients With (N=22), Without TD (N=25), and Normal Comparison Subjects (N=37)
TD=tardive dyskinesia
 |
1 Kane JM, Woerner M, Lieberman J: Tardive dyskinesia: prevalence, incidence, and risk factors. J Clin Psychopharmacol 1988; 8:52–56Crossref, Medline, Google Scholar
2 Byne W, White L, Parella M, et al: Tardive dyskinesia in a chronically institutionalized population of elderly schizophrenic patients-prevalence and association with cognitive impairment. Int J Geriatr Psychiatry 1998; 13:473–479Crossref, Medline, Google Scholar
3 Wright AM, Bempong J, Kirby ML, et al: Effects of haloperidol metabolites on neurotransmitter uptake and release: possible role in neurotoxicity and tardive dyskinesia. Brain Res 1998; 788:215–222Crossref, Medline, Google Scholar
4 Casey DE: Tardive dyskinesia: pathophysiology and animal models. J Clin Psychiatry 2000; 61:5–9Google Scholar
5 Sachdev PS: The current status of tardive dyskinesia. Aust N Z J Psychiatry 2000; 34:355–369Crossref, Medline, Google Scholar
6 Silvestri S, Seeman MV, Negrete JC, et al: Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology 2000; 152:174–180Crossref, Medline, Google Scholar
7 Crow TJ, Cross AJ, Johnstone EC, et al: Abnormal involuntary movements in schizophrenia: are they related to the disease process or its treatment? are they associated with changes in dopamine receptors? J Clin Psychopharmacol 1982; 2:336–340Crossref, Medline, Google Scholar
8 Casey DE: Behavioral effects of long-term neuroleptic treatment in Cebus monkeys. Psychopharmacology 1985; 2:211–216Google Scholar
9 Cross AJ, Crow TJ, Ferrier IN, et al: Chemical and structural changes in the brain in patients with movement disorder. Psychopharmacology 1985; 2:104–110Google Scholar
10 Jenner P, Marsden CD: Is the dopamine hypothesis of tardive dyskinesia completely wrong? Trends Neurosci 1986; 9:259–260Crossref, Google Scholar
11 Adler CM, Malhotra AK, Elman I, et al: Amphetamine-induced dopamine release and post-synaptic specific binding in patients with mild tardive dyskinesia. Neuropsychopharmacology 2002; 26:295–300Crossref, Medline, Google Scholar
12 Newell KM, Wszola B, Sprague RL, et al: The changing effector pattern of tardive dyskinesia during the course of neuroleptic withdrawal. Exp Clin Psychopharmacol 2001; 9:262–268Crossref, Medline, Google Scholar
13 Peroutka SJ: Selective interaction of novel anxiolytics with 5-hydroxytryptamine1A receptors. Biol Psychiatry 1985; 20:971–979Crossref, Medline, Google Scholar
14 Meltzer HY, Flemming R, Robertson A: The effect of buspirone on prolactin and growth hormone secretion in man. Arch Gen Psychiatry 1983; 40:1099–1102Crossref, Medline, Google Scholar
15 Cowen PJ, Anderson IM, Grahame-Smith DG: Neuroendocrine effects of azapirones. J Clin Psychopharmacol 1990; 10:21–25Crossref, Google Scholar
16 Cherek DR, Moeller FG, Khan-Dawood F, et al: Prolactin response to buspirone was reduced in violent compared to nonviolent parolees. Psychopharmacology 1999; 142:144–148Crossref, Medline, Google Scholar
17 Bridge MW, Marvin G, Thompson CE, et al: Quantifying the 5-HT1A agonist action of buspirone in man. Psychopharmacology 2001; 158:224–229Crossref, Medline, Google Scholar
18 Marder SR, Essock SM, Miller AL, et al: The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull 2002; 28:5–16Crossref, Medline, Google Scholar
19 Schooler NR, Kane JM: Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry 1982; 39:486–487Medline, Google Scholar
20 Abi-Dargham A, Rodenhiser J, Printz D, et al: Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci 2000; 97:810–819Crossref, Google Scholar
21 Jenner P, Marsden CD: Chronic pharmacological manipulation of dopamine receptors in brain. Neuropharmacology 1987; 26:931–940Crossref, Medline, Google Scholar
22 Jenner P, Rupniak NM, Marsden CD: Differential alteration of striatal D-1 and D-2 receptors induced by the long-term administration of haloperidol, sulpiride or clozapine to rats. Psychopharmacology 1985; 2:174–181Google Scholar
23 Seeger TF, Thal L, Gardner EL: Behavioral and biochemical aspects of neuroleptic-induced dopaminergic supersensitivity: studies with chronic clozapine and haloperidol. Psychopharmacology 1982; 76:182–187Crossref, Medline, Google Scholar
24 LaHoste GJ, Wigal T, King BH, et al: Carbamazepine reduces dopamine-mediated behavior in chronic neuroleptic-treated and untreated rats: implications for treatment of tardive dyskinesia and hyperdopaminergic states. Exp Clin Psychopharmacol 2000; 8:125–132Crossref, Medline, Google Scholar
25 Kahn EM, Schulz SC, Perel JM, et al: Change in haloperidol level due to carbamazepine: a complicating factor in combined medication for schizophrenia. Clin Psychopharmacol 1990; 10:54–57Crossref, Medline, Google Scholar
26 Simpson GM, Angus JW: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand 1970; 22 (Suppl 44):11–9Google Scholar
27 Lane RD, Glazer WM, Hansen TE, et al: Assessment of tardive dyskinesia using the Abnormal Involuntary Movement Scales. J Nerv Ment Dis 1985; 173:353–357Crossref, Medline, Google Scholar



