Oxidative Stress During Treatment With First- and Second-Generation Antipsychotics
Abstract
Neurotoxicity of first-generation antipsychotics (FGAs) may be involved in lipid peroxidation, which is the pathogenesis of extrapyramidal symptoms, including tardive dyskinesia (TD). Blood samples at day 0, 7, and 21 drawn from patients taking antipsychotics were analyzed for malondialdehyde (MDA) in plasma, a marker of lipid peroxidation, by high-performance liquid chromatography. Of 115 patients enrolled, 92 patients completed the study. Most MDA levels were within normal ranges (<1.0 μmol/liter). Malondialdehyde levels in patients receiving clozapine (p=0.002), quetiapine (p=0.003), amisulpride (p=0.008), and risperidone (p=0.008) were significantly lower than within the first generation antipsychotic group. The authors conclude that lipid peroxidation is significantly higher in treatment with FGAs.
Tardive dyskinesia (TD) and extrapyramidal side effects are frequent side effects of treatment with first-generation antipsychotics (FGAs). Different hypotheses have been considered in determining the causes of TD, a disabling adverse iatrogenic effect of antipsychotics. One possible pathophysiological explanation is neuronal cell damage from free radicals induced by antipsychotics.1 This hypothesis is supported by evidence of elevated levels of lipid peroxidation products and decreased vitamin E levels in dyskinetic patients.2,3 In animal studies, oxidative stress and elevated levels of lipid peroxidation have been implicated in haloperidol toxicity.4 Evidence to support these animal studies includes elevated levels of lipid peroxidation with FGA treatment in psychotic patients.5 The clinical effects of vitamin E on TD are only positive in the animal model but not in patients with TD itself.6 However, the work of an Israeli group showed a significant improvement of TD in 22 patients with schizophrenia, with the antioxidant melatonin in a double-blind, placebo-controlled, crossover study.7,8 Oxidative stress has been assumed to be a pathogenetic mechanism associated with neurodegeneration.9 The peroxidation of membrane lipids is associated with a wide variety of toxicological effects. Lipid peroxidation itself reflects the interaction between molecular oxygen and polyunsaturated fatty acids, leading to oxidative deterioration of the latter with the production of several breakdown products. An established marker of oxidative stress is malondialdehyde (MDA), which can easily be detected.10,11,12 As patients with FGAs more often have extrapyramidal symptoms (EPS) and TD, our hypothesis is that FGAs might lead to higher lipid peroxidation, which might induce EPS, and with long-term treatment, TD. Active peroxidation can be recognized by increased levels of lipid peroxidation in plasma.2,13,14
METHOD
Patients
Volunteer patients being newly treated with FGAs or SGAs were recruited after positive approval of the ethics committee for a 3-week study. Recruiting criteria included: diagnosis of schizophrenia, schizoaffective disorders, or mood disorders; antipsychotic treatment; and above age 18 and under age 66. Exclusion criteria were: previous history of major head injuries or neurological disorder, diabetes, and current or previous substance misuse; planned and given combinations of antipsychotics; intake of vitamins and/or antioxidants; and TD. Patients with diabetes were excluded because there is increasing evidence suggesting a link between diabetes and oxidative stress.15,16 Patients were required to give informed consent for participation in the study after the procedures were fully explained. On day 0 (study begins), a novel treatment with an antipsychotic started, whether with an FGA or SGA. There was no washout period in patients who were treated previously with other antipsychotics, but combinations of antipsychotics were not allowed. Concomitant medications such as antidepressants, lithium, or mood stabilizers were allowed within the study. Blood samples, Brief Psychiatric Rating Scale (BPRS) and Abnormal Involuntary Movement Scale (AIMS) were scheduled for day 0, 7, and 21 for each individual patient.
Clinical Assessment
Extrapyramidal symptoms were assessed by the AIMS.17,18 Psychopathology was assessed with the BPRS.19 Fasting blood samples were taken at day 0, day 7, and day 21 between 7:00 a.m. and 9:00 a.m. Both scales were administered between 9:00 a.m. and noon.
Rater Training
Three experienced physicians worked as raters for the AIMS and BPRS, each rater working only in one center. All underwent a rater training together, but no interrater reliability measure was calculated after the training. V.K. conducted the rating at center 1 (Hannover); D.D. conducted the rating at center 2 (Göttingen); and S.B. at center 3 (Erlangen).
Laboratory Tests
Venous blood was drawn in the early morning after an overnight fast and centrifuged at 2000 g for 15 minutes to remove red blood cells (RBCs). The obtained clear plasma fraction was stored at −70°C until the time of the assay, which was conducted 3–5 months after the blood sampling. Plasma MDA, with a detection limit of 0.01 μmol/liter, was determined as previously described20 by the laboratory Schiwara & Co (Bremen, Germany). Mean and median MDA values were calculated from all blood tests and used for statistical analysis. All assays were performed blind to clinical information on the patients.
Statistical Analysis
Data were analyzed using nonparametric statistics. Group comparisons were examined using Mann-Whitney tests (two-tailed). Data concerning MDA were presented as medians with the 25th and the 75th percentiles because values were not evenly distributed. All statistical tests were considered significant at the 0.05 probability level. The SPSS 10.0 (Chicago, IL, USA) package was used throughout.
RESULTS
Demographic Characteristics
Patients with schizophrenic disorders (N=76), schizoaffective disorders (N=5) and mood disorders (N=11) were included in the study. The mean age and standard deviation (SD) of the FGA group was 38.5 years (SD=10.8) (nine women and 12 men; seven nonsmokers and 14 smokers). The second-generation antipsychotic (SGA) group incorporated 70 patients, aged 34.8 years (SD=12.1), 30 of whom were women and 40 of whom were men. Age within the subgroups of the SGAs were as follows: amisulpride (AMI) mean=37.8 (SD=7.6); risperidone (RIS) mean=35.0 (SD=16.4); clozapine (CLO) mean=36.3 (SD=14.4); olanzapine (OLA) mean=34.8 (SD=11.1). No significant statistical differences were found between age in the different SGA groups or between SGAs and FGAs. Smokers in the SGA group were dominant, with 49 patients. Twenty one patients were nonsmokers.
Treatment Groups
The FGA group consisted of patients treated with haloperidol (N=5) or flupentixol (N=17). Mean haloperidol dosage was 6.2 mg/day, and mean flupentixol dosage was 3.5 mg/day. In the SGA group, the 70 patients were divided in subgroups, with AMI (N=6; mean=447.2 mg/day); RIS (N=17; mean=4.7 mg/day); CLO (N= 12; mean=337.0 mg/day); OLA (N=22; mean=13.9 mg/day); and QUE (N=13; mean=363.4 mg/day).
Psychopathology
There was no difference concerning the BPRS scores on day 0 (FGAs mean=47.0, SD=13.3, versus SGAs mean=42.5, SD=9.7); day 7 (FGAs mean=42.1, SD=9.7, versus SGAs mean=39.7, SD=8.7); and day 21 (FGAs mean=38.8, SD=10.3, versus SGAs mean=35.4, SD=8.9) between both groups (Mann-Whitney tests: p=0.073 to 0.241, U 553 to 619, respectively).
Ratings of Extrapyramidal Symptoms
Mean AIMS scores on day 0, day 7, and day 21 differed significantly between FGAs and SGAs (each p<0.0001). The highest AIMS scores were found in the FGAs, which also increased during the 3-week study. In the AMI group, there was no change of EPS during the study, and patients on RIS and OLA changed insignificantly. Patients with QUE and CLO had the most pronounced benefit on the AIMS during this study. The results for the different compounds are shown in Table 1.
Laboratory Results
Most MDA levels were within normal range (<1.0 μmol/liter). No significant differences of MDA results were found between smokers and nonsmokers, between men and women, or among patients with concomitant medications. Other possible confounding factors such as duration of illness or age at onset had no influence on MDA levels (data not shown). At the end of the study on day 21, the highest MDA plasma levels were found in patients who received FGAs (mean 0.81). The lowest levels of MDA were localized in blood samples of patients with clozapine (mean 0.21) and amisulpride (mean 0.18). This was followed by the samples of quetiapine (mean 0.24) and risperidone (mean 0.37). Samples of patients with olanzapine had higher plasma levels of MDA within the SGAs (mean 0.80) and missed significant differences compared to FGAs. All other SGAs had significantly lower levels of MDA than patients with FGAs after 21 days. (For details see Table 2 and Figure 1.)
DISCUSSION
The most important finding of this study is that plasma levels of membrane lipid peroxidation products were found to be elevated significantly in patients treated with FGA, compared to most SGAs after a 3-week longitudinal study of antipsychotic treatment. It is well established that oxidative stress within the CNS is reflected in plasma.13,14 There is also evidence that psychotic disorders might impair antioxidant defense and increase lipid peroxidation, as antipsychotic treatment itself increases oxidative stress and induces irreversible neuropathological changes in animal models.21,22,23,24
To our knowledge, this is the first comparative study of oxidative stress in a population of patients being treated with FGAs and SGAs. An animal study with a similar hypothesis but different markers was published, of which the main results substantiate our conclusions.25 For most SGAs, we have shown significant differences to FGAs, which might explain the different incidence of EPS between FGAs and SGAs.26,27 Currently, we cannot explain why patients on olanzapine had higher MDA levels than other SGAs, but patients on olanzapine were not more indisposed than other patients in this study, according to BPRS ratings.
Oxidative stress induced by antipsychotic treatment is a hypothesis that should be taken into account concerning EPS and TD.2 There is also evidence of an impaired antioxidant defense and increased oxyradical-mediated cellular injury in patients with nonaffective psychoses who have never been treated with antipsychotics.21 For a long time, the theory that striatal postsynaptic dopamine receptor supersensitivity causes TD has been widely accepted, but new evidence may change this model.11 Recently, a new hypothesis combining the facts that antipsychotics enhance striatal glutamatergic neurotransmission by blocking presynaptic dopamine receptors and cause neuronal damage by oxidative stress was presented.3 Using the model of glutamatergic neurotransmission and the evidence of long-lasting persistence of FGAs in human brain tissue and the “fast dissociation hypothesis” of antipsychotics as explanation for “atypicality” of SGAs,3,28,29 our results may explain why SGAs, such as amisulpride, clozapine, and quetiapine, showed lower levels of oxidative stress in our study.
This study contains methodological limitations, however. One significant limitation is the open, nonblinded design, without a defined washout period. A washout-period for a longer time in acute patients would have been problematic from an ethical point of view. Further, the small sample size, the use of only one marker, and no measurement of the antioxidant defense system, as used by Parikh and co-workers, are limiting factors.25 For future studies on lipid peroxidation during treatment with SGAs and FGAs, isoprostanes could be used in combination with MDA.30,31
The role of free radicals and lipid peroxidation in different strategies of antipsychotic treatment requires further research. Obviously, these findings from an open, comparative study require replication.
ACKNOWLEDGMENTS
This study was supported in part by AstraZeneca Pharmaceuticals, the Eli Lilly and Company, and Janssen-Cilag Pharmaceutica-Cilag.
This study was presented in part at the Collegium Internationale Neuro-Psychopharmacologicum (CINP), Montreal, 24-27 June 2002; and the Nordic Psychiatric Congress (NPC), Reykjavik, 13–16 August 2003.
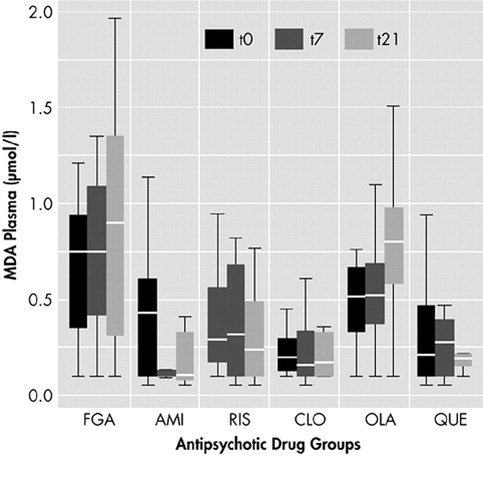
Figure 1. MDA Plasma Levels at Days 0, 7, and 21 With Different Antipsychoticsa
aFGA=first-generation antipsychotics; AMI=amisulpride; RIS=risperidone; CLO=clozapine; OLA=olanzapine; QUE=quetiapine. Box plots show 25th and 75th percentile, median, minimum, and maximum
 |
 |
1 Cadet JL, Lohr JB: Possible involvement of free radicals in neuroleptic-induced movement disorders. Evidence from treatment of tardive dyskinesia with vitamin E. Ann N Y Acad Sci 1989; 570:176–185Crossref, Medline, Google Scholar
2 Brown K, Reid A, White T, et al: Vitamin E, lipids, and lipid-peroxidation products in tardive dyskinesia. Biol Psychiatry 1998; 43:863–867Crossref, Medline, Google Scholar
3 Tsai G, Goff DC, Chang RW, et al: Markers of glutamatergic neurotransmission and oxidative stress associated with tardive dyskinesia. Am J Psychiatry 1998; 155:1207–1213Crossref, Medline, Google Scholar
4 Post A, Holsboer F, Behl C: Induction of NF-κB activity during haloperidol-induced oxidative toxicity in clonal hippocampal cells: suppression of NF-κB and neuroprotection by antioxidants. J Neurosci 1998; 18:8236–8246Crossref, Medline, Google Scholar
5 Pai BN, Janakiramaiah N, Gangadhar BN, et al: Depletion of gluthatione and enhanced lipid peroxidation in the CSF of acute psychotics following haloperidol administration. Biol Psychiatry 1994; 36:489–491Crossref, Medline, Google Scholar
6 Adler LA, Rotrosen J, Edson R, et al: Vitamin E treatment for tardive dyskinesia. Arch Gen Psychiatry 1999; 56:836–841Crossref, Medline, Google Scholar
7 Post A, Rücker M, Ohl F, et al: Mechanism underlying the protective potential of α-tocopherol (vitamin E) against haloperidol-associated neurotoxicity. Neuropsychopharmacology 2002; 26:397–407Crossref, Medline, Google Scholar
8 Shamir E, Barak Y, Shalman I, et al: Melatonin treatment for tardive dyskinesia. A double-blind, placebo-controlled, cross-over study. Arch Gen Psychiatry 2001; 58:1049–1052Crossref, Medline, Google Scholar
9 Pryor WA: Free Radicals and Lipid Peroxidation: What They Are and How They Got That Way?, In: Frei B, (edited by) Natural antioxidants in human health and disease. San Diego, Academic Press, 1994Google Scholar
10 Esterbauer H, Schauer RJ, Zollner H: Chemistry and biochemistry of hydroxynonenal, malondialdehyde and related aldehydes. Free Radic Biol Med 1991; 11:81–128Crossref, Medline, Google Scholar
11 Jenner P, Marsden CD: Is the dopamine hypothesis of tardive dyskinesias completely wrong? Trends Neurosci 1986; 9:259–260Crossref, Google Scholar
12 Richard MJ, Guiraud P, Meo J, et al: High Performance liquid chromatographic separation of malondialdehyde-thiobarbituric acid adduct in biological materials (plasma and human cells) using a commercially available reagent. J Chromatogr 1992; 577:9–18Crossref, Medline, Google Scholar
13 Arvindakshan M, Sitasawad S, Debsikdar V, et al: Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biol Psychiatry 2003; 53:56–64Crossref, Medline, Google Scholar
14 Zhang XY, Zhou DF, Cao LY, et al: The effects of risperidone treatment on superoxide dismutase in schizophrenia. J Clin Psychopharmacol 2003; 23:128–131Crossref, Medline, Google Scholar
15 Caimi G, Carollo C, Lo Presti R: Diabetes mellitus: oxidative stress and wine. Curr Med Res Opin 2003; 19:581–586Crossref, Medline, Google Scholar
16 Hoeldtke RD, Bryner KD, McNeill DR, et al: Oxidative stress and insulin requirements in patients with recent-onset type 1 diabetes. J Clin Endocrin Metabol 2003; 88:1624–1628Crossref, Medline, Google Scholar
17 Munitz MR, Benjamin S: How to examine patients using the abnormal involuntary movement scale. Hosp Community Psychiatry 1988; 39:1172–1177Medline, Google Scholar
18 Schooler NR, Kane JM: Research diagnosis for tardive dyskinesia. Arch Gen Psychiatry 1982; 39:486–487Medline, Google Scholar
19 Overall JE, Gorham DR: The brief psychiatric rating scale. Psychological Rep 1962; 10:799–812Crossref, Google Scholar
20 Bleich S, Kropp S, Degner D, et al: Creutzfeldt-Jakob disease and oxidative stress. Acta Neurol Scand 2000; 101:332–334Crossref, Medline, Google Scholar
21 Mahadik SP, Mukherjee S, Scheffer R, et al: Elevated plasma lipid peroxides at the onset of nonaffective psychosis. Biol Psychiatry 1998; 43:674–679Crossref, Medline, Google Scholar
22 Murthy JN, Laev H, Karpiak S, et al: Enzymes of oxyradical metabolism after haloperidol treatment of rat. Soc Neurosci 1989; 15:139Google Scholar
23 Cadet JL, Perumal AS: Chronic treatment with prolixine causes oxidative stress in rat brain. Biol Psychiatry 1990; 28:738–740Crossref, Medline, Google Scholar
24 Jeste DV, Lohr JB, Manley M: Study of neuropathological changes in the striatum following 4, 8 and 12 months of treatment with fluphenazine in rats. Psychopharmacology 1992; 106:154–160Crossref, Medline, Google Scholar
25 Parikh V, Khan MM, Mahadik SP: Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatric Res 2003; 37:43–51Crossref, Medline, Google Scholar
26 Schillevoort I, de Boer A, Herings RMC, et al: Risk of extrapyramidal syndromes with haloperidol, risperidone, or olanzapine. Ann Pharmacother 2001; 35:1517–1522Crossref, Medline, Google Scholar
27 Tollefson GD, Beasley CM, Tamura RN, et al: Blind, controlled, long-term study of the comparative incidence of treatment-emergent tardive dyskinesia with olanzapine or haloperidol. Am J Psychiatry 1997; 154:1248–1254Crossref, Medline, Google Scholar
28 Kapur S, Seeman P: Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics?: a new hypothesis. Am J Psychiatry 2001; 158:360–369Crossref, Medline, Google Scholar
29 Kornhuber J, Schultz A, Wiltfang J, et al: Persistence of haloperidol in human brain tissue. Am J Psychiatry 1999; 156:885–890Crossref, Medline, Google Scholar
30 Jackson Roberts L, Morrow JD: Products of the isoprostane pathway: unique bioactive compounds and markers of lipid peroxidation. Cell Mol Life Sci 2002; 59:808–820Crossref, Medline, Google Scholar
31 Block G, Dietrich M, Norkus E, et al: Factors associated with oxidative stress in human populations. Am J Epidemiol 2003; 156:274–285Crossref, Google Scholar



