Left Hippocampal Volume Inversely Correlates With Enhanced Emotional Memory in Healthy Middle-Aged Women
METHOD
This study was approved by the Institutional Review Board of the National Cancer Center. Twenty-seven healthy women were recruited by advertisements in newspapers and municipal information papers. They were between the ages of 35 and 61 (mean age=51.6 [SD=7.2]) and had 12 to 16 (mean education=13.9 [SD=1.7]) years of education. All gave written informed consent.
Most individuals were right-handed (N=24, 91.4%; mean handedness score=77.0 [SD=41.8]), as assessed by the Edinburgh Inventory. They were free of major medical illnesses and traumatic brain injury, free of psychopathology as determined by the Mini-International Neuropsychiatric Interview, 6 , 7 free of cognitive impairment as assessed by the Mini-Mental State Examination (MMSE), 8 free of gross abnormalities as assessed by MRI, free of potentially psychoactive medications, and free of a family history of psychiatric illness. After the experiment, all of the participants were given a gift voucher (4,000 JPY) for their participation.
On the first experimental day, participants viewed 11 slides 9 , 10 depicting an emotionally arousing short story. The story consisted of three phases. Phase 1 (images 1 to 4) depicted a mother taking her son to visit his father at work. In phase 2 (images 5 to 8), the boy was badly hurt in a motor vehicle accident and surgeons struggled to save his life. In phase 3 (images 9 to 11), the mother was shown leaving the hospital. Thus, the emotionally arousing narration occurred in phase 2. Participants returned 1 week later for a second day of experiments and were given a surprise memory-recall test consisting of five to nine multiple-choice questions per slide. 11 Recall achievement was expressed as a percentage of the maximum score for each phase because of the different numbers of questions given for each phase. The enhanced emotional memory was defined as the difference [Δ2–1] between percent recall achievement of the emotionally arousing story part (phase 2) and percent recall achievement of the neutral story part (phase 1).
MRI scans were obtained by using a 1.5-T General Electric Signa scanner (GE Medical Systems, Milwaukee) prior to the experiment with an identical protocol 2 , 3 and MRI-assessed brain volume measurement techniques of the hippocampus and amygdala 2 , 3 , 12 previously described. Intrarater and interrater reliabilities were determined by using the intraclass correlation coefficients (>0.90 for all measures). Intracranial volume measurements were made by using a semiautomatic volumetric procedure described in detail elsewhere. 13
Declarative memory ability was assessed using the Wechsler Memory Scale–Revised (WMS-R) 14 prior to the present experiment. We used the delayed recall indexes. Personality traits were measured using the revised Eysenck Personality Questionnaire (EPQ-R). 15 Since neuroticism refers to a general emotional overresponsiveness and a liability to develop stress-related psychiatric disorders, we used the EPQ-R neuroticism score.
We used the volumes of the left and right hippocampus and of the amygdala for the analysis. To analyze the specific effects of the amygdalar and hippocampal volumes on the enhanced emotional memory, a multiple linear regression partial correlation was used, with Δ2–1 as the dependent variable and the hippocampal and amygdalar volumes as the independent variables. We controlled the possible confounding effects of age, education, and variability in intracranial volume by entering these variables into the model. To eliminate the effect of cognitive function and personality, we subsequently entered the WMS-R delayed memory index, the MMSE score, and the EPQ-R neuroticism score into the model. All statistical analyses used two-tailed tests. The alpha levels of significance of all statistical analyses were p<0.05. The statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago).
RESULTS
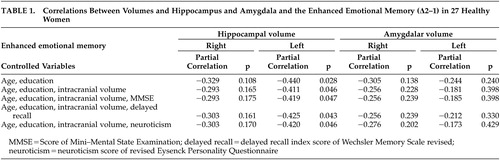
The mean percent recall achievements for phases 1, 2, and 3 were 51.98 (SD=8.82), 57.28 (SD=8.08), and 47.56 (SD=11.81), respectively. A one-way analysis of variance for repeated measures revealed a significant phase effect on recall achievement (F=11.21, df=2, 52, p<0.01). Regarding cognitive function and personality, the mean WMS-R delayed recall index was 103.26 (SD=14.54), the mean MMSE score was 29.19 (SD=0.83), the mean EPQ-R neuroticism score was 4.48 (SD=2.87). No significant correlation between these potential covariates and Δ 2–1 was noted (age, r=−0.104, p=0.61; delayed recall index, r=0.086, p=0.67; MMSE, r=−0.137, p=0.50; neuroticism, r=−0.61, p=0.72). The mean intracranial volume was 1284.4 cm 3 (SD=106.7) and no significant correlation between intracranial volume and Δ2–1 was noted. The left and right hippocampal volumes were 3326.2 mm 3 (SD=292.3) and 3423.4 mm 3 (SD=290.0), respectively. The left and right amygdalar volumes were 1125.4 mm 3 (SD=130.1) and 1208.5 mm 3 (SD=145.4), respectively. We found a significant Pearson’s correlation between the left hippocampal volume and Δ2–1 (r=−0.407, p=0.035). The hippocampal volume was significantly correlated with Δ2–1 irrespective of age, education, and intracranial volume ( Table 1 ). The effects of hippocampal volume on Δ2–1 remained significant after the effects of cognitive function and personality traits were controlled.
 |
DISCUSSION
The main finding in this study was that the enhanced emotional memory was predicted by the smaller left hippocampal volume but not the amygdalar volume in healthy middle-aged women. Furthermore, we have also addressed the potential impact of confounding factors in the interpretation of hippocampal volume variations. Our study design uniquely circumvented the impact of the participant’s own and familial psychiatric conditions. The enhanced emotional memory was negatively correlated with the left hippocampal volume irrespective of age, educational history, intracranial volume, cognitive function, delayed memory function, and neuroticism. These findings support that smaller left hippocampal volume might predispose women to acquire stronger emotional responses when exposed to an aversive stimulus. These findings also support that smaller left hippocampus in cancer survivors with intrusive recollections represent a preexisting (acquired until cancer experience) vulnerability factor rather than the neurotoxic effect of persistent intrusive recollections. Regarding the amygdala, it was suggested that smaller amygdalar volume in cancer survivors with intrusive recollections does not support a predisposition theory.
Since only left hippocampal volume, and not right, was significantly associated with the enhanced emotional memory, there was laterality in these results. Two previous studies have revealed smaller left hippocampal volume in women with a history of cancer-related intrusive recollections 2 and predominantly smaller right hippocampal volume in men with combat-related PTSD. 5 Taken together with these findings, we may assume the existence of gender difference in the laterality of hippocampal volume on emotional memory.
Since breast cancer, at times, occurs in middle-aged women, these findings have important implications for the understanding of the neurobiological basis underlying intrusive recollections in breast cancer survivors. Any interpretation of our results should take into account following limitations. First, there was a small sample size. Second, different populations, different conditions (healthy versus PTSD), and different stress-induction methods might have affected our findings. Finally, MMSE doses not test executive cognitive function.
1 . Matsuoka Y, Nagamine M, Uchitomi Y: Intrusion in women with breast cancer, in PTSD: Brain Mechanisms and Clinical Implications. Edited by Kato N, Kawata M, Pitman RK. Tokyo, Springer-Verlag, 2006, pp 169–178Google Scholar
2 . Nakano T, Wenner M, Inagaki M, et al: Relationship between distressing cancer-related recollections and hippocampal volume in cancer survivors. Am J Psychiatry 2002; 159:2087–2093Google Scholar
3 . Matsuoka Y, Yamawaki S, Inagaki M, et al: A volumetric study of amygdala in cancer survivors with intrusive recollections. Biol Psychiatry 2003; 54:736–743Google Scholar
4 . Kitayama N, Vaccarino V, Kutner M, et al: Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord 2005; 88:79–86Google Scholar
5 . Gilbertson MW, Shenton ME, Ciszewski A, et al: Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neurosci 2002; 5:1242–127Google Scholar
6 . Sheehan DV, Lecrubier Y, Sheehan KH, et al: The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33; quiz 34–57Google Scholar
7 . Otsubo T, Tanaka K, Koda R, et al: Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci 2005; 59:517–526Google Scholar
8 . Folstein MF, Folstein SE, McHugh PR: “Mini-mental state:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
9 . Kazui H, Mori E, Hashimoto M, et al: Enhancement of declarative memory by emotional arousal and visual memory function in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 2003; 15:221–226Google Scholar
10 . Kazui H, Mori E, Hashimoto M, et al: Impact of emotion on memory: controlled study of the influence of emotionally charged material on declarative memory in Alzheimer’s disease. Br J Psychiatry 2000; 177:343–347Google Scholar
11 . Cahill L, McGaugh JL: A novel demonstration of enhanced memory associated with emotional arousal. Conscious Cogn 1995; 4:410–421Google Scholar
12 . Matsuoka Y, Mori E, Inagaki M, et al: Manual tracing guideline for volumetry of hippocampus and amygdala with high-resolution MRI (in Japanese). No To Shinkei 2003; 55:690–697Google Scholar
13 . Mori E, Hirono N, Yamashita H, et al: Premorbid brain size as a determinant of reserve capacity against intellectual decline in Alzheimer’s disease. Am J Psychiatry 1997; 154:18–24Google Scholar
14 . Wechsler D: WMS-R: Wechsler Memroy Scale-Revised Manual. San Antonio, Psychological Corporation, 1987Google Scholar
15 . Eysenck SBG, Eysenck HJ, Barret P: A revised version of the psychoticism scale. Person Individ Diff 1985; 6:21–29Google Scholar



