Association Between Preoperative Anxiety in Spinal Stenosis Patients and Abnormal Cerebral Glucose Metabolism: Voxel-Based Statistical Analysis of F-18 FDG Brain PET
Preoperative psychological and emotional factors have been shown to be related to pain and functional outcomes in orthopedic patients. 3 Also, Rosenberger et al. 4 suggested that preoperative consideration of attitudinal and mood factors will assist the surgeon in estimating both the speed and extent of postoperative recovery. Interaction between psychological factors and body functions in humans has been discussed by various investigators. 5 Apart from the hypothalamic-pituitary-autonomic nervous system, the cerebral cortex and limbic system should not be ignored. 6 , 7 Although not in orthopedic patients, psychological factors have been related to the prognosis of cancer patients, and functional neuroimaging seems to be useful not only for psychiatric evaluation of major factors such as depression and anxiety but also for further psychological factors in cancer patients. 8 – 10
Preoperative and postoperative anxiety tend to be correlated. 2 Patients with high postoperative anxiety have longer hospitalization periods and report more postoperative pain. 11 One recent prospective study of 102 patients who underwent lumbar spine surgery found that lower presurgical anxiety, depression, and hostility predicted better outcome. 12
A recent study demonstrated that amygdala and insular hyperactivity is common to both social anxiety disorder and healthy subjects undergoing fear conditioning. 13 This conditioned fear provides a mechanism to explain fear and anxiety in public, and may in part be relevant in preoperative anxiety. 14
According to the model for conditioned fear, the amygdalae play a pivotal role in the elimination of conditioned responses, and the prefrontal cortex appears to have a modulatory effect on amygdala function. 15 , 16 From these findings, we can hypothesize that if patients are faced with a major operation—a stressful condition—these brain areas may be dysfunctional. Although a possible association between preoperative anxiety and abnormal cerebral glucose metabolism may exist, we are not aware of any report describing in detail preoperative anxiety and cerebral metabolism in patients with spinal stenosis. The primary purpose of the current study was to determine the association between preoperative anxiety and cerebral glucose metabolism in patients with spinal stenosis.
METHODS
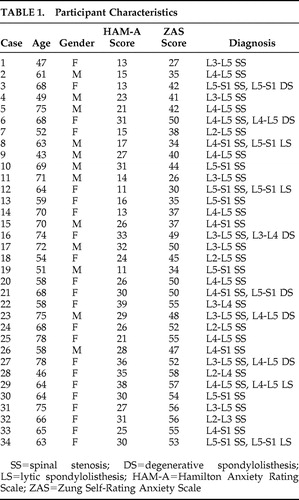
We studied 34 patients who had been admitted to our department for the surgical treatment of lumbar spinal stenosis. The diagnosis of lumbar spinal stenosis was based on clinical presentation and radiological findings (plain radiograph, computed tomography, and MRI). All patients were diagnosed as having lumbar spinal stenosis. All patients between 43 and 78 years old who presented spinal stenosis were asked to voluntarily complete a Hamilton Anxiety Rating Scale (HAM-A), Zung Self-Rating Anxiety Scale, and an analysis of their cerebral glucose metabolism. 17 , 18
The HAM-A consists of 14 items scored on a five-point scale. The Cronbach alpha for the HAM-A was 0.82. The Zung Self-Rating Anxiety Scale consists of 20 items scored on a four-point scale. The Cronbach alpha for the Self-Rating Anxiety Scale was 0.73. Thirty-four age- and gender-matched adults were used as the comparison group.
Before entering the study, all participants were also examined clinically to rule out any hidden metabolic disease or psychiatric disease that could affect the cerebral glucose metabolism. In addition, brain MRI studies were performed for all participants before entering the study in order to rule out organic brain lesions. Participants with a history of neuromuscular disease, endocrine disease, connective tissue abnormalities, organic brain disease, psychiatric disease, or previous spinal surgery were excluded from the study. All participants provided informed consent before the examination and measurements. The study was approved by the Clinical Research Ethics Committee of the university and the hospital.
FDG Brain PET
Brain positron emission tomography (PET) scans of a single frame of 15 minutes were acquired starting 60 minutes after the intravenous injection of 370 MBq (10 mCi) [ 18 F]fluorodeoxyglucose (FDG) using a Gemini PET/CT scanner (Philips, Milpitas, Calif.). Scans were performed while the participants were in a resting condition with their eyes closed and ears unplugged, comfortably lying in a darkened and quiet room. The participants fasted for at least 8 hours before PET imaging. The PET images were reconstructed using 3D RAMLA (2 repetition, 0.006 relaxation parameter) and displayed in a 128×128 matrix (pixel size=2×2 mm, with a slice thickness of 2 mm). Attenuation correction was performed with a uniform attenuation coefficient (μ=0.096 cm −1 ). In-plane and axial resolution of the scanner were 4.2 and 5.6 mm full width at half maximum (FWHM), respectively.
Statistical Parametric Map Analysis of Regional Cerebral Glucose Metabolism
Spatial preprocessing and statistical analysis were performed using the SPM2 implemented in Matlab 5.3 (The MathWorks, Inc., Natick, Mass.). All the reconstructed FDG brain PET images were spatially normalized into Montreal Neurological Institute (MNI, McGill University, Montreal, Quebec) standard templates by an affine transformation (12 parameters for rigid transformations) and a nonlinear transformation, and then smoothed with a FWHM 8 mm Gaussian kernel to increase the signal-to-noise ratio and to account for subtle variations in anatomic structures. To remove the effects of the difference in the overall counts, we normalized the voxel counts to the mean voxel count of the gray matter in each PET image using proportional scaling. Images of the spinal stenosis patients with preoperative anxiety were compared with those of healthy comparison subjects in a voxel-wise manner using SPM2 for between-group analysis (p<0.001, uncorrected; extent threshold, k=100). The clusters that passed this threshold were considered significant at p<0.05 corrected for multiple comparisons using family-wise error correction (Height threshold T=5.02, extent of cluster=100). The Talairach brain coordinates were estimated from a nonlinear transformation from MNI space to Talairach space (Talairach Daemon Client, Version 1.1, Research Imaging Center, University of Texas Health Science Center at San Antonio). We examined the differences between the spinal stenosis patients with preoperative anxiety and comparison subjects using an extent threshold level of 100 voxels with significance at p<0.001 to illustrate the group differences in statistical voxel-based analysis, as well as for illustrating the result of the registration between spinal stenosis patients with preoperative anxiety and comparison subjects. For the visualization of the t-score statistics (SPM{t} map), significant voxels were projected onto the 3-dimensional rendered brain or a standard high-resolution MRI template provided by SPM2, thereby allowing anatomic identification. In addition, we investigated linear correlations of the regional brain glucose metabolism with HAM-A and Zung Self-Rating Anxiety Scale scores using “single subject: covariates only” analysis and simple regression analysis, which are statistical models in SPM2 based on the general linear model.
Statistical Analysis
Statistical analysis was performed with SPSS 11.5 software for Windows (SPSS, Chicago). Data were expressed by mean and standard deviation (SD). We compared groups using the t test. Statistical significance was set at p<0.05.
RESULTS
Patient Characteristics
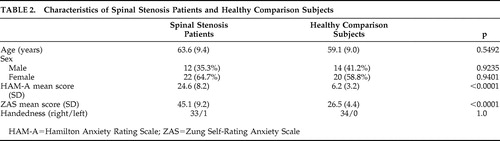
Table 1 and Table 2 show the details of the participants. The spinal stenosis group consisted of 34 patients (22 women and 12 men) with a mean age of 63.6 years old (SD=9.4). The mean HAM-A score in the spinal stenosis group was 24.6 (SD=8.2) and the mean Zung Self-Rating Anxiety Scale score was 45.1 (SD=9.2). The comparison group consisted of 34 participants (20 women and 14 men) with a mean age of 59.1 years old (SD=9.0). The mean HAM-A score in the comparison group was 6.2 (SD=3.2), and the mean Zung Self-Rating Anxiety Scale score was 26.5 (SD=4.4). The mean HAM-A score and Zung Self-Rating Anxiety Scale score were significantly lower in the comparison group than in the spinal stenosis group (p<0.0001 in both cases).
 |
 |
Regional Cerebral Metabolism Abnormalities by Statistical Parametric Map Analysis
As shown in Figure 1 and Table 3 , several voxel clusters of significantly decreased cerebral metabolism were observed in the spinal stenosis patients with preoperative anxiety. The largest clusters were areas of left insula and left prefrontal cortex (Brodmann’s areas 9 and 11). The second largest cluster area was left prefrontal cortex (Brodmann’s area 10). The other clusters were right insula (Brodmann’s area 13), right superior temporal gyrus (Brodmann’s area 22), and right middle frontal gyrus (Brodmann’s area 8).
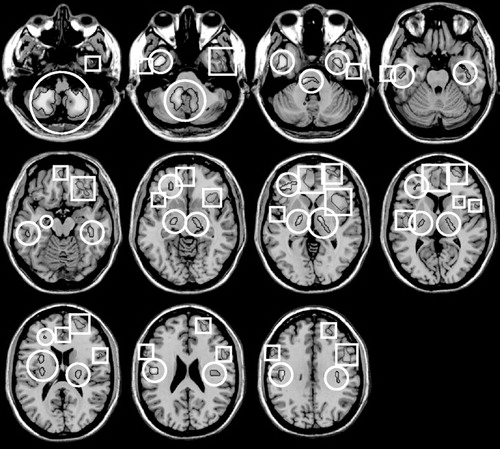
Circled regions: increased cerebral glucose metabolism; Squared regions: decreased cerebral glucose metabolism.
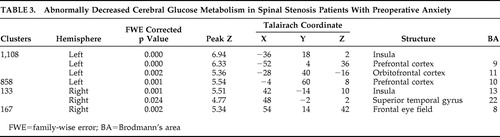 |
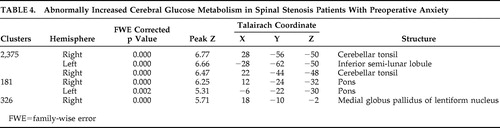
Table 4 reveals the several clusters of significantly increased cerebral glucose metabolism in spinal stenosis patients with preoperative anxiety relative to healthy comparison subjects. These areas are right cerebellar tonsil, left inferior semilunar lobule, right cerebellar tonsil, both pons, and right internal globus pallidus.
 |
Correlations of HAM-A Scores, Zung Self-Rating Anxiety Scale Scores, and Brain Metabolism
No significant correlation was found between HAM-A and Zung Self-Rating Anxiety Scale scores and cerebral glucose metabolism in spinal stenosis patients with preoperative anxiety.
DISCUSSION
The major finding of our study is that spinal stenosis patients with preoperative anxiety showed decreased cerebral glucose metabolism in several areas, including the insula, temporal lobe, and frontal brain regions. Our study especially demonstrated left frontal brain hypometabolism.
Frontal brain asymmetry is suggested to be associated with differences in the basic dimensions of emotion. 19 The left prefrontal cortex is associated with the approach-related emotion, anger, whereas the right prefrontal area is associated with the withdrawal-related emotion, anxiety. 19 , 20 Our study also showed hypometabolism of left prefrontal cortex and left orbitofrontal cortex in spinal stenosis patients with preoperative anxiety. Morinaga et al. 21 examined regional cerebral oxygenated hemoglobin (O 2 Hb) levels in human medial prefrontal cortex prior to and during anticipatory anxiety. They found that right medial prefrontal cortex O 2 Hb was significantly increased relative to left medial prefrontal cortex O 2 Hb during anticipation of the shock. Right-sided O 2 Hb increases were significantly correlated with the Temperament and Character Inventory Harm Avoidance subscale.
The orbitofrontal cortex and amygdala are supposed to be components of the neural circuitry involved in the adaptive processing of emotion and in psychopathology. 22 , 23 A study of adolescent primates demonstrates involvement of the orbitofrontal cortex in mediating threat-induced freezing, a behavior that is similar to human behavioral inhibition and is a characteristic of trait-like anxiety. 24 They demonstrated that the orbitofrontal cortex lesions significantly increased left frontal asymmetric brain electrical activity, which is similar to findings in our study. 24
Our study also revealed insular involvement in preoperative anxiety processing in spinal stenosis patients. Insular regions are known to be associated with modulation of affective processing and are important for linking emotions to cognitive processes and behavioral responses. 25 Several studies have noted that the insular cortex may be central for understanding anxiety proneness. In particular, the insular cortex is supposed to process visceral responses accessible to awareness as subjective feeling states. 26 Some studies have found the altered insular function in anxiety disorders. The insular is activated during anticipatory anxiety paradigms in healthy subjects and in patients with social anxiety disorders. 27 – 29
In our study, decreased cerebral glucose metabolism was noted in the right superior temporal gyrus (Brodmann’s area 22) of preoperative anxiety patients with spinal stenosis. This finding is consistent with prior studies which found structural abnormalities of the temporal lobe in panic disorder patients. 30 , 31 Other studies also have reported the structural abnormalities of the temporal lobe in patients with panic disorder. The right temporal lobe is believed to be associated with an earlier onset and frequent panic attacks in patients with panic disorder. 32 Similar to the current study, a PET study noted the decreased cerebral glucose metabolism in the right inferior parietal and right superior temporal regions. 33 The authors assumed that the temporal lobe played a mediating role between affect and behavioral responses with input from the limbic system. The authors also suggested that decreased metabolism in the right superior temporal region would be associated with an elevated γ-amino-butyric acid (GABA) activity receptor system in the right prefrontal cortex. In addition to metabolic changes, hemodynamic alteration in panic disorder patients is also reported. A recent study demonstrated that there is decreased cerebral blood flow in temporal regions of the brain in panic disorder and that this decrease may, in part, reflect the clinical severity of panic disorder. 34
Interestingly, spinal stenosis patients with preoperative anxiety showed increased cerebral glucose metabolism in several brain areas, including right cerebellar tonsil, left inferior semilunar lobule of cerebellum, right cerebellar tonsil, both pons, and right internal globus pallidus. These findings suggest the hyperactivity of two neurocircuits: afferent pathways to the amygdala and part of the startle circuit. The afferent pathways of the fear network include the viscerosensory information conveyed by the nucleus of the solitary tract, the visuospatial/auditory or cognitive information from the thalamus, and fear-signifying memories from the hippocampus. 35 Previous preclinical studies have found an efferent amygdalofugal pathway to the primary startle circuit at the level of the caudal pontine reticular formation, which is relayed by the periaqueductal gray matter. Higher FDG uptake may have been detected in the lower dorsal pons and midbrain, associated with the anticipatory anxiety of panic disorder patients. 36
Sacchetti et al. 37 reported on the relationship between cerebellar regions and panic disorder, which is a role of the cerebellum in fear-conditioning consolidation. The study showed that interpositus nucleus functional integrity was necessary for acoustic, conditioned stimulus fear response memory formation and that vermis functional integrity was necessary for memory formation of both context and acoustic, conditioned stimulus fear responses. Also, similar results were found in the study by Sakai et al. 38 in which panic disorder patients showed appreciably high-state anxiety before scanning, and exhibited significantly higher levels of glucose uptake in the bilateral amygdala, hippocampus, and thalamus, and in the midbrain, caudal pons, medulla, and cerebellum than comparison subjects.
Interestingly, there are recent reports of panic with autonomic disturbances occurring in patients receiving hypothalamic deep brain stimulation for cluster headaches and for deep brain stimulation in the subthalamic nucleus region. 39 , 40 Data indicate that subthalamic nucleus deep brain stimulation can both impair fear recognition and induce fear and panic. 39 , 41 A similar finding was reported in 2006. 42 The authors of that study showed that deep brain stimulation of the anterior limb of the internal capsule and nucleus accumbens region caused severe panic, and this response may result from the activation of limbic and autonomic networks.
A major drawback of our study is that the difference in the severity of the anxiety between the healthy comparison group and the spinal stenosis patients with preoperative anxiety is a potential confound. In other words, the lack of participant anxiety scores is the major limitation of the current study. It is possible that stability in anxiety in pre- and postoperative participants might be related to stability in trait anxiety in this group. In other words, perhaps people who are by nature highly anxious are more likely to undergo surgical procedures or are at greater risk for health-related problems. Therefore, this major problem should be addressed in a future study.
Spinal stenosis patients with preoperative anxiety showed decreased cerebral glucose metabolism in several brain areas including the left insula, left prefrontal cortex, right insula, right superior temporal gyrus, and right middle frontal gyrus. Increases were also noted in the right cerebellar tonsil, left inferior semilunar lobule, right cerebellar tonsil, both pons, and right internal globus pallidus. The findings of our study provide functional neuroimaging support of abnormal cerebral glucose metabolism in spinal stenosis patients with preoperative anxiety.
1. Cooke M, Chaboyer W, Schluter P, et al: The effect of music on preoperative anxiety in day surgery. J Adv Nurs 2005; 52:47–55Google Scholar
2. de Groot KI, Boeke S, Van den Berge HJ, et al: The influence of psychological variables on postoperative anxiety and physical complaints in patients undergoing lumbar surgery. Pain 1997; 69:19–25Google Scholar
3. Trief PM, Ploutz-Snyder R, Fredrickson BE: Emotional health predicts pain and function after fusion: a prospective multicenter study. Spine 2006; 31:823–830Google Scholar
4. Rosenberger PH, Jokl P, Ickovics J: Psychological factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg 2006; 14:397–405Google Scholar
5. Eysenck HJ: Personality, stress and cancer: prediction and prophylaxis. Br J Med Psychol 1988; 61:57–75Google Scholar
6. Musselman DL, McDaniel JS, Porter MR, et al: Psychoneuroendocrinology and cancer, in Psycho-oncology. Edited by Holland JC. New York, Oxford University Press, 1998, 135–146Google Scholar
7. Tashiro M, Itoh M, Kubota K, et al: Relationship between trait anxiety, brain activity and natural killer cell activity in cancer patients: a preliminary PET study. Psycho-oncology 2001; 10:541–546Google Scholar
8. Greer S, Morris T, Pettingale KW, et al: Psychological response to breast cancer and 15-year outcome. Lancet 1990; 335:49–50Google Scholar
9. Tashiro M, Juengling F, Moser E, et al: High social desirability and prefrontal cortical activity in cancer patients: a preliminary study. Med Sci Monit 2003; 9:CR119–124Google Scholar
10. Watson M, Haviland JS, Greer S, et al: Influence of psychological response on survival in breast cancer: a population-based cohort study. Lancet 1999; 354:1331–1336Google Scholar
11. Boeke S, Duivenvoorden HJ, Verhage F, et al: Prediction of postoperative pain and duration of hospitalization using two anxiety measures. Pain 1991; 45:293–297Google Scholar
12. Trief PM, Grant W, Fredrickson B: A prospective study of psychological predictors of lumbar surgery outcome. Spine 2000; 25:2616–2621Google Scholar
13. Etkin A, Wager TD: Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488Google Scholar
14. LeDoux JE: Emotion circuits in the brain. Ann Rev Neurosci 2000; 23:155–184Google Scholar
15. Phelps EA, Delgado MR, Nearing KI, et al: Extinction learning in humans: role of the amygdala and VMPFC. Neuron 2004; 43:897–905Google Scholar
16. Milad MR, Quirk GJ, Pitman RK, et al: A role for the human dorsal anterior cingulated cortex in fear expression. Biol Psychiatry 2007; 62:1191–1194Google Scholar
17. Riskind JH, Beck AT, Brown G, et al: Taking the measure of anxiety and depression: validity of the reconstructed Hamilton scales. J Nerv Ment Dis 1987; 175:474–479Google Scholar
18. Zung WW: A rating instrument for anxiety disorders. Psychosomatics 1971; 12:371–379Google Scholar
19. Davidson RJ: Anxiety and affective style: role of prefrontal cortex and amygdale. Biol Psychiatry 2002; 51:68–80Google Scholar
20. Schmidt LA, Trainor LJ: Frontal brain electrical activity (EEG) distinguishes valence and intensity of musical emotions. Cogn Emot 2001; 15:487–500Google Scholar
21. Morinaga K, Akiyoshi J, Matsushita H, et al: Anticipatory anxiety-induced changes in human lateral prefrontal cortical activity. Biol Psychol 2007; 74:34–38Google Scholar
22. Davidson RJ, Lewis DA, Alloy LB, et al: Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry 2002; 52:478–502Google Scholar
23. Drevets WC: Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 2000; 126:413–431Google Scholar
24. Kalin NH, Shelton SE, Davidson RJ: Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry 2007; 62:1134–1139Google Scholar
25. Nitschke JB, Sarinopoulos I, Mackiewicz KL, et al: Functional neuroanatomy of aversion and its anticipation. Neuroimage 2006; 29:106–116Google Scholar
26. Critchley HD, Wiens S, Rotshtein P, et al: Neural systems supporting interoceptive awareness. Nat Neurosci 2004; 7:189–195Google Scholar
27. Lorberbaum JP, Kose S, Johnson MR, et al: Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport 2004; 15:2701–2705Google Scholar
28. Simmons A, Matthews SC, Stein MB, et al: Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport 2004; 15:2261–2265Google Scholar
29. Simmons A, Strigo I, Matthews SC, et al: Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry 2006; 60:402–409Google Scholar
30. Massana G, Serra-Grabulosa JM, Salgado-Pineda P, et al: Amygdalar atrophy in panic disorder patients detected by volumetric magnetic resonance imaging. Neuroimage 2003; 19:80–90Google Scholar
31. Vythilingam M, Anderson ER, Goddard A, et al: Temporal lobe volume in panic disorder: a quantitative magnetic resonance imaging study. Psychiatry Res 2000; 99:75–82Google Scholar
32. Ontiveros A, Fontaine R, Breton G, et al: Correlation of severity of panic disorder and neuroanatomical changes on magnetic resonance imaging. J Neuropsychiatry Clin Neurosci 1989; 1:404–408Google Scholar
33. Bisaga A, Katz JL, Antonini A, et al: Cerebral glucose metabolism in women with panic disorder. Am J Psychiatry 1998; 155:1178–1183Google Scholar
34. Lee YS, Hwang J, Kim SJ, et al: Decreased blood flow of temporal regions of the brain in subjects with panic disorder. J Psychiatr Res 2006; 40:528–534Google Scholar
35. Coplan JD, Lydiard RB: Brain circuits in panic disorder. Biol Psychiatry 1998; 44:1264–1276Google Scholar
36. Fendt M, Koch M, Schnitzler HU: Lesions of the central gray block conditioned fear as measured with the potentiated startle paradigm. Behav Brain Res 1996; 74:127–134Google Scholar
37. Sacchetti B, Baldi E, Lorenzini CA, et al: Cerebellar role in fear-conditioning consolidation. Proc Natl Acad Sci USA 2002; 99:8406–8411Google Scholar
38. Sakai Y, Kumano H, Nishikawa M, et al: Cerebral glucose metabolism associated with a fear network in panic disorder. Neuroreport 2005; 16:927–931Google Scholar
39. Okun MS, Raju DV, Walter BL, et al: Pseudobulbar crying induced by stimulation in the region of the subthalamic nucleus. J Neurol Neurosurg Psychiatry 2004; 75:921–923Google Scholar
40. Schoenen J, Di Clemente L, Vandenheede M, et al: Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain 2005; 128:940–947Google Scholar
41. Biseul I, Sauleau P, Haegelen C, et al: Fear recognition is impaired by subthalamic nucleus stimulation in Parkinson’s disease. Neuropsychologia 2005; 43:1054–1059Google Scholar
42. Shapira NA, Okun MS, Wint D, et al: Panic and fear induced by deep brain stimulation. J Neurol Neurosurg Psychiatry 2006; 77:410–412Google Scholar



