Education Effect on Depression and Quality of Life in Nondemented Parkinson’s Disease Patients
Depression is the most consistent determinant of poorer health-related quality of life in Parkinson’s disease 1 , 2 and accounts for 50%–60% of the variability in health-related quality of life measures on different depression measuring scales. 5 – 7 Depression is also closely associated with cognitive impairment in Parkinson’s disease, from the common manifestation of reduced cognitive performance in nondemented patients to the most severe manifestation of dementia. 8 Dementia in Parkinson’s disease patients 5 and poorer cognitive performance in patients without dementia 9 are each independently associated with poorer health-related quality of life.
In Parkinson’s disease, more years of education is associated with a lower risk of dementia 10 , 11 and with better cognitive performance. 12 – 14 Despite the links between education and cognition, and cognition and health-related quality of life, an association between longer education and better health-related quality of life in Parkinson’s disease patients has not been unequivocally demonstrated. Two studies did not identify education as relevant for health-related quality of life, 5 , 7 while two others did. 6 , 15 In another study, univariate association between more years of education and better health-related quality of life was lost in a multivariate model that included cognitive performance and level of depression as covariates. 9
A comparison of Parkinson’s disease patients with lower, intermediate, and higher educational levels (based on length of formal education) 14 indicated fewer hallucinations, delusions, and sleep disturbances in the more well-educated patients, suggesting that education modified some neuropsychological aspects of Parkinson’s disease. However, the relationship between education and depression, one of the most prominent neuropsychological symptoms in Parkinson’s disease, has been only scarcely addressed. Our study evaluates education as a determinant of depression and health-related quality of life by comparing nondemented Parkinson’s disease patients divided into groups based on length of education. 14
METHODS
Three educational level groups were defined based on length of formal education (completion of educational programs in licensed elementary/high schools and universities): 14 Lower—patients completing ≤8 years of education (i.e., elementary/primary school or less); Intermediate—patients completing 9–12 years of education (i.e., from one year of high school to completing high school); Higher—patients completing ≥13 years of education (i.e., at least one year of college or more). The patients in each group were compared separately for depression level and health-related quality of life. To prevent multiplicity problems, the comparison-wise alpha level was set at 0.025 (two-sided). We expected approximately twice the number of intermediate education patients as either lower or higher education patients. 9 Based on standard deviation (SD) of depression level and health-related quality of life for lower education patients from previous data, 9 we estimated that a sample of 112 patients (28 lower, 56 intermediate, 28 higher education) would achieve 80%–85% power to detect a 15% difference between the lower education and either of the remaining two groups regarding either of the outcomes in analysis of variance (ANOVA). Actual distribution of eligible patients in respect to education was slightly out of the expectations, and the ratio in the enrolled sample was approximately 1:2:1.5. Poststudy, we decided to compare the groups for cognitive performance. We therefore reduced the comparison-wise alpha level to 0.017. This diminished the power of the sample: 80% power was retained for a smallest detectable pairwise difference of around 30%. The study was approved by the institution’s ethics committee.
Patients
Candidates were consecutive idiopathic Parkinson’s disease patients diagnosed according to standard criteria. 16 Inclusion criteria were informed consent and a lack of dementia. Exclusion criteria were somatic diseases with a potential relevant effect on mood, cognition, or quality of life (advanced cerebrovascular diseases or sequelae; chronic pain syndromes; chronic inflammatory/infectious diseases; advanced diabetes mellitus; malignancy; renal, hepatic, or heart failure; severe anemia or any other severely debilitating or life-threatening disease/state); neurodegenerative diseases other than Parkinson’s disease; history of or an ongoing psychotic disorder or presence of hallucinations, delusions or a delirium; history of or an ongoing antipsychotic treatment; and use of antidepressants/anxiolytics within 6 months before the study (at least 3 weeks of continuous treatment). Patients were considered nonpsychotic based on psychiatric evaluation (DSM-IV) and considered nondemented based on both psychiatric evaluation (DSM-IV) and a Mini Mental State Examination (MMSE) score equal to or higher than the median value established in a reference population-based group of the corresponding age and education. 17
Patient Evaluations
Patients were evaluated during the “on” (motor symptom-free) state. The Beck Depression Inventory (BDI) (validated Croatian version) was used to measure the level of depression (11 cognitive-affective items as specified by Visser et al.: 18 mood, pessimism, sense of failure, lack of satisfaction, guilty feeling, sense of punishment, self-hate, self-accusations, suicidal ideas, irritability, and social withdrawal; higher score indicates more severe depression). The Croatian version of a 39-item self-reporting quality of life questionnaire validated for use in Parkinson’s disease (Parkinson’s Disease Questionnaire, PDQ-39) 19 was used to assess health-related quality of life (higher score indicates poorer health-related quality of life). Neuropsychological evaluation included standardized tests for cognitive domains typically affected in Parkinson’s disease (memory/attention, visuospatial and executive functions): 8 Stroop interference test (attention, mental speed, mental control); 20 Symbol Digit Modalities (visuoverbal substitution speed); 21 Rey Auditory Verbal Learning test (verbal memory) 22 with five learning trials of a 15-word list and the immediate-recall task; Rey-Osterreith Complex Figure test (visuomotor organization and memory) 23 with the two testing conditions freehand copy of the figure and reproduction of the figure after 30 minutes (recall); and Trail Making tests A and B (visual, conceptual, and visuomotor tracking). 24 Higher scores indicated better performance in all tests except Trail Making tests A and B. Tests were implemented by an investigator unaware of the patients’ educational level and BDI scores.
Parkinsonism was evaluated using the Unified Parkinson’s Disease Rating Scale (UPDRS) Part II and Part III and modified Hoehn and Yahr Parkinson’s disease staging scale. 25 UPDRS Part II evaluates activities of daily living by scoring 13 items from 0 to 4 points (0=normal, 4=most impairment for each item); the scale is 0 to 3 for the salivation item. UPDRS Part III is a detailed motor exam that evaluates 27 items on a five-point scale (0=normal, 4=most impairment). UPDRS Part I was not considered since its items were either assessed by more detailed testing or were part of the exclusion criteria (thought disorders such as hallucinations, delusions, or psychosis). Modified Hoehn and Yahr Parkinson’s disease grading has 8 levels based on unilateral or bilateral motor symptoms and ability to walk/stand (0=no disease, 1, 1.5, 2, 2.5, 3, 4, 5=wheelchair-bound or bedridden unless assisted).
Data on Parkinson’s disease history, education, and other sociodemographic characteristics were collected from a structured interview. We calculated the average daily L -dopa dose over the 4 weeks preceding the evaluation.
Statistics
Unadjusted group comparisons were based on appropriate univariate tests for trends or differences in proportions, medians, or means. A number of sociodemographic and Parkinson’s disease-related characteristics were considered as covariates in the adjusted comparisons (analysis of covariance). Their number was partly reduced by the means of principal components analysis (varimax rotation, Kaiser normalization). To check whether the patients could be separated based on depression level and health-related quality of life, cluster analysis was performed that used least p th powers clustering criterion (p=1) to reduce the effect of outliers on cluster centers. To validate the results of the main analysis, cluster allocation prevalence was analyzed using modified Poisson regression with robust error variance. We used SAS for Windows 9.1 software (SAS Inc., Cary, NC).
RESULTS
Patient Characteristics and Univariate Comparison of Educational Level Groups
A total of 114 patients (24 lower, 53 intermediate, 37 higher education level) from a broad metropolitan area were enrolled. Groups were comparable regarding MMSE score and sociodemographic characteristics ( Table 1 ). Motor symptoms were more severe in the lower education group than in the remaining two groups (UPDRS Part III), whereas other Parkinson’s disease-related characteristics appeared comparable: duration, early onset, activities of daily living (UPDRS Part II) and Hoehn and Yahr Parkinson’s disease score ( Table 1 ) (distribution across Hoehn and Yahr stages χ 2 =9.85, df=10, p=0.454). Cognitive tests performance and health-related quality of life (PDQ-39 score) were better, while depressive difficulties (cognitive-affective BDI items score) were less severe, with increasing educational level ( Table 1 ). Use of L -dopa, dopaminergic agonists (ropinirole or pramipexole) or monoamine oxidase B inhibitors (selegiline) was comparable across the groups ( Table 2 ).
 |
 |
Principal Components Analysis
In the first run, age had a communality less than 0.5 and was thereafter considered as a separate variable. The remaining variables were conceptually close and correlated: UPDRS Part II, Part III, and Hoehn and Yahr score (pairwise Kendall’s nonparametric correlation coefficients τ-b between 0.340 and 0.435, p<0.001 in all cases); Parkinson’s disease duration, history of use and L -dopa dose (pairwise correlations τ-b between 0.532 and 0.733, p<0.001 in all cases); and cognitive tests (pairwise correlations τ-b between 0.282 and 0.532, p<0.001 in all cases). Cognitive tests loaded onto the first component named “cognition” (cognitive performance, higher is better); Parkinson’s disease duration, history of use, and L -dopa dose loaded onto the second component named “duration-dopa” (higher is worse); UPDRS Part II, Part III, and Hoehn and Yahr loaded onto the third component named “Parkinson’s disease severity” (higher is worse). All communalities were ≥0.55, loadings were between 0.705 and 0.912, total variance explained was 65.9%, there were no outliers, and each variable loaded onto a single component. Similar communalities, eigenvalues, and loadings were obtained in randomly selected data (random number seed based on the analysis date) confirming validity of the model.
Adjusted Comparison of Educational Level Groups With Respect to Level of Depression
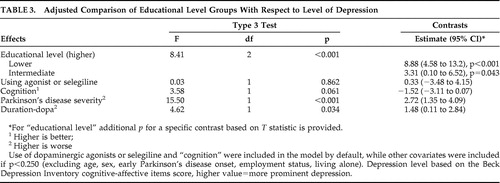
In our analysis of covariance (ANCOVA) with adjustment for “cognition,” use of dopaminergic agonists or selegiline (by default), and covariates with p<0.250 ( Table 3 ), educational level groups were significantly different (F=8.41, df=2, p<0.001) with respect to cognitive-affective BDI items score, and depression level progressively decreased from lower to higher education level group ( Table 3 ). More severe depression appeared associated with worse “Parkinson’s disease severity” and “duration-dopa” ( Table 3 ).
 |
Adjusted Comparison of Educational Level Groups With Respect to Cognitive Performance
In ANCOVA, with adjustment for cognitive-affective BDI score, age (F=41.8, df=1, p<0.001) (by default) and covariates with p<0.250 (“Parkinson’s disease severity,” using dopaminergic agonists or selegiline), educational level groups were significantly different regarding “cognition” (F=12.7, df=2, p<0.001), and cognitive performance progressively increased from lower to higher education level group (not shown).
Adjusted Comparison of Educational Level Groups with Respect to Health-Related Quality of Life
Health-related quality of life (PDQ-39, higher score=poorer quality of life) was compared among the groups in ANCOVA with different adjustment for “cognition” and depression level ( Table 4 ). First, “cognition” and depression level were disregarded; “Parkinson’s disease severity,” “duration-dopa,” and using agonists/selegiline were included by default, while other covariates were included if p<0.250. Educational level groups significantly differed (F=6.45, df=2, p=0.002), and health-related quality of life was progressively better from lower to higher education group ( Table 4 ). Then, depression level was disregarded, whereas “cognition” was added by default (other covariates if p<0.250). Higher education patients had somewhat better health-related quality of life but differences were no longer significant (F=2.73, df=2, p=0.069). Better “cognition” was associated with better health-related quality of life (F=9.98, df=1, p=0.002) ( Table 4 ). Next, “cognition” was disregarded, whereas depression level was included by default (other covariates if p<0.250). Again, higher education level patients had somewhat better health-related quality of life, but differences were not significant (F=1.15, df=2, p=0.321). Higher level of depression was associated with poorer health-related quality of life (F=21.4, df=1, p<0.001) ( Table 4 ). Finally, both “cognition” and depression level were added as default covariates (other covariates if p<0.250) ( Table 5 ). Differences among educational level groups were not significant (F=0.93, df=2, p=0.396), whereas better health-related quality of life was associated with better “cognition” (F=14.7, df=1, p<0.001) and lower depression level (F=29.4, df=1, p<0.001) ( Table 5 ). The effect of “cognition” was conditional on age (F = 10.2, df=1, p=0.002), and the effects of “cognition” and depression were conditional on each other (F = 6.56, df=1, p = 0.012) ( Table 5 ). The score on the PDQ-39 decreased with better “cognition” only in younger patients (below the mean age); PDQ-39 decreased with better “cognition” only in patients with lower BDI scores (below the mean value), whereas PDQ-39 increased with higher BDI scores only in patients with better “cognition” (above the mean value). Poorer health-related quality of life appeared associated with female sex, worse “Parkinson’s disease severity,” and “duration-dopa” ( Table 5 ).
 |
 |
Patient Clustering Based on Depression Level and Health-Related Quality of Life
Cognitive-affective BDI items score and PDQ-39 score were correlated ( τ-b =0.650, p<0.001). Patients formed two clusters: A, with a low depression level and better health-related quality of life (n=51, median BDI=2, quartiles 0–4; median PDQ-39=33, quartiles 20–50), and B, with a higher depression level and poorer health-related quality of life (n=63, median BDI=9, quartiles 5–17; median PDQ-39=86, quartiles 74–100). Prevalence of patients allocated to cluster B was 79.2% for lower, 58.5% for intermediate, and 35.1% for higher education level groups. In a regression analysis, risk of allocation to cluster B was greater for lower and intermediate than higher education level patients ( Table 6 ).
 |
DISCUSSION
In our study, nondemented nonpsychotic Parkinson’s disease patients showed a gradually better cognitive performance, lower degree of depression, and better health-related quality of life across three educational level groups based on length of education. Between-group differences in cognition and depression were significant with adjustment for sociodemographic and Parkinson’s disease-related characteristics (history, severity/motor symptoms, and activities of daily living/treatment modalities) and for degree of depression when cognition was an outcome, or for cognition when depression was an outcome. Differences in health-related quality of life were significant with adjustment for sociodemographic and Parkinson’s disease-related characteristics, but not with additional adjustment for cognitive performance and particularly for the depression level. Data suggest that in nondemented nonpsychotic Parkinson’s disease patients, more years of education “dose-dependently” favors milder depressive difficulties and better health-related quality of life. Regarding the latter conclusion, we argue that education and health-related quality of life are linked indirectly, through the effect of education on cognition and particularly on depression level. In elaboration of this view, we use the term “effect” only to describe the relationship between independent and dependent variable(s) without implications of causality, considering the cross-sectional nature of the data. Also, we consider health-related quality of life as a “final” measure of the burden conveyed by the motor and nonmotor Parkinson’s disease aspects, although poorer health-related quality of life clearly may inversely influence the level of depression.
Longer (better) education is a well-established determinant of cognitive performance in Parkinson’s disease patients. 10 – 14 Based on medical histories, it appeared that in our study participants, academic achievements preceded their ongoing difficulties (including depression) and the estimated “education effect” on depression level was large in size (around 1.8-fold difference in least-square BDI mean values between each two of the three consecutive educational levels) and reasonably unbiased and unconfounded considering the covariates accounted for. Hence, with the limitations intrinsic to retrospective data collection, the study suggests that education might also be viewed as a determinant of depressive difficulties (in nondemented nonpsychotic Parkinson’s disease patients). Depression in Parkinson’s disease is considered to be partly due to awareness about the Parkinson’s disease diagnosis. 3 , 4 The association between better cognition and less severe depression in Parkinson’s disease has been well established. 8 Present data suggest that the effect of education on depression goes beyond the influence it may have on cognitive abilities and that it is independent of disease severity and Parkinson’s disease treatment modalities (some of which may affect mood, particularly dopaminergic agonists and monoamine oxidase B inhibitors). We hypothesize that for a given cognitive status (and Parkinson’s disease severity and treatment), more well-educated nondemented nonpsychotic Parkinson’s disease patients might be more capable of rationalization and/or appropriate disease and self perception and that these factors could contribute to less severe depressive difficulties.
In our analysis of health-related quality of life, degree of depression (less so cognitive performance) showed all the characteristics defined by Baron and Kenny 26 as indicative of its “intervening” or “mediator” nature; it was significantly (and independently) associated with both health-related quality of life (a dependent variable) and educational level (an independent variable), and when introduced into the model fitted to health-related quality of life, the association between education and health-related quality of life was no longer significant. We performed an ad hoc analysis of “mediation”; 27 accounting for all the covariates depicted in Table 5 , total effect of education on health-related quality of life (T = −3.538, p<0.001) was 84% indirect, and the direct effect was insignificant (T=−0.498, p=0.619). Therefore, it seems justified to suggest that education has a beneficial effect on health-related quality of life that is due to its effects on cognition and, particularly, on depression. Such reasoning appears plausible as the concept of self-perceived life satisfaction rating is inherently susceptible to emotional status (mood) and (in)ability of rationalization. We find the present cluster analysis to further support the relevance of education for degree of depression and health-related quality of life; patients were clearly separated based on depression level/health-related quality of life, and the risk of allocation into a “high depression/poor health-related quality of life” cluster was independently higher in poorly educated patients than in more well-educated patients.
In addition to the limitations of cross-sectional design, present data are also of limited generalizability (relatively small sample gathered at a single center, restricted to nondemented patients free of hallucinations, delusions, and antidepressant treatment). Still, we believe that the presented estimates are reasonably unbiased and that the study convincingly points to education as a relevant element to consider in studies of depression and health-related quality of life in Parkinson’s disease patients.
1. Dowding CH, Shenton CL, Salek SS: A review of the health-related quality of life and economic impact of Parkinson’s disease. Drugs Aging 2006; 23:693–721Google Scholar
2. Schrag A: Quality of life and depression in Parkinson’s disease. J Neurol Sci 2006; 248:151–157Google Scholar
3. Veazey C, Aki SOE, Cook KF, et al: Prevalence and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2005; 17:310–323Google Scholar
4. Lieberman A: Depression in Parkinson’s disease: a review. Acta Neurol Scand 2006; 113:1–8Google Scholar
5. Schrag A, Jahanshahi M, Quinn N: What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry 2000; 69:308–312Google Scholar
6. Carod-Artal FJ, Vargas AP, Martinez-Martin P: Determinants of quality of life in Brazilian patients with Parkinson’s disease. Mov Disord 2007; 22:1408–1415Google Scholar
7. Carod-Artal JF, Ziomkowski S, Mesquita HM, et al: Anxiety and depression: main determinants of health-related quality of life in Brazilian patients with Parkinson’s disease. Parkinsonism Relat Disord 2008; 14:102–108Google Scholar
8. Emre M: Dementia associated with Parkinson’s disease. Lancet Neurol 2003; 2:229–237Google Scholar
9. Klepac N, Trkulja V, Relja M, et al: Is quality of life in non-demented Parkinson’s disease patients related to cognitive performance? A clinic-based cross-sectional study. Eur J Neurol 2008; 15:128–133Google Scholar
10. Glatt SL, Hubble JP, Lyons K, et al: Risk factors for dementia in Parkinson’s disease: effect of education. Neuroepidemiology 1996; 15:20–25Google Scholar
11. Levy G, Jacobs DM, Tang MX, et al: Memory and executive function impairment predict dementia in Parkinson’s disease. Mov Disord 2002; 17:1221–1226Google Scholar
12. Green J, McDonald WM, Vitek JL, et al: Cognitive impairment in advanced PD without dementia. Neurology 2002; 59:1320–1324Google Scholar
13. Pai MC, Chan SH: Education and cognitive decline in Parkinson’s disease: a study of 102 patients. Acta Neurol Scand 2001; 103:243–247Google Scholar
14. Cohen OS, Vakil E, Tanne D, et al: Educational level as a modulator of cognitive performance and neuropsychiatric features in Parkinson’s disease. Cogn Behav Neurol 2007; 20:68–72Google Scholar
15. Cubo E, Rojo A, Ramos S, et al: The importance of education and psychological factors in Parkinson’s disease quality of life. Eur J Neurol 2002; 9:589–593Google Scholar
16. Hughes AJ, Daniel SE, Kilford L, et al: Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55:181–184Google Scholar
17. Crum RM, Anthony JC, Bassett SS, et al: Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993; 18:2386–2391Google Scholar
18. Visser M, Leentjens AFG, Marinus J, et al: Reliability and validity of the Beck Depression Inventory in patients with Parkinson’s disease. Mov Dis 2006; 21:668–672Google Scholar
19. Jenkinson C, Fitzpatrick R, Peto V, et al: The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing 1997; 26:353–357Google Scholar
20. Golden CJ: Stroop Color and Word Test. Chicago, Stoelting Company, 1978Google Scholar
21. Smith A: Symbol Digit Modalities Test: Manual. Los Angeles, Western Psychological Services, 1991Google Scholar
22. Schmidt M: Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, Western Psychological Services, 1996Google Scholar
23. Lezak MD: Neuropsychological Assessment, 2nd ed. New York, Oxford University Press, 1983Google Scholar
24. Reitan RM: Trail Making Test: Manual for Administration and Scoring. South Tuscon, Ark, Reitan Neuropsychology Laboratory, 1992Google Scholar
25. Fahn S, Elton RL, UPDRS Development Committee: Unified Parkinson’s Disease Rating Scale, in Recent Developments in Parkinson’s Disease, vol 2. Edited by Fahn S, Marsden CD, Goldstein M, et al. New York, MacMillan, 1987, pp 153–163Google Scholar
26. Baron RM, Kenny DA: The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986; 51:1173–1182Google Scholar
27. Preacher KJ, Hayes AF: Asymptomatic and resampling strategies for assessing and comparing indirect effect in multiple mediator models. Behav Res Methods 2008; 40:879–891Google Scholar



