SPECT Imaging of Dopamine Transporters With 99mTc-TRODAT-1 in Major Depression and Parkinson's Disease
Abstract
To investigate dopamine transporter in major depressive disorder and Parkinson's disease, the authors obtained single photon emission computed tomography (SPECT) brain images from 13 patients with major depression, 17 Parkinson's disease patients, and 10 healthy volunteers by using 99mTc-TRODAT-1. The authors found the 99mTc-TRODAT-1 radio signal in the striatum was reduced in the majority of patients with major depressive disorder, and this decrease was even more severe in patients with Parkinson's disease. The results support the hypothesis of dopamine hypofunction in major depressive disorder and suggest that deficient dopamine transporter may be involved in the etiology of severe major depressive disorder.
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized pathologically by a loss of dopaminergic neurons in the substantia nigra with resultant depletion of striatal dopamine and presence of Lewy bodies in the remaining neurons. Clinically, patients with schizophrenia and major depressive disorder sometimes have psychomotor disturbances similar to those found in patients with PD. Dysfunction of the dopamine system is believed to play a vital role in the psychomotor disturbances.1,2 Like PD, diagnosis of major depressive disorder is currently mainly based on clinical symptoms. Computed tomography (CT) and magnetic resonance imaging (MRI) do not show any characteristic changes in these two disorders, so that they do not offer significant help in clinical diagnosis. Nonetheless, sensitive and specific techniques for diagnosis of major depressive disorder and PD are urgently required. In recent years, single photon emission computed tomography (SPECT) has been extensively used to study brain dopamine transporter in PD.3,4
According to the monoamine hypothesis of major depressive disorder based on the abnormality of the 5-hydroxytryptamine (5-HT) system, depression may result from norepinephrine hypofunction. It was proposed that dysfunction of mesolimbic dopamine system may play an important role in major depressive disorder,5 whereas hypofunction of dopamine D1 receptors is also considered to be involved in the etiology of major depressive disorder.5 The striatum of the normal human brain contains a variety of neurotransmitters, including dopamine and its metabolic product homovanillic acid (HVA). With increase in age, the activity of dopamine hydroxylase decreases, and this may further lead to deficiencies in the dopamine system. Since brain dopamine transporter in patients with major depressive disorder (MDD) is poorly understood, this study aimed to investigate the dopamine transporter damage in major depressive disorder and to evaluate the clinical usage of 99mTc-TRODAT-1 SPECT imaging in the diagnosis of major depressive disorder and PD.
METHOD
Participants
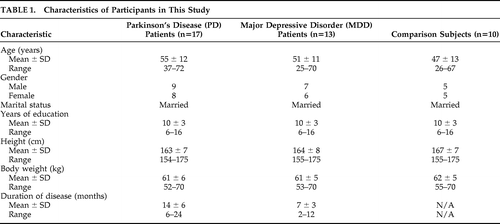
A total of 17 PD patients, 13 MDD patients, and 10 healthy-comparison subjects from our hospital were enrolled in this study. Characteristics of these patients and healthy subjects were summarized in Table 1. No statistical differences (p>0.05) were observed in gender, age, education level, marital status, height, or weight, among PD patients, MDD patients, and healthy participants. All patients met the International Classification of Disease, Version 10 (ICD–10), and PD patients met the U.K. Brain Bank Diagnostic Criteria for Parkinson's disease. Moreover, MDD patients also met the Chinese Classification of Mental Disorders Version 3 (CCMD–3)6 and the DSM–IV criteria for a major depressive episode.7 PD patients had scores of <14 on the 17-item Hamilton Rating Scale for Depression (Ham-D), and MDD patients scored >24. All patients included in this study were first seen in our clinic and initially diagnosed. None of them had received dopaminergic drugs, antidepressants, or antipsychotic drugs within the previous 2 years. Those who had a disease history for the brain or other major organs, including liver, heart, lung, intestine, and kidney, were excluded from the study. Informed consent was obtained from all subjects.
 |
Procedure
To block the 99mTc-TRODAT-1 uptake in the choroid plexus, 400 mg of KClO4 was administered orally 30 minutes before intravenous injection of 99mTc-TRODAT-1 (GMS Pharmaceutical Co., Ltd, Shanghai). SPECT (Millennium VG8 SPECT, GE Medical Systems, Milwaukee, U.S.) imaging was taken 3 hour after 99mTc-TRODAT-1 injection. All subjects remained in a supine resting state. The parameters were as follows: 128×128 matrices; each frame covered 360°, and scanning time lasted for 30 to 40 sec. A total of 64 images were collected and analyzed. Images were reconstructed with attenuation correction. After reorientation of the individual slices parallel to the orbitomeatal line, thickness per slice was 3.125 mm. For semiquantitative analysis, the slices of trans-axial images containing the most intense activity in the striatal area were collected. The region of interest included the bilateral striatum and the cerebellum (CB). The radioactivity ratios of right to left striatum, right striatum to cerebellum, and left striatum to cerebellum were calculated.
Statistical analysis was performed with MANOVA, using SPSS 10.0 software (Chicago, IL; 2003). A p value of <0.01 was considered to be statistically significant.
RESULTS
SPECT dopamine transporter imaging of the brain showed high-density radioactive uptake in corpora striatum, and moderate distribution in brain stem and certain regions of frontal lobe, but little in the occipital lobe and cerebellum. Notably, the dopamine transporter imaging in corpora striatum was different among the healthy-comparison, MDD, and PD groups. SPECT brain imaging indicated that the specific uptake of 99mTc-TRODAT-1 binding was significantly higher in the basal ganglia of brain. There was no significant 99mTc-TRODAT-1 binding in the other areas of the brain in healthy-comparison, MDD, or PD groups. In the healthy-comparison group, most cases showed symmetric, well-defined and even 99mTc-TRODAT-1 radio signals of striata (Figure 1; panel A), whereas in the majority of MDD patients, the signals were reduced, and the outlines of radioactive striatum structures were not well defined (Figure 1; panel B). In PD patients, the radio signals were further reduced, and the full striatum structure was rarely seen (Figure 1; panel C). Only isolated radio dots were seen in the area of striata.
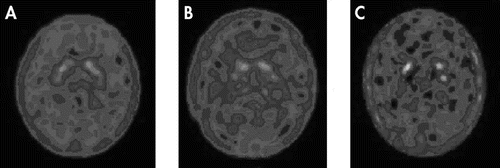
(A) Healthy comparison subject, (B) major depressive disorder (MDD) patient, and (C) Parkinson's disease (PD) patient.
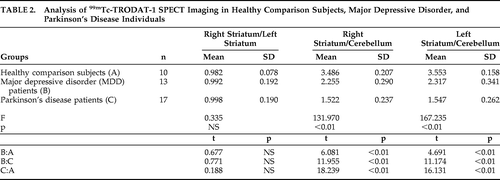
There was no significant difference in the 99mTc-TRODAT-1 signals between the two hemispheres in any of the three groups, so that the right striatum/left striatum ratios were close to 1 in all groups (Table 2). However, when the 99mTc-TRODAT-1 signals were normalized by those in the internal reference cerebellum, we found that both the right striatum/cerebellum and left striatum/cerebellum ratios were significantly decreased in MDD and PD, relative to comparison subjects (Table 2). This decrease was more severe in PD than in MDD. These results suggest that dopamine transporter is deficient in the striatum of patients with MDD, but this deficiency is not as severe as in the brains of patients with PD.
 |
DISCUSSION
Dopamine transporter is a glycoprotein localized in the presynaptic membrane of the dopaminergic neuron, and it plays a key role in maintaining normal brain function, including movement, emotion, and other higher-level cognitive functions. The major function of the dopamine transporter is to take dopamine from the synaptic cleft back to dopaminergic neurons and thereby regulate dopamine level in the synaptic cleft. Dopamine transporter imaging is an important indicator in diagnosing PD.8 At present, 99mTc-TRODAT-1, an agent with high affinity to dopamine transporter, has been used as a brain-imaging agent for assisting in the diagnosis of PD.8–11 Several recent studies have documented monoamine loss for multiple monoamines, excessive clearance of monoamines through greater monoamine transporter binding, and excessive metabolism of monoamines in MDD.12 These observations have led to a monoamine hypothesis of major depressive disorder.
To our knowledge, the present study is the first to compare specific nigrostriatal dopamine system deficits between major depressive disorder and Parkinson's disease. We used the cerebellum as an internal reference and found that the radioactive 99mTc-TRODAT-1 uptake in the striatum over cerebellum (striatum/cerebellum) was markedly decreased in both MDD and PD, relative to age-matched, healthy-comparison subjects. An outstanding feature of the present study is that none of patients had received any treatments with dopaminergic, antidepressants, or antipsychotic drugs within 2 years. The absence of medical treatment for depression and psychomotor symptoms was not uncommon for people age 50 years and older in some areas of China, because it was thought that these symptoms are part of the normal aging process, given the lack of knowledge of mental health issues. This feature of our subjects eliminated any possible effects of the treatments on technetium-99m-TRODAT-1 SPECT imaging. Our findings support the idea of hypofunction of dopamine transporter in the striatum in PD and MDD, and are consistent with findings of previous studies.12,13
Laine et al.14 investigated the relationship between depressive symptoms during alcohol withdrawal and decrease of dopamine transporter availability by using β-CIT SPET. They found a significant correlation between dopamine transporter and depressive symptom scores during withdrawal and after sobriety. The findings suggest a possible role of dopaminergic neurotransmission in depression. In the present study, we found that the radioactivity right striatum/cerebellum and left striatum/cerebellum ratios in MDD patients were significantly lower than those in healthy subjects, although they were not as low as in PD patients. However, the lower dopamine transporter signal in the PD group relative to the MDD group might also be attributed to the longer disease duration (7 months) of Parkinson's disease than major depressive disorder. Leenders15 reported that the estimated annual striatal dopaminergical neuronal loss in PD patients is 6%–11% per year, as assessed with dopamine transporter tracers, whereas the average annual loss in healthy-comparison subjects is about 0.8%.16 Remy et al.17 found no difference in carbon-11-RTI-32 uptake in the striatum or the substantia nigra between Parkinson's disease patients with and without depression. It appears that the presence of Parkinson's disease is more likely to influence the results than the disease duration.
In this study, we found that the dopamine transporter of the basal ganglia region in patients with major depressive disorder was decreased, supporting the dopamine-lesion model of major depressive disorder. Several studies on dopamine transporter imaging in major depressive disorder have been previously reported, but the results did not reach consensus. Some studies indicated that the dopamine transporter of basal ganglia region in patients with major depressive disorder was decreased,18 but other studies showed normal levels both before and after treatment19 or even demonstrated that the dopamine transporter of basal ganglia region in patients with major depressive disorder was increased.20,21 In the present study, the patients had been ill for 7 months, and the eligibility criterion was a score of at least 24 on the 17-item Ham-D, plus psychomotor retardation. This suggests that the patients included in the present study had a longer interval between the onset of disease and the first clinical visit and were more severe in symptoms. Furthermore, various endogenous dopamine levels under different conditions could also affect the number of receptors available, reducing the radio ligand uptake in these studies.
Tzen et al.11 studied dopamine transporter levels in the striatum and putamen by 99mTc-TRODAT-1 and reported that dopamine transporter level at the side opposite to the diseased limb is decreased in PD patients. We did not find any significant difference in dopamine transporter uptake between the two hemispheres of the brain in PD patients. The discrepancy between our study and Tzen et al.'s may be the result of the research subjects included in the two studies. We also found that there was no difference in dopamine transporter signals between the two hemispheres in major depressive disorder, suggesting doparminergic hypofunction in the striatum of both sides of the brain.
In conclusion, we demonstrated a deficient dopamine transporter in the striatum in major depressive disorder. These results support the hypothesis of dopamine hypofunction in major depressive disorder and suggest that deficient dopamine transporter may be involved in the mechanism of severe major depressive disorder.
1. : Brain-derived neurotrophic factor: the neurotrophin hypothesis of psychopathology. CNS Spectr 2008; 13:945–949Crossref, Medline, Google Scholar
2. : Psychomotor symptoms in depression: a diagnostic, pathophysiological and therapeutic tool. J Affect Disord 2008; 109:1–20Crossref, Medline, Google Scholar
3. : Comparison between a dual-head and a brain-dedicated SPECT system in the measurement of the loss of dopamine transporters with [123I]FP-CIT. Eur J Nucl Med Mol Imaging 2008; 35:1343–139Crossref, Medline, Google Scholar
4. : Optimized, automated striatal uptake analysis applied to SPECT brain scans of Parkinson's disease patients. J Nucl Med 2007; 48:857–864Crossref, Medline, Google Scholar
5. Shen YT (ed): Psychiatry, 5th ed. Beijing, The People's Health Publishing, 2009, pp 548–551Google Scholar
6.
7.
8. : Dopamine transporter distribution in patients with Parkinson disease of different stages detected using single-photon emission computed tomography brain imaging. Neural Regen Res 2007; 2:18–21Crossref, Google Scholar
9. : Imaging of dopamine transporters in humans with technetium 99m-TRODAT-1. Eur J Nucl Med 1996; 23:1527–1530Crossref, Medline, Google Scholar
10. : [99mTc]TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson disease. Neurology 2005; 64:1716–1720Crossref, Medline, Google Scholar
11. : Differential diagnosis of Parkinson's disease and vascular parkinsonism by (99m)Tc-TRODAT-1. J Nucl Med 2001; 42:408–413Medline, Google Scholar
12. : Applying neuroimaging ligands to study major depressive disorder. Semin Nucl Med 2008; 38:287–304Crossref, Medline, Google Scholar
13. : Imaging of dopamine transporters in the human brain in healthy and Parkinsons disease with 99Tcm-TRODAT-1 SPECT. J Nucl Med 1999; 40:27–30Google Scholar
14. : Dopamine transporter availability and depressive symptoms during alcohol withdrawal. Psychiatry Res 1999; 90:153–157Crossref, Medline, Google Scholar
15. : Neuroimaging methods applied in Parkinson's disease. J Neurol 2004; 251(suppl 6): VI/7-VI/12Crossref, Google Scholar
16. : Age-related decline in striatal dopamine transporter binding with iodine-123-beta-CIT SPECT. J Nucl Med 1995; 36:1175–1181Medline, Google Scholar
17. : Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 2005; 128:1314–1322Crossref, Medline, Google Scholar
18. : Lower dopamine transporter binding potential in striatum during depression. Neuroreport 2001; 12:4121–4125Crossref, Medline, Google Scholar
19. : Dopamine transporter availability in medication free and in bupropion treated depression: a 99mTc-TRODAT-1 SPECT study. J Affect Disord 2005; 89:115–123Crossref, Medline, Google Scholar
20. : Greater availability of brain dopamine transporters in major depression shown by [99m Tc]TRODAT-1 SPECT imaging. Am J Psychiatry 2003,Oct 160:1836–1841Crossref, Medline, Google Scholar
21. : Striatal dopamine transporter density in major depression. Psychopharmacology 1999; 144:282–285Crossref, Medline, Google Scholar



