HIV and Age Do Not Substantially Interact in HIV-Associated Neurocognitive Impairment
Abstract
The authors investigated the combined age and HIV effects on cognitive functions in 146 individuals, 116 of whom had HIV infection. Forty-two percent had HIV-associated neurocognitive disorder, and all were receiving highly active antiretroviral therapy. Using linear and nonlinear regression modeling, the authors found only a trending effect of the quadratic term HIV status × age, both including dementia cases (p=0.12) and excluding dementia cases (p<0.06). Our results suggest that either this early-2000 cohort is not old enough to detect a clear interactive age and HIV effect or that there may be a survivor bias for individuals with long-term infection. Further longitudinal studies are warranted.
The prevalence of HIV-associated neurocognitive disorders (HAND) has not changed despite the introduction of highly active antiretroviral therapy (HAART), in sharp contrast to other AIDS-defining illnesses.1–4
There has been increasing interest in the effect of age as a contributing factor to the persistence of HIV-associated neurocognitive disorder in the HAART era.5,6 Several studies have shown increased cognitive impairment in older HIV-positive (HIV+) individuals receiving HAART compared to younger HIV+ individuals receiving HAART.7–10
However, one important limitation in the studies conducted to-date has been that they have simply compared groups of older with younger HIV+ individuals, and none has included age-matched comparison groups. Therefore the cognitive impairment observed in the older patients reflects both the effect of HIV and the effect of age. Furthermore, splitting a continuous dimension such as age on the basis of some arbitrary cut-point reduces the power of the studies to detect any true effects of age. Greater statistical power would be obtained by treating age as a covariate in the statistical analysis of performance on cognitive tests (e.g., standard regression method). It is also possible that relationships between age and cognitive performance are not linear, and, therefore, statistical analysis should consider the potential for nonlinear effects of age on neuropsychological performance.11
The aim of our study was to investigate whether there was a linear and/or nonlinear differential age effect on neuropsychological performance between a group of advanced HIV+ individuals relative to HIV-negative (HIV−) healthy volunteers with similar demographic and risk-factor characteristics. We also investigated whether a linear and/or nonlinear differential effect of age was dependent on the degree of cognitive dysfunction by comparing neuropsychologically-normal to mildly neuropsychologically-impaired individuals, and to severely neuropsychologically-impaired individuals within the HIV+ group. The age effect was tested both dichotomously and continuously. A significant differential effect of age would imply that HIV and age have additional or synergistic effects on cognitive functions. A nonsignificant finding would suggest that the issue should be reinvestigated in older individuals and/or that a survivor bias may be at play.
METHOD
Participants
The HIV+ HAART cohort was initially composed of 115 HIV+ men and 1 HIV+ woman recruited from St. Vincent's Hospital in Sydney, Australia.1 These participants had been enrolled in a prospective study of neurocognitive function if they had advanced HIV infection (1993 CDC Classification Criteria C3) and were receiving highly active antiretroviral therapy (HAART).
The exclusion criteria were neurological and psychiatric disorders (predating or unrelated to HIV), head injury with loss of consciousness greater than 1 hour, current alcohol abuse or drug dependence, and current active opportunistic infection. Eight individuals (6%) were co-infected with hepatitis C.
Seronegative comparison subjects (30 men) were screened for significant neurological or psychiatric disorders. They self-reported being HIV-negative within the last 3 months. If not tested during this time-frame, the participant was requested to have a test performed by their general practitioner.
Procedure
All participants were assessed with a standard neuropsychological battery.1 Also, cognitive complaints were collected to determine the clinical meaningfulness of neuropsychological impairment. For this, we asked each participant before the assessment whether they had memory complaints (coded as a Yes/No answer). All participants provided their informed consent before study entry. Local ethics committees approved the research protocols.
Raw neuropsychological scores were transformed into T scores using published normative data inclusive of corrections for age, education level, gender, and Caucasian ethnicity.11 Six T-score domain abilities were defined by averaging individual neuropsychological measures: speed of information-processing, executive function, learning, memory, motor, and verbal. HIV-associated neurocognitive disorder was determined following Antinori et al.11 nomenclature. Using memory complaints as a surrogate of functional decline, we were able to classify each case as either having asymptomatic neurocognitive impairment, mild neurocognitive disorder, or HIV-associated dementia.
Global rate of impairment reached 42.2% in the HIV+ cohort (49/116). Using the HIV-associated neurocognitive disorder (HAND) nomenclature, we found that 7.7% (9/116) had asymptomatic neurocognitive impairment; 23.3% (27/116) had mild neurocognitive disorder; and 11.2% (13/116) had HIV-associated dementia. Rate of neuropsychological-impairment in the HIV-negative comparison subjects was 10% (3/30; Global Deficit Score method13). As expected, this was significantly different from the HIV+ cohorts (p<0.0001). Demographic and clinical characteristics are presented in Table 1.
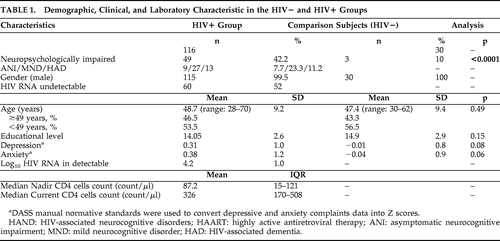 |
Data Analysis
Group comparisons for demographic and clinical variables were performed using t-tests, ANOVAs, or chi-square analyses. For the following analyses, we used a summary scaled score (averaged scaled score across all neuropsychological measures) to test the effect of age across the groups because it provides a common metric, is normally distributed and is not corrected for demographic factors. This scaled score transformation also cancels out potential outliers that may overinfluence the subsequent regression analyses.14
We developed regression models to determine linear and nonlinear effects of age on neuropsychological performance. We ran the first analyses (see Cysique et al.1) using a Group factor (as HIV+ versus HIV–). Second, the data were reanalyzed, treating cognitive performance as a grouping factor (as neuropsychologically-normal, neuropsychologically-impaired—mild, and neuropsychologically-impaired—moderate-to-severe) within the HIV+ group to investigate effect on HIV-associated neurocognitive-disorder severity. These analyses used age as a continuous variable. The analyses using age as a dichotomous variable did not include the quadratic term and the second-order interaction. Our central question was to test whether there was a differential age effect between the HIV+ and HIV− groups. A significant age × performance interaction would indicate that the HIV+ older individuals performed much worse than their HIV− counterparts. A significant quadratic age interaction would indicate that the effect of age is steeper at the right-hand side of slope curvature for the HIV+ than for the HIV− subjects. An inverse U-shape of the term is expected because of the group coding (Table 2). In the HIV+ group model, an interaction of HIV+ impairment × age and/or quadratic term for the same interaction would indicate that the effect of age is steeper as a function of impairment severity.
Linear and Nonlinear (Quadratic) Effect of Age Model

Where Yp is the predicted mean scaled score, β1 is the regression coefficient (slope) for predictor X1 (group), β2 for predictor X2 (age) and, their interaction (group × age), age to a degree =2 (age2), and the interaction to a degree =2 ([group× age]2), and a is the intercept.
This model was reconducted in the same groups while excluding the 13 individuals diagnosed with HIV-associated dementia. These extra analyses were performed because HIV-associated dementia severity in relation to age may mask differential age effect that could only be present in a milder form of HIV-associated neurocognitive disorder.
This model was rerun separately to determine whether there was any linear effect of education or self-reported depressive and anxiety symptoms.
We then investigated the effect of the following HIV disease markers on neuropsychological performance (defined by the Global Deficit Score) in the complete HIV+ group, using correlation analyses: nadir CD4, current CD4, number of AIDS-defining illnesses and HIV duration, as well as log10 plasma viral load as a surrogate of systemic antiretroviral treatment efficacy. We then used the HIV disease markers found to be associated with neuropsychological performance as covariates in our regression models.
Age was distributed normally. Depression and anxiety scores were transformed into Z scores using published norms.15 No cases had missing data. Statistical analyses were conducted using the statistical package JMP 7 (SAS Institute, Inc.; 2007)
RESULTS
As expected, we found significant HIV-status and age effects in all analyses; namely, HIV+ individuals had lower neuropsychological performance than comparison subjects and that older participants had lower neuropsychological performance than younger individuals, independently of their HIV status.
A trending quadratic interaction for age × group (p=0.12) was found in the HIV+ subjects relative to HIV− comparison subjects, showing a trend for worse neuropsychological performance in older HIV+ individuals (Table 2).
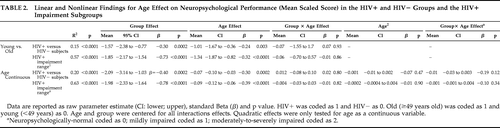 |
A trending quadratic interaction for age × group (p<0.06) was found for worse neuropsychological performance in older HIV+ individuals relative to HIV− comparison subjects, while excluding individuals with HIV-associated dementia (Table 3). The contribution of this term to the model R2 was 13%. In comparison, the total age effect contributed to 45% (see Figure 1).
 |
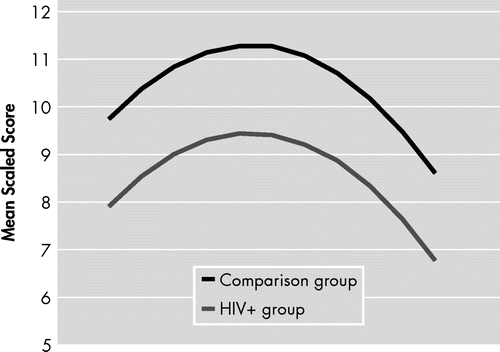
For all models, the comparisons Old versus Young did not yield any significant finding regarding the interaction between age and HIV status (see Table 2 and Table 3).
As expected, for all models, education contributed significantly (p<0.01). The trending quadratic interaction for age × group (p=0.10) remained as such only in the model involving the HIV+ to HIV− comparison subjects, while excluding individuals with HIV-associated dementia. In all other models, this quadratic term was nonsignificant.
The severity of depressive complaints did not contribute significantly to the model; however, the severity of anxiety complaints did contribute (p<0.01) in all analyses. All other findings remained similar.
Among the HIV-related disease markers, only viral load was negatively related with neuropsychological performance as measured by the Global Deficit Score (r=−0.20, p=0.03). Plasma viral load tended to be associated in the same manner with neuropsychological performance (mean scaled score) in our HIV+ -only regression models (including the HIV-associated dementia cases [p<0.06] and excluding the HIV-associated dementia cases [p<0.03]). However, the age-and-HIV interaction remained nonsignificant; the quadratic term of HIV × age was nonsignificant in the model including all HIV+ and remained trending (p=0.09) in the model excluding the HIV-associated dementia cases.
DISCUSSION
The main finding of this study was that no clear combined effect of HIV and age on cognitive function was detected. This was despite thorough statistical exploration (both linear and nonlinear) as well as with the use of age as both a continuous variable and a dichotomous variable. As such, this study is the first, in the HAART era, to explore potential multidimensional effects of age on neuropsychological performance in both an HIV+ sample and an HIV− demographically comparable sample.
However, when we focused on mild HIV-associated neurocognitive disorder, and versus HIV− comparison subjects, we found a trending effect for a steeper curvature of age × HIV effect on neuropsychological performance (contributing up to 13% of variance explanation in the regression model), meaning that, for some older HIV+ individuals, there is a precipitation of neuropsychological performance in the impairment range. This trending effect was still present after adjustment for plasma viral load in the analyses testing the effect of impairment range within the HIV+ group.
The first reason for a mostly negative finding may be that, as in previous studies,7–10 our cohort did not include many individuals in their 60s or 70s (12% ≥60 years old in the HIV+ sample and 8% ≥60 years old in the HIV− sample). Indeed, as for other neurological conditions, it may be that it is only after the age of 60 or even 70 that age may increase HIV disease-related cognitive impairment in an additive or synergistic fashion. As such, the current negative finding should not preclude further long-term analysis of this question. Some have argued that a “threshold effect” may be at stake.16 An adverse effect of age and HIV may only happen for individuals with long enough HIV duration and advanced age. Moreover, the time of HIV infection may also play a role, in that individuals infected after their 50s have more rapid HIV progression than individuals infected at a younger age.16 Whether this could also represent an accelerated aging and HIV effect in the brain remains to be investigated.
The second reason that we were not able to detect an obvious combined effect of age and HIV on brain function is that there may have been some sampling bias. It is possible that this sampling bias may actually affect all current middle-aged HIV+ cohorts and occur because these include mostly individuals who have survived the first two decades of HIV infection, including the time when suboptimal treatment was the only option. Thus, most of these currently middle-aged individuals could be considered as survivors and therefore be particularly resistant to any exacerbation of cognitive effects by age. Modeling “survivor bias” is difficult, however.17 In the current study, the analyses involving the HIV+ group without the HIV-dementia individuals could arguably represent a surrogate testing of this effect in that the more severely impaired could be the least resistant to age effect (note that there was no age difference between the subgroups). The results of the current study suggest that the linear effect of age across all groups explained most of the variance related to age. In other words, the survivor bias may even apply to the most neuropsychological-impaired or these analyses remain an imperfect surrogate for testing a survivor bias.
Interestingly, when considering the number of AIDS-defining illnesses (other than HIV-associated neurocognitive disorder) as another surrogate marker of survivor bias, we found that age was inversely correlated with a higher number of AIDS-defining events (r=−0.24, p<0.008). This control analysis supports the hypothesis that a proportion of current middle-aged HIV+ individuals could be considered survivors, and they could therefore bias the sample. This may be especially the case in metropolitan cohort composed of gay and bisexual men, such as in Australia,18 because those also represent individuals who are generally well educated (as it is the case for the current cohort), and have universal healthcare access. These last two factors have been consistently associated with greater life-expectancy in the general population.19 Higher educational level has also been associated with greater cognitive reserve in HIV populations.20
Our nonlinear and trending finding could still imply that an effect of age may happen as a sudden drop in neuropsychological performance for some older HIV+ individuals. We believe that we observed this slightly more robust finding in individuals with mild HIV-associated neurocognitive disorder for two potential reasons: 1) In HIV-dementia cases, the age effect may have contributed to the occurrence of dementia, rather than its current characteristics in terms of neuropsychological performance; and 2) HIV-associated dementia, because of its severity, is or has become a different neuropathological entity. Although this question is not central to the current study, our finding suggests that HIV-associated dementia cases may need to be discriminated in some instances in NeuroAIDS studies.
If it were the case that age precipitates rather than only amplifies HIV-related cognitive impairment in some individuals, and more likely in those considerably over the age of 60, then there is reasonable concern that, within a few years, these persons may develop a more aggressive form of HIV-associated neurocognitive disorders with or without involving a direct neurodegenerative pathway.16 The nonlinear trending finding also suggests that some older individuals well over 60 may develop sudden or acute cognitive decline because a threshold of brain injury has been reached, even if these individuals were somewhat more resistant to the effect of aging during their middle-aged years.
Several limitations to our study should be mentioned. Our analysis was cross-sectional in nature. A similar multidimensional exploration of HIV and age will be necessary in longitudinal studies. Our control group was of small size, potentially increasing the neuropsychological performance variance in this group and reducing our power to detect an age-and-HIV effect. Although this is possible, despite the sample size, our control group performed well within the normal range, therefore minimizing the adverse effect of large variance on model predictions. Moreover, at the time of neuropsychological testing, we did not collect cardiovascular disease-related information on our participants. It could be that, for some individuals, the age effect may be mediated by greater cardiovascular risks. This issue has recently been emphasized in HIV infection21 and will need greater investigation.
1. : Prevalence and pattern of neuropsychological impairment in HIV/AIDS-infection across pre and post- highly active antiretroviral therapy eras: a combined study of 2 cohorts. J Neurovirol 2004; 10:350–357Crossref, Medline, Google Scholar
2. : Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol 2003; 9:205–221Crossref, Medline, Google Scholar
3. : The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007; 21:1915–1921Crossref, Medline, Google Scholar
4. : Neurobehavioral effects of human immunodeficiency virus infection among former plasma donors in rural china. J Neurovirol 2008; 7:1–14Google Scholar
5. : Evidence for a change in aids dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of aids dementia complex. AIDS 2004; 18:S75–S78Crossref, Medline, Google Scholar
6. : HIV infection and dementia in older adults. Clin Infect Dis 2006; 42:1449–1454Crossref, Medline, Google Scholar
7. : Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS 2004; 18:S11–S18Crossref, Medline, Google Scholar
8. : Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. AIDS 2004; 18:S27–S34Crossref, Medline, Google Scholar
9. : Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. J Neurovirol 2007; 13:203–209Crossref, Medline, Google Scholar
10. : Higher frequency of dementia in older HIV-1 individuals. The hawaii aging with HIV-1 cohort. Neurol 2004; 63:822–827Crossref, Medline, Google Scholar
11. : Updated research nosology for HIV-associated neurocognitive disorders. Neurol 2007; 69:1789–1799Crossref, Medline, Google Scholar
12. : Revised comprehensive norms for an expanded Halstead-Reitan Battery: demographically adjusted neuropsychological norms for African American and Caucasian adults Scoring Program. Odessa, Fla, Psychological Assessment Resources, 2004Google Scholar
13. : Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004; 26:307–319Crossref, Medline, Google Scholar
14. : Using Multivariate Statistics, 5th edition. Boston, Person International Edition, 2007Google Scholar
15. : Manual for the Depression Anxiety Stress Scales, 2nd edition. Sydney, Australia, Psychology Foundation, 1995Google Scholar
16. : Neurodegeneration and ageing in the HAART Era. J Neuroimmune Pharmacol 2008; 6:6Google Scholar
17. : Strengths and barriers in conducting research among older adults with HIV. Psychol Rep 2003; 92:949–950Crossref, Medline, Google Scholar
18. : Rapidly ageing HIV epidemic among men who have sex with men in Australia. Sex Health 2009; 6:83–86Crossref, Medline, Google Scholar
19. : Differential impacts of health care in Australia: trend analysis of socioeconomic inequalities in avoidable mortality. Int J Epidemiol 36:157–165, 2007Crossref, Medline, Google Scholar
20. : Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Arch Neurol 1996; 53:148–153Crossref, Medline, Google Scholar
21. : Pathogenesis of cardiovascular disease in HIV infection. Curr Opin HIV AIDS 2008; 3:234–239Crossref, Google Scholar



